Abstract
Timely recognition and elimination of invasive group A Streptococcus (GAS) by innate immunity is crucial to control infection. The intracellular pattern recognition receptor Toll-like receptor 9 (TLR9) promotes macrophage hypoxia-inducible factor-1α levels, oxidative burst and nitric oxide production in response to GAS. TLR9 contributes to GAS clearance in vivo in both localized cutaneous and systemic infection models.
Key Words: Innate immunity, Toll-like receptor 9, Group A Streptococcus
Introduction
The Gram-positive bacterium group A Streptococcus (GAS) is a leading human pathogen causing 1700 million superficial infections such as pharyngitis or pyoderma and 1650,000 invasive infections worldwide every year [1]. The host's initial defense mechanisms against GAS involve the physical barriers of mucosa or skin and their commensal microflora. Once GAS has breached the epithelium, eradication by soluble factors and phagocytes is required, and quick and accurate recognition of GAS by the host innate immune system can prevent disease spread. Pattern recognition receptors such as Toll-like receptors (TLRs) recognize conserved molecular patterns from pathogens; most TLRs will initiate signal transduction via the central adaptor protein myeloid differentiation factor 88 (MyD88). MyD88 has recently been shown to play an important role in innate defense against GAS infection [2].
Though classically described as an extracellular pathogen, studies have shown that GAS may survive intracellularly for several hours within epithelial cells [3, 4, 5], macrophages [6, 7] and neutrophils [8, 9]. These data led us to hypothesize that the intracellularly localized TLR9 could be important for GAS detection and elimination. TLR9 is found in endosomes and is stimulated by nonmethylated DNA and synthetic CpG oligodeoxynucleotides [10, 11] or during infection with obligate intracellular bacteria including Listeria monocytogenes and Legionella pneumophila[12, 13]. To date, the role of TLR9 in innate defense against bacteria that exist mainly extracellulary has not been fully elucidated [14, 15] and remains controversial [16, 17, 18, 19].
Methods
Mice and Bacterial Strains
C57BL/6 wild-type (WT) and TLR9-deficient mice in the C57BL/6 background were used in this study. TLR9-deficient mice were a gift from Dr. Shizuo Akira (Osaka University, Japan) and were bred and handled in our facilities under the approved protocols of the University of California San Diego Animal Care Committee. WT mice were purchased from Charles River.
GAS M1T1 strain 5448 was originally isolated from a patient with necrotizing fasciitis and toxic shock syndrome [20]. GAS were propagated in Todd-Hewitt broth (Difco, BD Diagnostics) or Todd-Hewitt agar plates. In macrophage and mouse challenge studies, bacteria were grown to logarithmic phase in Todd-Hewitt broth [optical density at 600 nm = 0.4 = approx. 2 × 108 colony-forming units (CFU)/ml], pelleted, washed and resuspended in PBS or in tissue culture media at the desired concentration.
Macrophage Killing Assays
Peritoneal macrophages were isolated from the peritoneal cavity of WT and TLR9-deficient mice 72 h after injection of thioglycollate, as previously described [21]. Harvested macrophages were washed and resuspended in RPMI-1640 containing 10% heat-inactivated fetal bovine serum (FBS). In a 48-well plate, 106 macrophages were added per well and incubated for 24 h at 37°C with 5% CO2. Then cells were washed twice and resuspended in RPMI-1640 containing 2% FBS. Where indicated, the TLR9 antagonist guanosine-rich inhibitory oligonucleotide (G-ODN; 5 μM; Invivogen, Carlsbad, Calif., USA) was added. After 1 h, logarithmic phase bacteria were added to the wells at a final multiplicity of infection (MOI) of 0.1–0.2 and centrifuged for 5 min at 1,500 rpm. After 4 h of incubation at 37°C with 5% CO2, macrophages were detached with trypsin and lysed with 0.025% Triton X in PBS for serial dilutions to enumerate surviving bacteria (plated on Todd-Hewitt agar).
