Abstract
Natural Killer (NK) cells are a subset of lymphocytes that kill virus-infected and cancerous cells and influence adaptive immune responses via production of inflammatory cytokines. Recent studies have identified multiple subsets of NK cells with differing potential for cytolysis or cytokine production and restricted tissue distribution. However, it remains to be determined whether all of these subsets represent truly distinct fates or transitory activation states and how these subsets arise from the conventional pathway of NK cell development. The transcriptional programs promoting NK cell lineage specification or commitment have not been identified and emerging data indicate that transcription factor redundancy may be pervasive at early stages of NK cell development.
The innate and adaptive immune systems are essential for effective resistance to the wide range of pathogenic substances encountered by vertebrates. The innate immune system provides a rapid but pre-programmed response whereas the adaptive immune response is delayed but is antigen specific and leads to prolonged memory of the pathogen [1–3]. While B and T lymphocytes are the primary mediators of adaptive immunity, natural killer (NK) lymphocytes have been considered a component of the innate immune system. Over the past 20 years much attention has been focused on understanding the mechanisms that control the development and function of B and T lymphocytes. In contrast our understanding of NK cell development and function have lagged behind. The relatively recent identification of the receptors used by NK cells to recognize virus-infected, stressed or cancerous cells has led to a resurgence in the interest in NK cell development and identification of functionally distinct NK cell subsets. Here we discuss recent evidence supporting the existence of multiple functionally distinct subsets of NK cells and the transcriptional regulators that are known to guide their development.
An overview of NK cell development
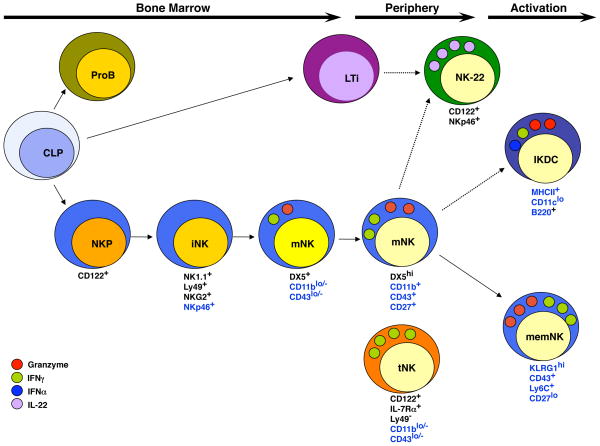
NK cell development has been divided into stages based on the differential expression of three cell surface receptors, CD122 (also known as IL2Rβ), NK1.1 (NKR-P1C) and DX5 (CD49b) (Figure 1) [4,5]. The most immature but NK cell lineage-restricted progenitors (NKP) are Lin(CD3 and CD19)−CD122+NK1.1−DX5− although this phenotype identifies a heterogeneous subset in which only some cells express the NK cell receptors 2B4 (CD244) and NKG2D. However, a subset of these cells are T lymphocytes as demonstrated by intracellular expression of CD3ε and a dependence on the recombinase activating gene Rag2 (Boos and Kee et al., submitted). NKPs give rise to immature (i) NK cells that are Lin−CD122+NK1.1+DX5− and dependent on IL15 for survival and later for expansion [6]. These cells, and their more mature progeny, also express the pan-NK cell activating receptor NKp46/NCR1 [7]. Mature NK cells are Lin−CD122+NK1.1+DX5+ but also acquire expression of multiple activating and inhibitor receptors of the NKG2 and Ly49 (KIR in humans) family of receptors [4,5]. Expression and signaling via these receptors during NK cell development plays a pivotal role in the education of NK cells that either “licenses” or “arms/disarms” the cells making them self-tolerant yet effective against relevant targets [8,9].
Figure 1.
