Abstract
The Agr quorum-sensing system represents the master regulator for staphylococcal virulence factors and is known to have a strong impact on the release of pathogen-associated molecular pattern (PAMP) molecules. Among the various staphylococcal PAMPs, phenol-soluble modulin (PSM) peptides have attracted increasing interest because they are crucial for staphylococcal virulence and have neutrophil-recruiting properties. The latter depend on recognition of PSMs by the neutrophil formyl peptide receptor 2 (FPR2/ALX), for which PSMs are highly efficient agonists. We demonstrate that Agr inactivation in Staphylococcus aureus or S. epidermidis leads to strongly reduced neutrophil responses, which is in agreement with the previously reported strict control of PSM expression by Agr. Agr had a distinct and profound impact on activation of FPR2/ALX but not of the related FPR1 receptor that senses bacterial formylated peptides. S. epidermidis PSMs had similar FPR2/ALX-activating properties but differed in their dependence on N-terminal formylation compared to S. aureus PSMs. Moreover, S. aureus and S. epidermidis PSMs upregulated the neutrophil complement receptor CD11b via FPR2/ALX stimulation in an Agr-dependent fashion. Hence, Agr controls the capacity of staphylococcal pathogens to activate FPR2/ALX-dependent neutrophil responses, underscoring the crucial role of FPR2/ALX and PSMs in staphylococcus-host interaction.
Key Words: Staphylococci, Formyl peptide receptor 2, Phenol-soluble modulins, Quorum-sensing system Agr
Introduction
Most staphylococcal species control exoprotein biosynthesis by the Agr quorum-sensing system, which activates gene expression upon accumulation of an autocrine pheromone peptide [1, 2]. In facultative pathogens, such as Staphylococcus aureus and S. epidermidis, Agr is known to regulate the production of surface adhesins and toxins and also many other genes [3]. The latter include the phenol-soluble modulin (PSM) peptide toxins whose expression is controlled by direct binding of the Agr response regulator AgrA to the PSM promoter regions [4]. An S. epidermidis Agr mutant has also been shown to exhibit strongly reduced proinflammatory capacities toward human leukocytes [5], indicating that major staphylococcal pathogen-associated molecular patterns (PAMPs) are controlled by agr. Staphylococci produce a variety of PAMPs including lipoproteins, peptidoglycan fragments, formylated peptides and PSM peptides, which are sensed by human Toll-like receptor 2 (TLR2) [6], nucleotide-oligomerization domain 2 (NOD2) receptor [7], formyl peptide receptor 1 (FPR1) [8], and formyl peptide receptor 2 (FPR2/ALX) [9]. It has remained unclear if Agr has a general impact on the release of PAMPs or if it controls only specific classes of staphylococcal proinflammatory molecules.
S. epidermidis belongs to the class of coagulase-negative staphylococci which are usually less pathogenic than the coagulase-positive S. aureus. Among the coagulase-negative staphylococci, S. epidermidis causes the largest number of diseases, which are often associated with the infection of indwelling medical devices [10, 11]. S. epidermidis infections develop only rarely into life-threatening diseases, but they are often difficult to eradicate because of antibiotic resistance genes and the formation of biofilms [12, 13]. PSMs were initially identified in S. epidermidis culture filtrates [13, 14, 15]. Related peptides have subsequently been described in S. aureus [16, 17] and, only recently, in virtually all staphylococcal pathogens [18]. The production of PSMs appears to correlate with the virulence potential of a given staphylococcal species [18]. PSMs are short, amphiphatic peptides, which are secreted by an unknown mechanism as formylated peptides in an Agr-regulated fashion [4, 19, 20]. S. aureus produces 5 shorter PSM peptides (α-type PSMs), with 21–26 amino acids, and 2 longer (β-type) PSMs with 44 amino acids [17]. An additional α-type PSM, PSMmec, has recently been identified in culture filtrates of some hospital-acquired methicillin-resistant S. aureus (MRSA) and S. epidermidis strains [16]. In S. epidermidis, 4 α-type PSMs (PSMα, PSMδ, PSMε and δ-toxin) and 2 β-type PSMs have been identified [13, 14]. Both S. epidermidis and S. aureus produce δ-toxin, which corresponds in size and structure to the α-type PSMs [17, 21, 22, 23]. It has been shown to be the most abundantly secreted PSM in both organisms [5, 17].
Translation starts in bacteria with formylated methionine, which distinguishes bacterial from eukaryotic proteins [24]. Many proteins and N-terminal protein fragments from protein degradation retain the N-terminal formyl group. Formylated peptides with hydrophobic amino acids adjacent to the N-terminal formylmethionine, such as f-Met-Leu-Phe, are very efficient ligands for FPR1 [25]. δ-Toxin and most other S. aureus PSMs are secreted predominantly with formylated N-termini, but deformylated δ-toxin has been observed to accumulate during the exponential and early postexponential phase [26].
Most α-type PSMs are strongly cytotoxic for neutrophils at micromolar concentrations, while β-type PSMs have lower cytotoxicity and seem to have an additional role in the release of individual bacteria from biofilms [17, 23, 27]. Moreover, PSMs have profound proinflammatory capacities in the nanomolar range that encompass the induction of neutrophil chemotaxis, respiratory burst and IL-8 release [17, 23, 28]. S. aureus and S. lugdunensis PSMs have been shown to stimulate neutrophils via FPR2/ALX [9, 18]. Whether or not the various formylated or nonformylated S. epidermidis PSMs also activate FPR2/ALX or FPR1 remains unknown. FPR1 and FPR2/ALX are present on neutrophils and other leukocytes. They belong to the class of G-protein-coupled seven-transmembrane receptors [29]. Many S. aureus strains produce specific inhibitors of the human FPR1 (CHIPS) and FPR2/ALX (FLIPr) [30, 31]. These proteins are thought to enable bacteria to multiply unrecognized by human immune defenses in the early stages of infection.