Oxidative Burst and Nitric Oxide Expression
For reactive oxygen species (ROS) determination, bone marrow (BM)-derived macrophages were used. The BM cells of femurs and tibias of WT and TLR9-deficient mice were collected. Cells were plated in Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated FBS and 30% conditioned medium as described elsewhere [22]. Mature adherent BM-derived macrophages were harvested by gentle scraping after 7 days in culture and reseeded for the assay. On day 10, macrophages were washed twice, resuspended in Hanks' balanced salt solution and incubated with 30 μm 2‘,7’-dichlorofluorescein diacetate for 30 min of rotation at 37°C. Cells were washed 3 times with PBS and resuspended in Hanks' balanced salt solution for a final concentration of 5 × 105 in 100 μl of solution per well of a 96-well plate. Where indicated, logarithmic phase bacteria were added at an MOI of 1:1. After 270 min, the fluorescence signal was measured with excitation at 485 nm and emission at 535 nm using a fluorescent plate reader (Wallac II, Perkin Elmer). Nitric oxide (NO) expression was determined by the Griess reaction in peritoneal macrophages as previously described [23]; baseline values (no GAS stimulation) were subtracted from indicated values.
Hypoxia-Inducible Factor-1α Detection by Western Blot and Immunostaining
BM-derived macrophages were harvested and cultured as described above. After 7 days in culture, mature macrophages were reseeded into 100-mm2 wells for Western blot analysis or onto 12-mm2 glass cover slides in 24-well plates for immunostaining. Macrophages were then either challenged with WT GAS (MOI 10) or were not exposed to bacteria. After 1 h of incubation, penicillin and gentamicin (10 and 100 μg/ml, respectively) were added to all the wells and macrophages were incubated for an additional 3 h. For Western blot analysis, cells were harvested and washed (in PBS), and proteins were extracted with RIPA buffer. Nuclear extracts (20 μg) were loaded onto a 12% Tris-tricine gel in a 2-(N-morpholino)ethanesulfonic acid buffer (Invitrogen) for hypoxia-inducible factor (HIF)-1α Western blot using standard methodologies [24]. For immunostaining, cells were immediately fixed with 4% paraformaldehyde and kept at 4°C until further analysis. After washing, cells were permeabilized for 45 min with PBS containing 2% BSA, 0.2% Triton X-100 and 5% goat serum.
After additional washes, rabbit anti-HIF-1α (Novus Biologicals; diluted 1:100 in PBS-Tween) was added for 60 min at room temperature followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG (green; Invitrogen; diluted 1:500 in PBS-Tween) for 45 min at room temperature. After washing, samples were embedded in Prolong Gold antifade containing DAPI for counterstaining (blue; Invitrogen). Mounted samples were examined using an inverted confocal laser scanning 2-photon microscope (Olympus Fluoview FV1000, Olympus × 20/0.7 UPlanSApo objective) with FluoviewTM Spectral Scanning technology (Olympus America, Center Valley, Pa., USA) or, alternatively, using a Zeiss Axiolab microscope (Zeiss × 20/0.5 Plan-Neofluor objective; Carl Zeiss, San Diego, Calif., USA) with an attached Sony Digital Photo Camera DKC-5000 (Sony, USA) at calibrated magnifications. Mean fluorescence intensities as markers for HIF-1α expression levels were measured at equal exposure times and quantified using Image J (National Institutes of Health, Bethesda, Md., USA). HIF-1α levels were calculated by dividing the HIF-1α-specific signal by the background subtracted DAPI-related signal.
Mouse Infection Models
Logarithmic phase GAS were resuspended in PBS and mixed 1:1 with sterile Cytodex beads (Sigma, St. Louis, Mo., USA), and an inoculum of 5 × 107 CFU of GAS was injected subcutaneously into one flank of 10- to 12-week-old WT or TLR9-deficient mice (total injected volume per mouse 100 μl). The size of developing necrotic lesions was monitored daily for 4 days, at which time mice were euthanized and skin lesions were harvested for bacterial counts. In systemic challenge experiments, 12-week-old mice (WT and TLR9-deficient) were given intraperitoneal injections of mid-log phase GAS (5 × 107 CFU) in 500 μl of PBS or PBS alone as a control. After 24 h, blood was collected for bacterial counts.