A Schematic Representation of Natural Killer Cell Development. Conventional NK cell development in the mouse occurs in the bone marrow where common lymphoid progenitors (CLPs) undergo NK cell lineage specification and commitment resulting in CD122+ NK cell progenitors (NKP). Acquisition of activating and inhibitory receptors such as NK1.1, Ly49, NKG2 and NKp46 occurs at the iNK cells stage. Subsequent acquisition of DX5 and CD27 occurs at the transition to the mNK cell stage as the cells undergo IL15-driven expansion. In the periphery mNK cells undergo further maturation as detected by increased expression of CD11b and CD43 and transient up-regulation of CD27 and become efficient at target directed cytolysis and cytokine production. Additional subsets of NK cells can be identified based on differential surface receptor expression including NK22 cells, which express CD122 and NKp46 but, for the most part, not NK1.1 and produce IL22. IKDCs express B220 and low levels of CD11c and MHC Class II but may represent an activation state rather than distinct NK cell fate. Memory NK cells are similar in phenotype to peripheral mNK cells except that they are KLRG1+ and have a heightened capacity for cytolysis and cytokine production. A subset of mNK cells (tNK) that lacks Ly49 receptors, CD11b and CD43 but expresses IL7Rα develops in the thymus and is found primarily in lymph nodes. These cells are inefficient killer cells but produce large quantities of cytokines. Dashed arrows represent lineage relationships that have not yet been fully characterized. Markers noted in black continue to be expressed on the progeny of this cell. Markers noted in blue are differentially expressed.
Mature NK cells reside in the bone marrow as well as in peripheral tissues such as the spleen, lymph nodes and liver. However, CD122+NK1.1+DX5+ mNK cells are heterogeneous and higher expression of CD43 (sialophorin) and CD11b (Mac1) correlates with functional maturity and the ability to produce IFNγ after stimulation with cytokines in vitro [5]. Recent studies determined that there is a transient increase of CD27 during progression of mature NK cells from CD43loCD11blo to CD43highCD11bhigh stage implying that expression of CD27 may be associated with the acquisition of effector functions [10,11] The activating receptor KLRG1 is expressed on a subset of CD11b+ mNK cells, notably on NK cells undergoing proliferation in development or homeostatic expansion [12]. These KLRG1+ mNK cells are less responsive to IL15 and show poor expansion capacity in adoptive transfer experiments suggesting that they may be the terminal progeny of KLRG1− mNK cells.
The developmental scheme proposed in Figure 1 represents a most likely path for differentiation from multipotent progenitors to mature NK cells but may not represent an absolute differentiation route. While the majority of NK cells in the bone marrow seem to progress through these stages of receptor expression, the patterns of NK cell receptor expression during development span a continuum. There are clear populations of putative NK cells that, by surface marker expression, do not fit into this developmental scheme. For example, some cells express DX5 but not NK1.1 yet still express a diverse array of Ly49 and NKG2 receptors, a pathway that is not strictly allowed in the presented scheme [4]. In addition, some subsets of mNK cells may not have a mature cell phenotype (for example, see the section on thymic NK cells). And finally, cells other than NK cells, particularly activated T lymphocytes, can share many of the characteristics of mNK cells [13]. Therefore, the developmental scheme shown in Figure 1 is intended to be a guide but not an absolute for analysis of NK cell development in vivo.
Transcriptional regulators of NK cell development
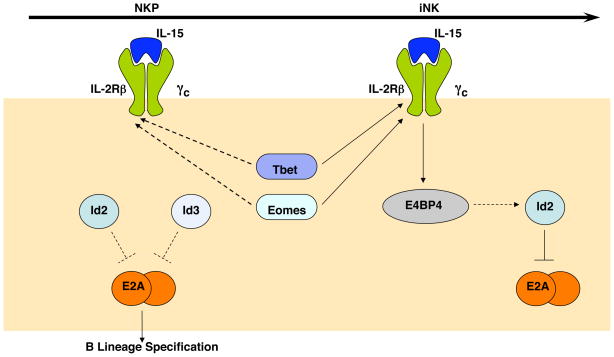
Several transcription factors have been identified that are essential for NK cell development or function but specifying or commitment factor for the NK cell program, that is, transcription factors that drive multipotent cells to activate the NK gene expression program and repress non-NK cell fates, have not been identified [14]. As discussed below, we propose that one reason for the failure to identify an NK cell lineage-specifying factor may be that the transcriptional regulators that are essential in this process are members of multi-protein families with redundant representation in NKPs. At least two examples of this will be highlighted here: the Id proteins (Id2 and Id3) and the T-box proteins (T-bet and Eomesodermin) although it is not yet clear whether these proteins are necessary for NK cell lineage specification or commitment (Figure 2). We will also discuss a newly identified NK cell transcription factor E4BP4 (Nfil3) that plays an essential role in NK cell development beyond the iNK cell stage.