To investigate how the Agr system controls the proinflammatory capacity of staphylococci, we compared the culture filtrates of S. epidermidis and S. aureus Agr mutants with those from parental strains for their capacity to stimulate neutrophils or receptor-transfected cell lines. Agr had a profound, distinct impact on FPR2/ALX activation in both species, while FPR1 activation was hardly affected. All tested S. epidermidis isolates from catheter infections or nasal swabs activated the human FPR2/ALX. The S. epidermidis PSMs were efficient FPR2/ALX ligands, but the various peptides differed substantially in their dependence on N-terminal formylation. Moreover, S. aureus and S. epidermidis PSMs upregulated the complement receptor CD11b on neutrophils in an Agr-dependent manner.
Experimental Procedures
Synthetic Peptides
PSM peptides with the recently published sequences [13, 14, 15, 17], the FPR2/ALX-specific control ligand MMK1 (LESIFRSLL-FRVM-NH2) [32] and the FPR2/ALX inhibitor WRW4 (WRW-WWW-NH2) [33, 34] were synthesized (EMC Microcollections). The peptides were synthesized using solid-phase Fmoc/tBu chemistry. All peptides were purified to homogeneity by preparative RP-HPLC and characterized by RP-HPLC-ESI-MS (purity 190%). S. aureus PSMα2 (MGIIAGIIKFIKGLIEKFTGK), PSMα3 (MEFVAKLFKFFKDLLGKFLGNN) and δ-toxin (MAQDIISTISDLVKWIIDTVNKFTKK) as well as S. epidermidis PSMα (MADVIAKIVEIVKGLIDQFTQK), PSMδ (MSIVSTIIEVVKTIVDIVKKFKK), PSMε (MFIINLVKKVISFIKGLFGNNENE), δ-toxin (MAADIISTIGDLVKWIIDTVNKFKK), PSMβ1 (MSKLAEAIANTVKAAQDQDWTKLGTSIVDIVESGVSVLGKIFGF) and PSMβ2 (MEQLFDAIRSVVDAGINQDWSQLASGIAGIVENGISVISKLLG) were synthesized in their formylated and nonformylated forms. fMLF was purchased from Sigma.
Bacteria and Cell Lines
USA300 is a prevalent community-associated methicillin-resistant S. aureus (CA-MRSA) strain [35]. Defined psm mutants have recently been described in detail [17]. S. aureus RN6911 is an isogenic Agr mutant derived from laboratory strain RN6390 [36]. S. epidermidis 1457 and its isogenic Agr mutant have been described previously [20]. S. epidermidis isolates were either obtained from infected catheters or from the nasal swabs of healthy volunteers. Bacterial culture filtrates were obtained by centrifugation of overnight cultures grown in tryptic soy broth and filtered through 0.2-µm-pore-size filters. No chemotactic or stimulatory activity was detected in the noninoculated medium at the used concentrations.
Recently, HL60 cells stably transfected with human FPR1, FPR2/ALX, or FPR3 have been described [37, 38]. These cells were grown in RPMI medium (Biochrom) supplemented with 10% FCS (Sigma-Aldrich), 20 mM Hepes (Biochrom), penicillin (100 units/ml), streptomycin (100 μg/ml) (Gibco) and 1 × Glutamax (Gibco). Transfected cells were grown in the presence of G418 (Biochrom) at a final concentration of 1 mg/ml.
Neutrophil Chemotaxis
Human neutrophils and peripheral blood mononuclear cells were isolated by standard Ficoll/Histopaque gradient centrifugation. Chemotaxis of neutrophils towards staphylococcal culture filtrates or synthetic peptides was determined by using fluorescence-labeled neutrophils that migrated through a 3-μm-poresize polycarbonate transwell filter as described recently [8]. Synthetic chemoattractants were used at concentrations in the linear range of the dose-response curves. Initial control experiments were performed to verify that PSM peptides mobilize neutrophils by chemotactic rather than by undirected chemokinetic stimulation. Neutrophil migration from the upper to the lower transwell chamber was only observed when the peptides were added to the lower chamber. When an equal PSM concentration was present in the two chambers, no such migration was observed, confirming that PSMs do induce chemotactic migration in neutrophils. For chemotaxis, peptides were used at concentrations of 250 nM for formylated PSMα and PSMδ, 2.5 μM for formylated PSMβ1, 1 μM for δ-toxin and 100 nM for nonformylated and formylated PSMε and for MMK1. The relative fluorescence measured was corrected for the buffer control (i.e. buffer added only to lower compartment) and divided by 1,000.
Measurement of Calcium Ion Fluxes in Human Neutrophils and HL60 Cells
Since calcium fluxes can be measured more robustly and accurately than chemotaxis, they were monitored in certain experiments as a surrogate marker for it. They were analyzed by stimulating cells loaded with Fluo-3-AM (Molecular Probes) and monitoring fluorescence with a FACSCalibur flow cytometer (Becton Dickinson) as described recently [30]. In order to study the influence of pertussis toxin (PTX), cells were preincubated with 1 μg/ml PTX (List Biological Laboratories) for 3 h at 37°C under 5% CO2. To measure the influence of CHIPS or FLIPr, 1 × 106 cells/ml were preincubated with CHIPS or FLIPr at final concentrations of 1.4 or 0.5 μg/ml, respectively, for 20 min under agitation at room temperature. The CHIPS and FLIPr proteins were prepared as described recently [30,31]. Synthetic chemoattractants were used at concentrations in the linear range of the dose-response curves. In order to stimulate neutrophils and HL60 cells, the following concentrations were used: formylated PSMα (300 nM), PSMδ (200 nM), formylated and nonformylated PSMβ1 (1.5 μM), PSMβ2 (1.8 μM), formylated δ-toxin (500 nM), formylated PSMε (200 nM) and nonformylated PSMε (300 nM). fMLF and MMK1 were used at final concentrations of 20 and 10 nM, respectively. Culture filtrates for neutrophil calcium influx were used at concentrations of 1.5% for S. epidermidis 1457 and S. aureus RN6390 and their isogenic Agr mutants, or at 0.37% for USA300 and its isogenic Agr mutant. Measurements of 2,000 events were performed and calcium flux was expressed as relative fluorescence corrected for buffer controls.