Statistics
For comparisons of two groups of either paired or unpaired samples we used the nonparametric Wilcoxon test or t test, and for 3-sample comparison we used nonparametric Kruskal-Wallis one-way ANOVA on ranks. If the ANOVA analysis was significant, Bonferroni pair-wise group comparison was performed. The log rank test was used for survival analysis. p values were calculated using the software packages NCSS 2007 (NCSS, Kaysville, Utah, USA) and SPSS Statistics 17.0 (SPSS, Chicago, Ill., USA).
Results and Discussion
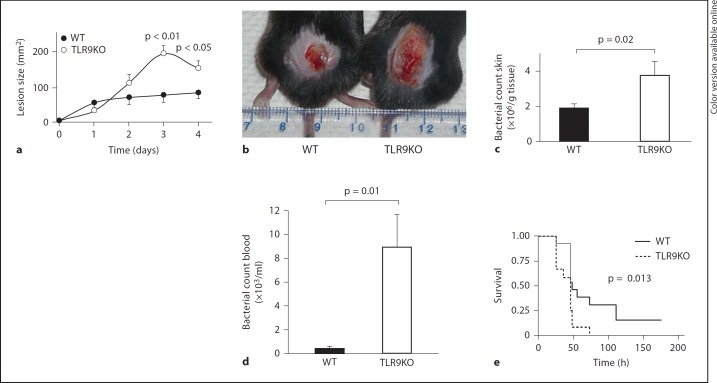
To directly test the importance of TLR9 for the recognition and clearance of GAS in vivo, we challenged WT and TLR9-deficient mice with the invasive M1T1 GAS strain 5448 using a previously described model of necrotizing subcutaneous infection [25] and monitored the development of lesions over 4 days. TLR9-deficient mice developed significantly larger necrotizing skin lesions than did WT mice (fig. 1a, b). Mice were sacrificed on day 4, and the bacterial load in the excised skin lesions of TLR9-deficient mice was significantly higher than that in WT controls (fig. 1c).
Fig. 1.
TLR9 is important for controlling GAS infection in vivo. a C57BL/6 (WT) and TLR9-deficient mice were injected subcutaneously with equivalent inocula of the M1 GAS strain, and skin lesion progression was measured for 4 days. b Representative photograph of skin lesions seen in the subcutaneous challenge experiment. c After 4 days, there were twice as many bacteria in the skin in the TLR9-deficient mice compared to the WT mice. d Using an intraperitoneal infection model, a subset of the mice (5 WT and 4 TLR9-deficient mice) were bled 24 h after GAS infection, and bacteremia was evaluated by serial dilution of the blood. e Survival of WT (n = 13) and TLR9-deficient (n = 12) mice followed over 1 week after intraperitoneal infection. Data are presented as means ± SEM. TLR9KO = TLR9-deficient.
GAS can cause bacteremic infections and toxic shock syndrome with high morbidity and mortality. To test the importance of TLR9 in a systemic infection model, WT and TLR9-deficient mice were injected intraperitoneally with logarithmic phase M1T1 GAS, and blood was collected at 24 h for enumeration of CFUs. TLR9-deficient mice resolved GAS bacteremia less effectively than did WT mice (fig. 1d), and the impaired GAS clearance observed in TLR9-deficient mice was reflected in increased mortality (fig. 1e). Altogether, these animal data show for the first time that TLR9 is important for controlling GAS in vivo during localized cutaneous or systemic infection.
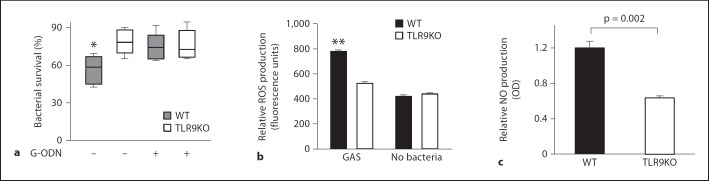
Macrophages, which express TLR9 [26], play a key role in the defense against GAS infections [27, 28]. We hypothesized that the impaired clearance of GAS in TLR9-deficient animals could be explained by inefficient killing of GAS by macrophages lacking TLR9 signaling. We thus studied the contribution of TLR9 to GAS clearance using in vitro killing assays and genetically or pharmacologically modified murine macrophages. GAS was killed efficiently when incubated with WT macrophages; however, killing was significantly impaired if TLR9 was absent or pharmacologically blocked by the TLR9 antagonist G-ODN (fig. 2a).