Figure 2.
Transcription Factors in Early Natural Killer Cell Development. At the present time there are only a few transcription factors that are known to play a role in NK cell development. Within NKPs Id2 and Id3 are thought to function redundantly to repress E2A and prevent activation of the B lymphocyte gene program, although this hypothesis remains to be tested. T-bet and Eomes are expressed in NKPs and are likely required for expression of CD122, the β-chain of the IL15 receptor, as they are known to do in iNK cells. IL15 is essential at the iNK cell stage and activates the bZIP transcription factor E4BP4 (also known as Nfil3), which has been implicated in the up-regulation of Id2. At the iNK cell stage Id3 is down-regulated and Id2 is essential for repression of E2A.
Id2, an inhibitor of E protein transcription factors, was postulated to be an essential commitment factor for NK cells based on its potential to antagonize E2A-mediated induction of the B lymphocyte gene program in common lymphoid progenitors (CLPs) [15,16]. Id2−/− mice do have a defect in the generation of mNK cells and lymphoid tissue inducing (LTi) cells, which also develop from CLPs [17,18]. However, a recent detailed analysis of Id2−/− mice revealed that NK cell development is blocked at the iNK to mNK cell transition and that NKPs develop in normal numbers in the absence of Id2 [19]. Importantly however, Id3 is also expressed in NKPs (and increased in Id2−/− NKPs) and the requirement for Id2 coincides with the stage at which Id3 is down-regulated. Therefore, it remains to be determined whether Id2 and Id3 play redundant roles in commitment to the NK cell fate.
A clear example of redundancy between transcriptional regulators in NK cell development comes from the T-box transcription factors T-bet (encoded by Tbx21) and Eomesodermin (Eomes) (Figure 2). While mice lacking T-bet have some defects in NK cell maturation and function, combined deletion of Tbx21 and heterozygous deletion of Eomes leads to a severe NK cell deficiency [20,21]. Most notably, these mice lack IL15 –responsive cells suggesting that NK cell development is perturbed at the iNK cell stage. Analysis of double deficient animals may reveal a role for these proteins in NK cell lineage specification or commitment, however, this analysis awaits the development of conditional alleles for Eomes.
The bZIP transcription factor E4BP4 (encoded by Nfil3) was recently identified as an essential regulator of NK cell development [22]. Like many NK cell transcription factors, E4BP4 is expressed in NK cells, NKT cells and memory CD8 cells. However, mice that lack E4BP4 specifically lack mNK cells, which in this study were defined as CD122+NK1.1+CD11b+ cells and consequently they have a reduced capacity to kill NK cell targets in vivo. This paper reports a decrease in iNK cells in E4BP4−/− mice, however, iNK cells were defined as CD122+NK1.1+CD11b− cells which includes the portion of mNK cells defined as CD122+NK1.1+DX5+CD11b−. Indeed, the majority of CD122+NK1.1+CD11b− cells express DX5 and are considered mNK cells in most schemes of NK cell development. Therefore, the arrest in NK cell development observed in E4BP4−/− mice likely occurs at or after the classically defined iNK cell stage as observed in IL15−/− and Id2−/− mice. This timing would be consistent with the observation that E4BP4 functions downstream of IL15 receptor signaling. NKP are present in normal numbers in E4BP4−/− mice indicating that E4BP4 is not a “master regulator” of the NK cell fate and a role in NK cell lineage specification or commitment remains to be demonstrated. Indeed, identification of the specification and commitment factors for the NK cell lineage is a major challenge for understanding the process of NK cell differentiation from multipotent progenitors.