IL-8 Production
Human IL-8 was measured using an ELISA kit (R&D Systems) according to the manufacturer's instructions. Neutrophils were incubated with the indicated culture filtrates for 5 h at 37°C and 5% CO2 with or without preincubation of FLIPr (1 μg/ml) for 20 min. Then, culture filtrates were centrifuged for 10 min at 250 g and 4°C and culture filtrates were stored at −20°C before use. Media contained polymyxin B (Sigma) at a final concentration of 10 μg/ml to exclude lipopolysaccharide contamination.
Priming of Human Neutrophils
Priming of human neutrophils was determined by measuring surface expression of CD11b. After the preincubation of neutrophils with FLIPr (1 μg/ml) for 20 min under agitation at room temperature, neutrophils were further incubated with different concentrations of PSMs or culture filtrates at 37°C with rotation for 60 min, as described elsewhere [16]. Cells were stained with a phycoerythrin-labeled antibody against CD11b (mAb 44, BD Biosciences) or isotype control antibody (BD Bioscience). Then, neutrophils were analyzed on an FACSCalibur flow cytometer (Becton Dickinson). Media contained polymyxin B at a final concentration of 10 μg/ml to exclude lipopolysaccharide contamination.
Statistical Methods
Statistical analyses were performed with the Prism 4.0 package (GraphPad Software), and the between-group differences were analyzed for significance with the two-tailed paired Student t test (culture filtrates; the same filtrates were used for matched experiments) or the unpaired Student t test (synthetic peptides).
Results
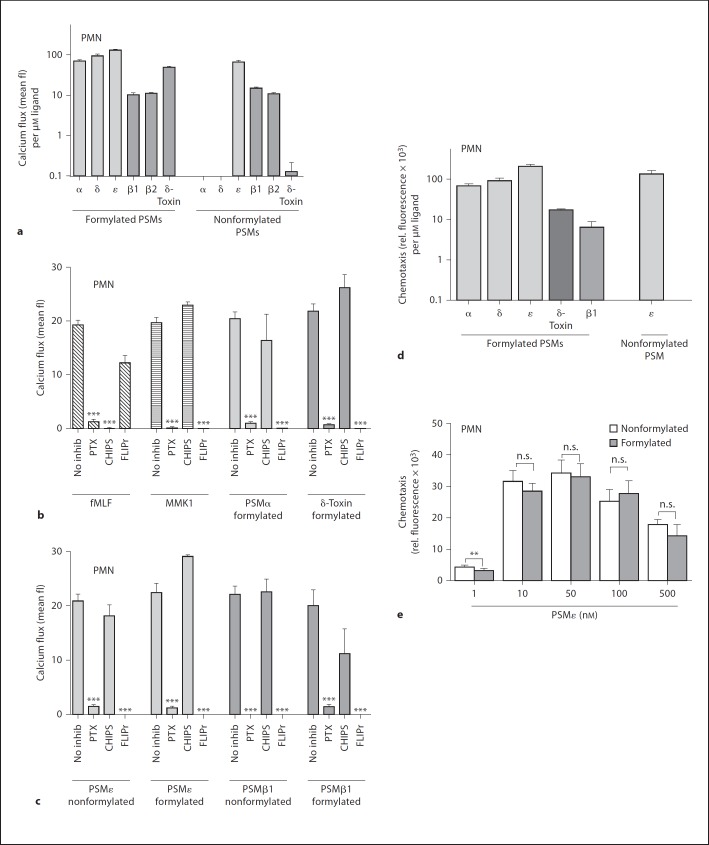
S. epidermidis PSM Are Specific FPR2/ALX Ligands Inducing Ca2+ Influx and Chemotaxis in Neutrophils S. epidermidis culture filtrates can stimulate FPR2/ALX [18] and S. epidermidis PSM peptide complexes have been shown to elicit proinflammatory reactions in human neutrophils [28]. However, it has remained unknown if the individual formylated or nonformylated S. epidermidis PSMs can activate neutrophils via FPR1 or FPR2/ALX and if they differ in activity. In order to clarify these questions, chemotaxis of human neutrophils in response to the various PSMs was measured in the presence or absence of receptor-specific inhibitors. In addition, Ca2+ influx, which usually accompanies chemotactic migration and can be measured very sensitively [39, 40], was monitored. The various synthetic peptides displayed large differences in activity. All formylated PSM peptides induced Ca2+ influx (fig. 1a–c) and the same was observed for chemotaxis with selected PSMs (fig. 1d, e). The longer β-PSMs showed lower activity than the shorter α-PSMs, which is in agreement with previous reports on the activities of the S. aureus PSMs [17] (fig. 1d). Most PSMs were much more active in their formylated forms except for PSMε, β1, and β2 (fig. 1a). PSMε was the most active peptide and its activity was similar in the formylated and nonformylated forms (fig. 1e). In contrast, the other α-PSMs were almost completely inactive as deformylated peptides.
Fig. 1.
S. epidermidis PSM peptides induce chemotaxis and calcium ion fluxes in human neutrophils. a Formylated PSMs and nonformylated PSMε, PSMβ1 and PSMβ2 induce Ca2+ flux in human neutrophils (values normalized to 1 μM). b, c Neutrophil stimulation by PSMs (formylated PSMα 300 nM, PSMδ 200 nM, δ-toxin 500 nM, and PSMε 200 nM, formylated and nonformylated PSMβ1 1.5 μM, PSMβ2 1.8 μM and nonformylated PSMε 300 nM) is sensitive to PTX and the FPR2/ALX-specific inhibitor FLIPr, but not the FPR1-specific inhibitor CHIPS. fMLF (10 nM) and MMK1 (20 nM) are synthetic control ligands of FPR1 and FPR2/ALX, respectively. d Formylated PSMα (250 nm), PSMδ (250 nm), PSMε (100 nm), δ-toxin (1 μm) and nonformylated PSMε (100 nm) induce chemotaxis in human neutrophils. e Formylated and nonformylated PSMε stimulates chemotaxis in human neutrophils at nanomolar concentrations with a typical bell-shaped dose-response curve. Data represent means ± SEM of 3 independent experiments. * p < 0.05; ** p < 0.005; *** p < 0.001 versus no inhibition (b, c). a–c All values were significant versus buffer control in (a). δ = δ-Toxin; fl = fluorescence; inhib = inhibitor; n.s. = not significant.