Fig. 2.
TLR9 mediates killing of GAS by macrophages. a Surviving GAS after incubation with WT macrophages or TLR9-deficient macrophages at an MOI of 1, with and without the TLR9 antagonist G-ODN. b, c WT and TLR9-deficient macrophages were incubated with GAS at an MOI of 1, and ROS (b) and NO (c) was quantified. Data are given as box plots with n = 6 per point and 3 repeats (a) or as means ± SEM with n = 6 (b) and n = 3 (c) (representative experiment from 3 independent experiments). ANOVA was significant at p = 0.01, with Bonferroni pair-wise group comparison at * p < 0.05 and ** p < 0.005. TLR9KO = TLR9-deficient; OD = optical density at 540 nm.
Macrophages kill GAS efficiently in part through production of ROS and NO [28]. Macrophages from WT and TLR9-deficient mice were incubated with GAS, and ROS and NO production was quantitated after 30 min. TLR9-deficient macrophages produced significantly less ROS (fig. 2b) and NO (fig. 2c) in response to GAS than did WT macrophages. These results suggest that impaired control of GAS infection in TLR9-deficient mice may in part be related to diminished NO- and ROS-mediated macrophage killing.
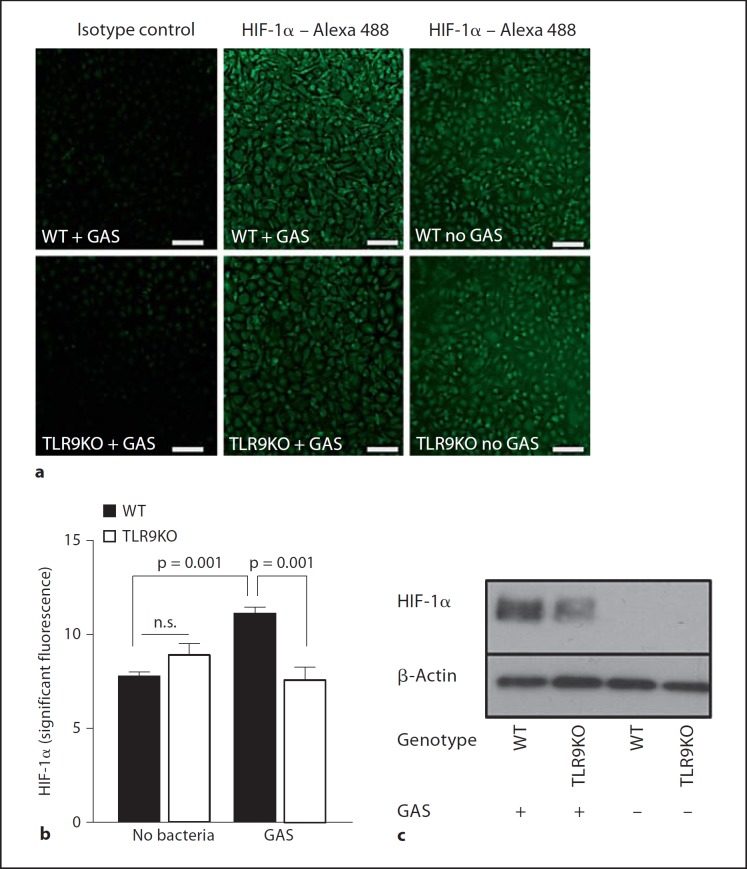
HIF-1α is a key transcriptional regulator orchestrating myeloid cell inflammatory and innate immune responses [29]. GAS infection leads to increased levels of HIF-1α in macrophages [23], and HIF-1α is important for regulating macrophage production of ROS and NO in response to infection [23, 30]. We challenged WT and TLR9-deficient macrophages with GAS and determined the intranuclear content of HIF-1α by immunostaining (fig. 3a, b; online suppl. fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000329550) and Western blotting (fig. 3c). After GAS challenge, HIF-1α stabilization was less pronounced in TLR9-deficient compared to WT mouse macrophages. The residual HIF-1α induction still detectable in the TLR9-deficient mouse macrophages after GAS challenge could be due to the redundant effects of other pattern recognition receptors such as TLR2, TLR4 and TLR6, which contribute to HIF-1α induction and stabilization [31, 32]. likely through NF-κB-dependent increases in HIF-1α transcript [33]. Also, GAS has been shown to be able to induce multiple inflammatory responses via MyD88-dependent TLR2/TLR4/TLR9-independent signaling [19], as has been shown for other Gram-positive pathogens [16, 34].