Heterogeneity among mNK cells
Over the past few years multiple NK cell subsets have been identified that have distinct effector functions (for comprehensive reviews see [23,24]. The origin of these cells and their developmental requirements are currently under investigation and many questions remain to be answered. The first functional subdivision in mNK cells was identified in humans based on expression of CD56 and CD16. CD56dimCD16+ cells are efficient killer cells whereas CD56brightCD16− cells are better at producing cytokines such as IFNγ[25,26]. Until recently it was unclear whether this functional subdivision was specific to human NK cells; however, a subset of mouse NK cells that produces cytokine but is not efficient at cytolysis has been identified and these cells derive from the thymus rather than the bone marrow [27]. In addition, two potentially unique subsets of NK cells have recently been described called IKDCs (for Interferon-producing Killer Dendritic Cells) and NK22 cells, which denotes the ability of these NK cells to produce the cytokine IL22. Here we will briefly discuss these findings and the origin of these NK cell subsets.
In the mouse, distinct subsets of mNK cells with differential lytic or cytokine potential can be identified by expression of the receptor for IL7 (IL7Rα). The IL7Rα+ subset differs from conventional mNK cells in that they have low expression of the Ly49 receptors, CD43 and CD11b, fail to efficiently lyse NK cell targets and produce copious IFNγ and TNFα after stimulation [27]. In terms of their cell NK cell receptor expression and functional capacity these cells resemble immature NK cells. However, unlike conventional immature NK cells IL7Rα+ NK cells are IL7-dependent and they develop in the thymus. IL7Rα+ NK cells are present at very low frequency in the spleen (<5%) but represent a sizable fraction (20%) of lymph node NK cells. Importantly however, it should be noted that IL7Rα also marks a subset of activated T lymphocytes with NK cell characteristics and therefore it is advisable to analyze this subset of NK cells in T cell deficient animals [13], (Boos and Kee et al., submitted). How thymic NK cells influence immune responses and whether their function can be modulated by environmental cues remains to be determined.
Interferon-producing Killer Dendritic Cells (IKDCs), as the name implies, are a population of cells identified based on shared characteristics between plasmacytoid DCs and NK cells, two cell types that perform substantially different functions in the immune response [28,29]. IKDCs were initially identified as an antigen presenting plasmacytoid DC-like cell expressing CD11cloB220+MHCII+ but also expressing NK cell surface receptors such as NK1.1, DX5, NKG2D and Ly49. However, IKDC development was shown to depend on molecules that are central to NK cell, but not pDC, development including IL15 and Id2 raising questions about the proposed DC designation of these cells [30–32]. Indeed, the critical DC characteristic of potent antigen presentation to T cells was not born out in subsequent studies [31]. In addition, a substantial portion of mNK cells (>20%) express B220 and some NK cells were identified with a B220+CD11clo phenotype after activation leading to the conclusion that this phenotype identifies a subset of activated NK cells rather than DCs [31]. Whether cells with this IKDC phenotype perform a unique function during an immune response or whether this phenotype is a common property of all NK cell during activation is currently not resolved but the latter appears to be likely since the functional properties of these cells are similar to conventional mNK cells [31].
Recently a population of IL22-producing NK cells was identified in mouse and human mucosal tissues [33–37]. IL22 is not produced by conventional mNK cells but is known to be an important mediator of epithelial defense against mucosal pathogens [38–40]. In the mouse, IL22-producing NK cells express the pan-NK cell activating receptor NKp46, IL7Rα and c-kit but lack NK1.1 and Ly49 receptors and produce very little perforin or IFNγ. [35–37]. NK22 cells also express arylhydrocarbon receptor [34], a transcription factor involved in activation of IL22 in CD4+ Th17 cells [34]. Among NK cells AHR expression is unique to NK22 cells and NK22 cells differ from conventional mNK cells and other known NK cell subsets in that their development is IL15-independent and requires the transcription factor RORγt [33]. In many respects, the phenotype and developmental requirements of NK22 cells closely resemble lymphoid-tissue inducer cells (LTi cells), which also reside in some mucosal tissues (Figure 1) [3]. LTi cells develop from CLPs, require the transcription factor Id2 and have been shown previously to give rise to NK cells, at least at the bulk population level in vitro [41]. Therefore, it remains possible that NK22 cells are a subset of LTi cells that may or may not be part of the conventional NK cell lineage.