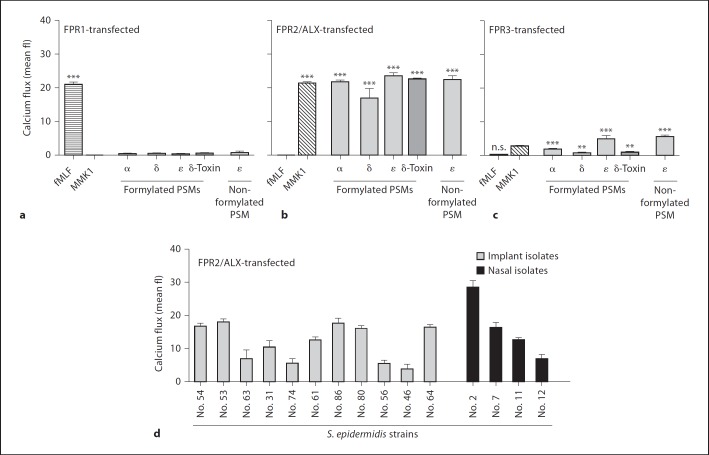
Preincubation of neutrophils with PTX, an inhibitor of Gi-coupled receptors, completely blocked the PSM-dependent activation of neutrophils (fig. 1b, c). CHIPS, a specific inhibitor of the human FPR1 and C5a receptors, had no major impact on PSM-induced Ca2+ influx, even though the activities of PSMα, PSMδ, and δ-toxin were entirely depended on formylation (fig. 1b, c). In contrast, the FPR2/ALX-specific inhibitor FLIPr completely blocked the activities of all formylated or nonformylated PSMs on neutrophils (fig. 1b, c). Moreover, HL60 cell lines transfected with FPR2/ALX exhibited strong responses to the tested S. epidermidis PSMs (fig. 2b), while untransfected HL60 or those transfected with FPR1 (fig. 2a) were unresponsive. HL60 cells transfected with FPR3, another FPR1 paralog of unknown ligand specificity expressed on monocytes but not on neutrophils, showed only weak responses to S. epidermidis PSM stimulation (fig. 2c) [41]. These results are reminiscent of those obtained with S. aureus PSMs [9] and confirm that S. epidermidis PSMs are specific ligands for FPR2/ALX but not for FPR1.
Fig. 2.
S. epidermidis PSMs specifically activate FPR2/ALX-transfected HL60 cells. a–c PSMs (formylated PSMα 300 nM, PSMδ 200 nM, δ-toxin 500 nM, PSMε 200 nM and nonformylated PSMε 300 nM) stimulated strong calcium fluxes in FPR2/ALX-transfected (b) but not in FPR1-transfected cells (a) and only slightly in FPR3-transfected cells (c). Nontransfected HL60 cells exhibited no significant responses (mean fluorescence values below 1; data not shown). fMLF (10 nM) and MMK1 (10 nM) are synthetic control ligands of FPR1 and FPR2/ALX, respectively. d Diluted culture filtrates (0.375%) of 11 clinical (catheter) isolates and 4 nasal isolates from healthy volunteers induce calcium fluxes in FPR2-transfected cells. Data represent means ± SEM of at least 3 independent experiments. * p < 0.05; *** p < 0.001 versus nontransfected cells. δ = δ-Toxin; fl = fluorescence; n.s. = not significant.
Culture filtrates of 15 S. epidermidis catheter-infection or nasal isolates activated FPR2/ALX, albeit with different potencies (fig. 2d) indicating that PSM production is a general but variable trait in S. epidermidis.
The Staphylococcal Capacity to Stimulate FPR2/ALX Depends on an Active Agr Quorum-Sensing System
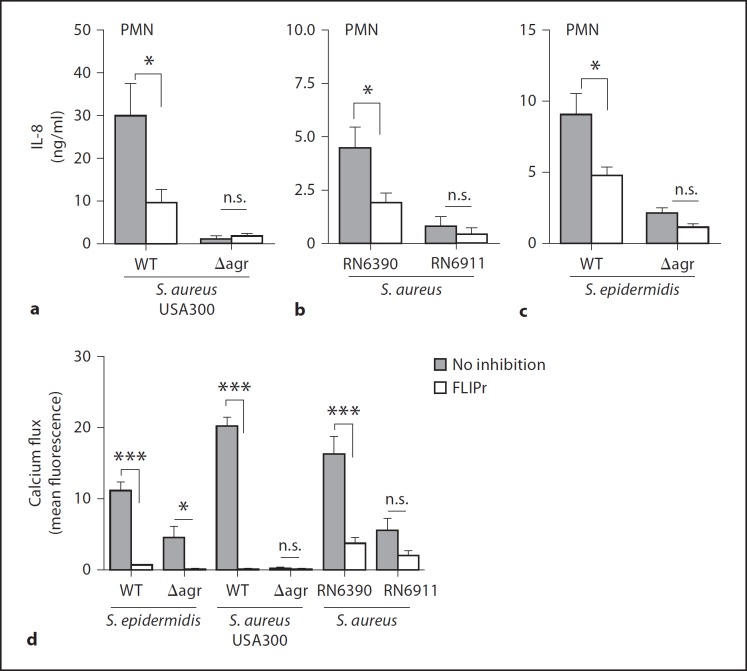
In order to investigate if the Agr quorum-sensing system has a general impact on staphylococcal proinflammatory molecules or if it specifically controls the release of FPR2/ALX ligands, we used isogenic strain pairs with or without functional Agr systems in the background of the laboratory strain S. epidermidis 1457 [42], the laboratory strain S. aureus RN6390 [43, 44] and the CA-MRSA strain USA300 [17]. Diluted culture filtrates from cultures grown to the stationary phase were incubated with neutrophils and analyzed for IL-8 induction. Inactivation of the Agr system led to severely reduced IL-8 release in all 3 bacterial strains (fig. 3a–c). The largest difference was found in S. aureus USA300, which is known to produce particularly high amounts of PSMs upon Agr activation [17, 45]. In agreement with this finding, S. aureus USA300 elicited 3- to 6-fold higher IL-8 production in neutrophils than S. aureus RN6390 or S. epidermidis 1457, respectively. The specific FPR2/ALX inhibitor FLIPr largely inhibited IL-8 induction by all 3 wild-type strains (fig. 3a–c). However, FLIPr did not influence the residual IL-8 induction by the 3 Agr mutants, which supports the notion that FPR2/ALX ligands are most critical proinflammatory staphylococcal molecules whose expression depends largely on Agr activation. Similar results were obtained with calcium flux experiments in neutrophils (fig. 3d). Only the residual calcium influx induced by culture filtrates of the S. epidermidis Agr mutant was slightly inhibited through FLIPr preincubation (fig. 3d).