Fig. 3.
TLR9 promotes HIF-1α expression. WT and TLR9-deficient macrophages were challenged with GAS. a Representative immunofluorescence micrograph. b Quantification of HIF-1α expression (infected cells, n = 14, images of 3 independent experiments; noninfected cells, n = 8, images of 2 independent experiments). c Nuclear extracts of the same cells were analyzed for HIF-1α expression by Western blot with analysis of β-actin as a loading control. Data are presented as means ± SEM. Scale bars = 100 μm. TLR9KO = TLR9-deficient; n.s. = not significant.
In summary, this study shows that TLR9 plays an important role in host defense against GAS infections and that stimulation of TLR9 improves macrophage killing of this leading human pathogen. TLR9-induced stabilization of the transcription factor HIF-1α and increased generation of bactericidal ROS and NO are likely contributing factors to the observed innate immune phenotypes.
Disclosure Statement
This research was supported by the Swiss National Foundation Grants SSMBS PASMA117303/1 (to A.S.Z.), PASMA-114623 (to P.H.) and PASM33-117302 (to R.A.S.), National Institutes of Health grants AI77780 (to V.N.) and AI077989 (to D.A.C.) and the ‘Deutsche Akademie der Naturforscher Leopoldina’ (BMBF-LPD 9901/8-187, to M.v.K.-B.). The authors declare no conflict of interest.
Supplementary Material
Supplementary data
Acknowledgement
We thank Dr. S. Akira (Osaka University, Japan) for providing the initial mouse strains.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Loof TG, Goldmann O, Gessner A, Herwald H, Medina E. Aberrant inflammatory response to Streptococcus pyogenes in mice lacking myeloid differentiation factor 88. Am J Pathol. 2010;176:754–763. doi: 10.2353/ajpath.2010.090422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. Cytolysin-dependent evasion of lysosomal killing. Proc Natl Acad Sci USA. 2005;102:5192–5197. doi: 10.1073/pnas.0408721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osterlund A, Engstrand L. Intracellular penetration and survival of Streptococcus pyogenes in respiratory epithelial cells in vitro. Acta Otolaryngol. 1995;115:685–688. doi: 10.3109/00016489509139387. [DOI] [PubMed] [Google Scholar]
- 5.Osterlund A, Engstrand L. An intracellular sanctuary for Streptococcus pyogenes in human tonsillar epithelium – studies of asymptomatic carriers and in vitro cultured biopsies. Acta Otolaryngol. 1997;117:883–888. doi: 10.3109/00016489709114219. [DOI] [PubMed] [Google Scholar]
- 6.Thulin P, Johansson L, Low DE, Gan BS, Kotb M, McGeer A, Norrby-Teglund A. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med. 2006;3:e53. doi: 10.1371/journal.pmed.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertzen E, Johansson L, Wallin R, Schmidt H, Kroll M, Rehn AP, Kotb M, Morgelin M, Norrby-Teglund A. M1 protein-dependent intracellular trafficking promotes persistence and replication of Streptococcus pyogenes in macrophages. J Innate Immun. 2010;2:534–545. doi: 10.1159/000317635. [DOI] [PubMed] [Google Scholar]
- 8.Medina E, Goldmann O, Toppel AW, Chhatwal GS. Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J Infect Dis. 2003;187:597–603. doi: 10.1086/373998. [DOI] [PubMed] [Google Scholar]
- 9.Staali L, Morgelin M, Bjorck L, Tapper H. Streptococcus pyogenes expressing M and M-like surface proteins are phagocytosed but survive inside human neutrophils. Cell Microbiol. 2003;5:253–265. doi: 10.1046/j.1462-5822.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 10.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 11.Huang LY, Aliberti J, Leifer CA, Segal DM, Sher A, Golenbock DT, Golding B. Heat-killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is Toll-like receptor 2 dependent. J Immunol. 2003;171:1441–1446. doi: 10.4049/jimmunol.171.3.1441. [DOI] [PubMed] [Google Scholar]