Fig. 3.
IL-8 secretion and calcium fluxes of neutrophils stimulated by staphylococcal culture filtrates are largely Agr and FPR2/ALX-dependent. IL-8 release from neutrophils induced by culture filtrates from S. aureus USA300 and the isogenic Agr mutant (a), S. aureus RN6390 and the isogenic Agr mutant RN6911 (b) and S. epidermidis and the isogenic Agr mutant (c) in the presence or absence of FLIPr. d Calcium influx in neutrophils stimulated by S. aureus or S. epidermidis culture filtrates in the presence or absence of FLIPr. Data represent means ± SEM of 3 independent experiments with neutrophils of different persons and at least 3 different culture filtrates. * p < 0.05; ** p < 0.005; *** p < 0.001. n.s. = Not significant.
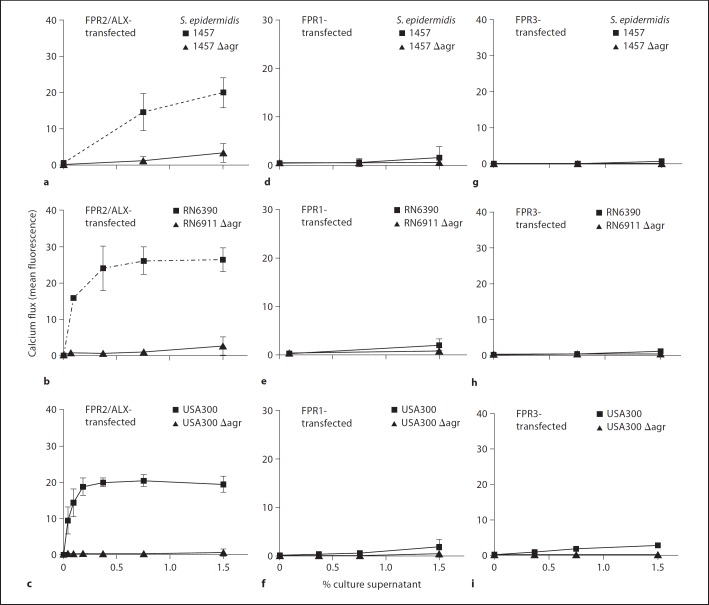
Ca2+ influx studies in FPR2/ALX- or FPR1-transfected HL60 cells further substantiated these findings. All bacterial strains stimulated FPR1- or FPR2/ALX-transfected cells in a dose-dependent manner (fig. 4). No significant difference in FPR1-mediated activation was detectable between wild-type and isogenic Agr mutants in any of the 3 strain backgrounds, indicating that Agr has no major impact on the release of formylated peptides, which constitute the most important bacterial FPR1 ligands [8]. FPR3-transfected cells showed even less activation than FPR1-transfected cells and there was no difference in receptor activation between wild-type and Agr mutants (fig. 4g–i) supporting the notion that FPR3 plays no considerable role in the detection of staphylococcal molecules [9, 18]. Of note, Ca2+ influxes in FPR2/ALX-transfected cells stimulated by culture filtrates of the S. epidermidis and S. aureus Agr mutants were strongly diminished compared to the corresponding wild-type strains (fig. 4a–c), confirming the important role of Agr in controlling FPR2/ALX-dependent leukocyte responses. Culture filtrates of S. aureus induced stronger activation of FPR2/ALX than those of S. epidermidis (fig. 4a–c) which is in agreement with the higher production of proinflammatory PSM by S. aureus compared to S. epidermidis [23].
Fig. 4.
Calcium influx stimulated by S. aureus and S. epidermidis culture filtrates are FPR2/ALX-dependent. FPR2/ALX-transfected HL60 cells respond strongly to S. epidermidis 1457 (a), S. aureus RN6390 (b) and USA300 (c) wild-type strains but only moderately to the corresponding isogenic Agr mutants, while FPR1- or FPR3-mediated responses differ only slightly between the various S. epidermidis (d, g) and S. aureus (e–h) strains. Nontransfected HL60 cells exhibited no significant responses (mean fluorescence values below 1; data not shown). Data represent means ± SEM of 3 independent experiments and at least 3 different culture filtrates.
Upregulation of the Complement Receptor CD11b by Staphylococcal Culture Filtrates Depends on Agr-Controlled Activation of FPR2/ALX
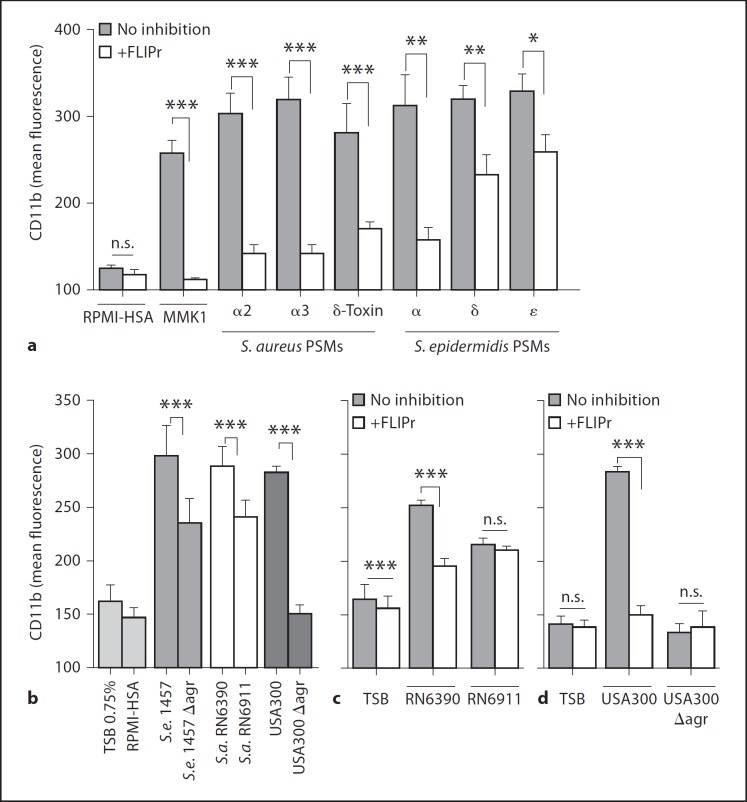
Previous studies have shown that S. aureus and S. epidermidis PSMs can prime neutrophils for upregulation of the complement receptor CD11b [17]. However, it has remained unclear if this process depends on FPR2/ALX and if it is controlled by Agr. After the preincubation of neutrophils with FLIPr, we observed a significant inhibition of CD11b upregulation after stimulation with S. aureus or S. epidermidis PSM peptides compared to the samples without FLIPr (fig. 5a). This inhibitory effect was slightly more pronounced with S. aureus than with S. epidermidis PSM peptides.
Fig. 5.
PSM-induced CD11b upregulation is inhibited by the FPR2/ALX antagonist FLIPr. CD11b upregulation of neutrophils induced by formylated PSM peptides of S. aureus (PSMα2 1 μM, PSMα3 500 nM, δ-toxin 250 nM) or S. epidermidis (PSMα 500 nM, PSMδ 500 nM, and PSMε 500 nM) with and without FLIPr preincubation (a) and culture filtrates from S. epidermidis 1457 (0.75%), S. aureus RN6390 (0.75%), and USA300 (0.09%) or the isogenic Agr mutants (b). CD11b upregulation of neutrophils with and without FLIPr preincubation induced by culture filtrates from S. aureus RN6390 and the isogenic Agr mutant (0.75%) (c) or USA300 and USA300 ∆agr (0.09%) (d). Data represent means ± SEM of 3 independent experiments with neutrophils of different individuals and at least 3 different culture filtrates. * p < 0.05; *** p < 0.001. HSA = Human serum albumin; n.s. = not significant; S.a. = S. aureus; S.e. = S. epidermidis; TSB = tryptic soy broth.
Culture filtrates of S. epidermidis and S. aureus also stimulated upregulation of CD11b in neutrophils (fig. 5b–d). The highly pathogenic S. aureus USA300 induced CD11b upregulation at a much lower concentration (0.09%-diluted culture filtrates), while S. aureus RN6390 and the less pathogenic S. epidermidis 1457 did not affect CD11b expression at such low amounts (significantly higher potency of USA300 with p values of <0.0001, RN6390 or 1457, respectively), but at around 8-fold higher concentrations (fig. 5b), which is in agreement with a crucial role of PSMs in the staphylococcal capacity to upregulate CD11b. Accordingly, preincubation of neutrophils with FLIPr led to a significant inhibition of CD11b upregulation by culture filtrates of S. aureus USA300 and RN6390 wild-type strains (fig. 5c). Culture filtrates of the S. epidermidis and S. aureus Agr mutants caused significantly lower stimulation of CD11b surface expression than those obtained from the corresponding wild-type strains (fig. 5b). Furthermore, FLIPr had no influence on the CD11b expression stimulated by culture filtrates of the S. aureus Agr mutants (fig. 5c, d), indicating that the upregulation of CD11b expression by staphylococcal molecules is fully dependent on FPR2/ALX and follows the same pattern as the induction of Ca2+ fluxes and IL-8 releases.
Discussion
Our study provides further evidence that the staphylococcal Agr system does not only regulate the expression of virulence factors and metabolic functions, but is also crucial for the proinflammatory properties of S. aureus and S. epidermidis. Accordingly, Agr mutants of both species exhibited strongly reduced capacities to induce calcium ion fluxes, IL-8 release and CD11b upregulation compared to the parental strains. The proinflammatory activities in culture filtrates of wild-type strains could be largely suppressed by FLIPr, while this FPR2/ALX-specific inhibitor had no/only weak impacts on the residual activities of Agr mutants, thereby supporting the notion that FPR2/ALX ligands represent the most important PAMPs for human neutrophils. These findings are in agreement with previous studies that used S. aureus mutants lacking PSM genes [5, 9]. Agr inactivation had no major impact on FPR1 activation, indicating that Agr is dedicated to the regulation of specific PAMPs such as FPR2/ALX agonists. Further studies will be necessary to investigate if additional PAMPs are affected by Agr.
The Agr system is present in almost all staphylococcal species for which genome sequences are available [46] and appears to be of particular importance for the ability of staphylococci to adapt to different environments [47]. It is interesting to note that both PSMs and Agr are widespread among staphylococcal species, emphasizing the basic role of PSM peptides and the Agr regulatory system in staphylococcal physiology and virulence [18]. Accordingly, the Agr system and PSM regulation are intimately connected in S. aureus, S. epidermidis and probably other species, and PSMs are regarded as particularly ancient targets for Agr regulation [4].
The level of PSM production correlates closely with the virulence potential of a given staphylococcal strain [18] and the differences in FPR2/ALX activation between S. aureus and S. epidermidis found in this study support these previous findings. They also confirm the central role of Agr in controlling the degree of virulence and inflammation in staphylococcal virulence. Accordingly, the Agr systems of highly pathogenic CA-MRSA are regarded as being dysregulated [45] and S. aureus clones from chronic infection, which usually have decreased aggressivity, often have inactive Agr systems [5]. During the early stage of infection and during colonization of the skin and nose, it may be advantageous for staphylococci to remain unrecognized by the innate immune system. During this stage, leukocidins may be dispensable and the secretion of FPR2/ALX ligands could be harmful, because neutrophils could easily detect and eradicate small numbers of bacteria. Accordingly, the importance of neutrophils for curing S. aureus infections and their superior role, compared to other leukocytes, have been documented repeatedly [48, 49]. However, at a later stage of infection, e.g. within an abscess, Agr will be activated, when the critical bacterial ‘quorum’ is reached, to produce PSMs as weapons against increasing numbers of infiltrating neutrophils. The FPR2/ALX-dependent neutrophilattracting properties may even be advantageous at such stages when leukocidins such as PSMs are abundant and provide efficient bacterial defenses. The ability of PSMs to upregulate CD11b via FPR2 may contribute to neutrophil recruitment, as CD11b is known to promote neutrophil diapedesis [50].