- 12.Bhan U, Trujillo G, Lyn-Kew K, Newstead MW, Zeng X, Hogaboam CM, Krieg AM, Standiford TJ. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect Immun. 2008;76:2895–2904. doi: 10.1128/IAI.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol. 2004;173:1179–1183. doi: 10.4049/jimmunol.173.2.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, Akira S, Normark S, Henriques-Normark B. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 15.Sjolinder H, Mogensen TH, Kilian M, Jonsson AB, Paludan SR. Important role for Toll-like receptor 9 in host defense against meningococcal sepsis. Infect Immun. 2008;76:5421–5428. doi: 10.1128/IAI.00615-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henneke P, Takeuchi O, Malley R, Lien E, Ingalls RR, Freeman MW, Mayadas T, Nizet V, Akira S, Kasper DL, Golenbock DT. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J Immunol. 2002;169:3970–3977. doi: 10.4049/jimmunol.169.7.3970. [DOI] [PubMed] [Google Scholar]
- 17.Wieland CW, Florquin S, van der Poll T. Toll-like receptor 9 is not important for host defense against Haemophilus influenzae. Immunobiology. 2010;215:910–914. doi: 10.1016/j.imbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Talati AJ, Kim HJ, Kim YI, Yi AK, English BK. Role of bacterial DNA in macrophage activation by group B streptococci. Microbes Infect. 2008;10:1106–1113. doi: 10.1016/j.micinf.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Gratz N, Siller M, Schaljo B, Pirzada ZA, Gattermeier I, Vojtek I, Kirschning CJ, Wagner H, Akira S, Charpentier E, Kovarik P. Group A streptococcus activates type I interferon production and MyD88-dependent signaling without involvement of TLR2, TLR4, and TLR9. J Biol Chem. 2008;283:19879–19887. doi: 10.1074/jbc.M802848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatellier S, Ihendyane N, Kansal RG, Khambaty F, Basma H, Norrby-Teglund A, Low DE, McGeer A, Kotb M. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect Immun. 2000;68:3523–3534. doi: 10.1128/iai.68.6.3523-3534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, Nizet V, Eckmann L. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci USA. 2009;106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CC, Hayashi T, Takabayashi K, Sabet M, Smee DF, Guiney DD, Cottam HB, Carson DA. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc Natl Acad Sci USA. 2007;104:3990–3995. doi: 10.1073/pnas.0611624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinkernagel AS, Peyssonnaux C, Johnson RS, Nizet V. Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J Infect Dis. 2008;197:214–217. doi: 10.1086/524843. [DOI] [PubMed] [Google Scholar]
- 25.Zinkernagel AS, Timmer AM, Pence MA, Locke JB, Buchanan JT, Turner CE, Mishalian I, Sriskandan S, Hanski E, Nizet V. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe. 2008;4:170–178. doi: 10.1016/j.chom.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–386. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Goldmann O, Rohde M, Chhatwal GS, Medina E. Role of macrophages in host resistance to group A streptococci. Infect Immun. 2004;72:2956–2963. doi: 10.1128/IAI.72.5.2956-2963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldmann O, von Kockritz-Blickwede M, Holtje C, Chhatwal GS, Geffers R, Medina E. Transcriptome analysis of murine macrophages in response to infection with Streptococcus pyogenes reveals an unusual activation program. Infect Immun. 2007;75:4148–4157. doi: 10.1128/IAI.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albiger B, Dahlberg S, Henriques-Normark B, Normark S. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med. 2007;261:511–528. doi: 10.1111/j.1365-2796.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 31.Spirig R, Djafarzadeh S, Regueira T, Shaw SG, von Garnier C, Takala J, Jakob SM, Rieben R, Lepper PM. Effects of TLR agonists on the hypoxia-regulated transcription factor HIF-1alpha and dendritic cell maturation under normoxic conditions. PLoS One. 2010;5:e0010983. doi: 10.1371/journal.pone.0010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 33.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol. 2007;245:103–110. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data