It remains enigmatic why most staphylococcal strains produce a variety of PSMs at one time and what renders a peptide an FPR2/ALX ligand, given that the various PSMs share only limited similarity [51]. Of note is that ligand variability is even larger among the various endogenous FPR2/ALX peptide agonists [52, 53, 54]. Our study demonstrates that the S. epidermidis PSMs activate human neutrophils in the same way as S. aureus PSMs via FPR2/ALX, despite major differences in peptide sequences [9, 17]. Thus, it is unclear which structural features, apart from amphipathicity and α-helicity [15, 17], render a peptide an FPR2/ALX ligand. S. aureus PSMs are much better FPR2/ALX agonists when they retain their N-terminal formylation, but residual FPR2/ALX activation has also been observed with the nonformylated variants [9]. Interestingly, the nonformylated S. epidermidis β-PSMs and PSMε were equally as active as the formylated versions indicating that formylation is only critical in certain ligand molecules. In contrast, S. epidermidis PSMα, PSM8, and δ-toxin possess strongly reduced activity without N-terminal formyl groups (data not shown). Although the FPR2/ALX receptor has been described as a low-affinity receptor for formylated peptides, most endogenous agonists described for this receptor possess no formylation [52, 53], so it can be speculated that some peptides require the formyl group for stability or appropriate folding.
Extensive inflammation is a hallmark of staphylococcal infections with severe consequences, such as sepsis [55, 56]. There is increasing evidence that PSM-mediated FPR2/ALX activation contributes to exuberant inflammation during staphylococcal infections [17, 28], which suggests that FPR2/ALX or components of the staphylococcal Agr system may represent attractive targets for anti-inflammatory therapies. The staphylococcal FPR2/ALX inhibitor FLIPr is probably not suitable for such therapies because of the high prevalence of FLIPr and cognate antibodies in the human population against FLIPr [31]. Nevertheless, the development of synthetic inhibitors may become a valuable strategy for the treatment of severe staphylococcal infections.
Acknowledgments
We thank Francois Boulay for FPR1-, FPR2/ALX- and FPR3-transfected cell lines and Kok van Kessel and Jos van Strijp for CHIPS and FLIPr. This research was supported by grants from the Fortüne program of the Medical Faculty, University of Tübingen (to D.K.), the German Research Foundation (SFB685, TRR34) and the German Ministry of Education and Research (SkinStaph, Menage) (to A.P.) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), US National Institutes of Health (to M.O.).
References
- 1.Otto M, Sussmuth R, Jung G, Gotz F. Structure of the pheromone peptide of the Staphylococcus epidermidis agr system. FEBS Lett. 1998;424:89–94. doi: 10.1016/s0014-5793(98)00145-8. [DOI] [PubMed] [Google Scholar]
- 2.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 3.Kong KF, Vuong C, Otto M. Staphylococcus quorum sensing in biofilm formation and infection. Int J Med Microbiol. 2006;296:133–139. doi: 10.1016/j.ijmm.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuong C, Durr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 2004;6:753–759. doi: 10.1111/j.1462-5822.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto M, Tawaratsumida K, Kariya H, Kiyohara A, Suda Y, Krikae F, Kirikae T, Gotz F. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol. 2006;177:3162–3169. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 7.Volz T, Nega M, Buschmann J, Kaesler S, Guenova E, Peschel A, Rocken M, Gotz F, Biedermann T. Natural Staphylococcus aureus-derived peptidoglycan fragments activate nod2 and act as potent costimulators of the innate immune system exclusively in the presence of tlr signals. FASEB J. 2010;24:4089–4102. doi: 10.1096/fj.09-151001. [DOI] [PubMed] [Google Scholar]
- 8.Durr MC, Kristian SA, Otto M, Matteoli G, Margolis PS, Trias J, van Kessel KP, van Strijp JA, Bohn E, Landmann R, Peschel A. Neutrophil chemotaxis by pathogen-associated molecular patterns – formylated peptides are crucial but not the sole neutrophil attractants produced by Staphylococcus aureus. Cell Microbiol. 2006;8:207–217. doi: 10.1111/j.1462-5822.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 9.Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uckay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. Foreign body infections due to Staphylococcus epidermidis. Ann Med. 2009;41:109–119. doi: 10.1080/07853890802337045. [DOI] [PubMed] [Google Scholar]
- 11.Otto M. Staphylococcus epidermidis – the ‘accidental' pathogen. Nat Rev Microbiol. 2009;7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Sturdevant DE, Otto M. Genome-wide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis. 2005;191:289–298. doi: 10.1086/426945. [DOI] [PubMed] [Google Scholar]
- 14.Mehlin C, Headley CM, Klebanoff SJ. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J Exp Med. 1999;189:907–918. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto M, O'Mahoney DS, Guina T, Klebanoff SJ. Activity of Staphylococcus epidermidis phenol-soluble modulin peptides expressed in Staphylococcus carnosus. J Infect Dis. 2004;190:748–755. doi: 10.1086/422157. [DOI] [PubMed] [Google Scholar]
- 16.Queck SY, Khan BA, Wang R, Bach TH, Kretschmer D, Chen L, Kreiswirth BN, Peschel A, Deleo FR, Otto M. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 2009;5:e1000533. doi: 10.1371/journal.ppat.1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 18.Rautenberg M, Joo HS, Otto M, Peschel A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J. 2011;25:1254–1263. doi: 10.1096/fj.10-175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 20.Vuong C, Gerke C, Somerville GA, Fischer ER, Otto M. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect Dis. 2003;188:706–718. doi: 10.1086/377239. [DOI] [PubMed] [Google Scholar]
- 21.McKevitt AI, Bjornson GL, Mauracher CA, Scheifele DW. Amino acid sequence of a deltalike toxin from Staphylococcus epidermidis. Infect Immun. 1990;58:1473–1475. doi: 10.1128/iai.58.5.1473-1475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janzon L, Lofdahl S, Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989;219:480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- 23.Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, Otto M. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffmann E, Corcoran BA, Wahl SM. N-Formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marasco WA, Phan SH, Krutzsch H, Showell HJ, Feltner DE, Nairn R, Becker EL, Ward PA. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984;259:5430–5439. [PubMed] [Google Scholar]
- 26.Somerville GA, Cockayne A, Durr M, Peschel A, Otto M, Musser JM. Synthesis and deformylation of Staphylococcus aureus delta-toxin are linked to tricarboxylic acid cycle activity. J Bacteriol. 2003;185:6686–6694. doi: 10.1128/JB.185.22.6686-6694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R, Khan BA, Cheung GY, Bach TH, Jameson-Lee M, Kong KF, Queck SY, Otto M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J Clin Invest. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liles WC, Thomsen AR, O'Mahony DS, Klebanoff SJ. Stimulation of human neutrophils and monocytes by staphylococcal phenol-soluble modulin. J Leukoc Biol. 2001;70:96–102. [PubMed] [Google Scholar]
- 29.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, van Strijp JA. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial anti-inflammatory agent. J Exp Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prat C, Bestebroer J, de Haas CJ, van Strijp JA, van Kessel KP. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J Immunol. 2006;177:8017–8026. doi: 10.4049/jimmunol.177.11.8017. [DOI] [PubMed] [Google Scholar]
- 32.Fu H, Karlsson J, Bylund J, Movitz C, Karlsson A, Dahlgren C. Ligand recognition and activation of formyl peptide receptors in neutrophils. J Leukoc Biol. 2006;79:247–256. doi: 10.1189/jlb.0905498. [DOI] [PubMed] [Google Scholar]
- 33.Onnheim K, Bylund J, Boulay F, Dahlgren C, Forsman H. Tumour necrosis factor (TNF)-alpha primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization. Immunology. 2008;125:591–600. doi: 10.1111/j.1365-2567.2008.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson J, Fu H, Boulay F, Bylund J, Dahlgren C. The peptide Trp-Lys-Tyr-Met-Val-D-Met activates neutrophils through the formyl peptide receptor only when signaling through the formylpeptide receptor-like 1 is blocked. A receptor switch with implications for signal transduction studies with inhibitors and receptor antagonists. Biochem Pharmacol. 2006;71:1488–1496. doi: 10.1016/j.bcp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–369. doi: 10.1016/j.tim.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamberlain NR, Imanoel B. Genetic regulation of fatty acid modifying enzyme from Staphylococcus aureus. J Med Microbiol. 1996;44:125–129. doi: 10.1099/00222615-44-2-125. [DOI] [PubMed] [Google Scholar]
- 37.Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F, Dahlgren C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem. 2001;276:21585–21593. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- 38.Dahlgren C, Christophe T, Boulay F, Madianos PN, Rabiet MJ, Karlsson A. The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-D-Met activates neutrophils preferentially through the lipoxin A(4) receptor. Blood. 2000;95:1810–1818. [PubMed] [Google Scholar]
- 39.Hartt JK, Barish G, Murphy PM, Gao JL. N-Formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med. 1999;190:741–747. doi: 10.1084/jem.190.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalmar JR, van Dyke TE. Effect of bacterial products on neutrophil chemotaxis. Methods Enzymol. 1994;236:58–87. doi: 10.1016/0076-6879(94)36009-x. [DOI] [PubMed] [Google Scholar]
- 41.Murphy PM, Ozcelik T, Kenney RT, Tiffany HL, McDermott D, Francke U. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J Biol Chem. 1992;267:7637–7643. [PubMed] [Google Scholar]
- 42.Vuong C, Gotz F, Otto M. Construction and characterization of an agr deletion mutant of Staphylococcus epidermidis. Infect Immun. 2000;68:1048–1053. doi: 10.1128/iai.68.3.1048-1053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick RP. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 45.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M, Payne SM. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 47.Shopsin B, Drlica-Wagner A, Mathema B, Adhikari RP, Kreiswirth BN, Novick RP. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J Infect Dis. 2008;198:1171–1174. doi: 10.1086/592051. [DOI] [PubMed] [Google Scholar]
- 48.von Kockritz-Blickwede M, Rohde M, Oehmcke S, Miller LS, Cheung AL, Herwald H, Foster S, Medina E. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol. 2008;173:1657–1668. doi: 10.2353/ajpath.2008.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmaler M, Jann NJ, Ferracin F, Landmann R. T and b cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-Myd88-dependent T cell activation. J Immunol. 2011;186:443–452. doi: 10.4049/jimmunol.1001407. [DOI] [PubMed] [Google Scholar]
- 50.Xia Y, Borland G, Huang J, Mizukami IF, Petty HR, Todd RF, 3rd, Ross GD. Function of the lectin domain of mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion. J Immunol. 2002;169:6417–6426. doi: 10.4049/jimmunol.169.11.6417. [DOI] [PubMed] [Google Scholar]
- 51.Joo HS, Cheung GY, Otto M. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J Biol Chem. 2011;286:8933–8940. doi: 10.1074/jbc.M111.221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. Ll-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, Dunlop NM, Gao JL, Murphy PM, Oppenheim JJ, Wang JM. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 2001;21:RC123. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matussek A, Strindhall J, Stark L, Rohde M, Geffers R, Buer J, Kihlstrom E, Lindgren PE, Lofgren S. Infection of human endothelial cells with Staphylococcus aureus induces transcription of genes encoding an innate immunity response. Scand J Immunol. 2005;61:536–544. doi: 10.1111/j.1365-3083.2005.01597.x. [DOI] [PubMed] [Google Scholar]
- 56.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]