Within the last year it has become appreciated that NK cells can demonstrate many of the features of adaptive immune cells including antigen specificity and a memory response. Two independent model systems were used to show antigen specificity in a memory response (lasting in the order of months); chemical hapten- delayed-type hypersensitivity and CMV infection using Ly49H-expressing cells adoptively transferred into Ly49H− recipients (Ly49H is specific for the CMV m157 protein) [42,43]. In the CMV model “Memory” Ly49H+ cells could be detected for at least 70 days after infection and had higher levels of Ly49H relative to other receptors than the “naïve” Ly49H+ population. These memory NK cells were KLRG1hiCD43hiCD27− consistent with the most mature NK cell population and produced more IFNγ and have a higher cytolytic capacity (measured by CD107a staining) before and after stimulation compared to naïve NK cells. Taken together these findings indicate that at least a subset of KLRG1hiCD43hiCD27− mNK cell population may be composed of previously antigen experienced or memory NK cells. Consistent with this hypothesis cytokine activated NK cells can persist after adoptive transfer into naïve hosts and show increased IFNγ production after re-stimulation [44]. Therefore, the NK cell response shows many of the hallmarks of memory that have been attributed to adaptive immune responses [45].
Conclusions
The studies summarized in this review suggest that NK cells wear multiple hats, performing unique functions in different tissues and under different conditions. These hats may be acquired as a consequence of cues received during development (bone marrow versus thymus-derived NK), in the periphery (NK22 cells versus conventional mNK) or as a consequence of prior antigen exposure (naïve versus memory mNK). However, additional studies are needed to clearly define the relationship between some of these subsets, particularly NK22 cells, and conventional NK cells. With respect to NK cell development, the transcription factors that promote lineage specification and commitment remain to be identified. In addition, how environmental cues that alter mNK cell function intersect with the transcriptional programs guiding development of NK cells remains to be investigated but cannot be achieved until the essential transcriptional networks are identified. Nonetheless, understanding how these transcriptional programs drive NK cell differentiation and function will be necessary in order to manipulate the outcome of pathogenic situations in which altering the balance of NK cell subsets is therapeutically advantageous.
Acknowledgments
We thank the members of the Kee lab for many helpful discussions. B.L.K. was supported by a Scholar Award from the Leukemia and Lymphoma Society. Work in the Kee lab was supported by grants from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 3.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol. 2001;31:1900–1909. doi: 10.1002/1521-4141(200106)31:6<1900::aid-immu1900>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 6.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 7.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 11.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 12.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 13.Stewart CA, Walzer T, Robbins SH, Malissen B, Vivier E, Prinz I. Germ-line and rearranged Tcrd transcription distinguish bona fide NK cells and NK-like gammadelta T cells. Eur J Immunol. 2007;37:1442–1452. doi: 10.1002/eji.200737354. [DOI] [PubMed] [Google Scholar]
- 14.Boos MD, Ramirez K, Kee BL. Extrinsic and intrinsic regulation of early natural killer cell development. Immunol Res. 2008;40:193–207. doi: 10.1007/s12026-007-8006-9. [DOI] [PubMed] [Google Scholar]
- 15.Bain G, Murre C. The role of E-proteins in B- and T-lymphocyte development. Semin Immunol. 1998;10:143–153. doi: 10.1006/smim.1998.0116. [DOI] [PubMed] [Google Scholar]
- 16.Kee BL. Helix-loop-helix proteins in lymphocyte lineage determination. Curr Top Microbiol Immunol. 2005;290:15–27. doi: 10.1007/3-540-26363-2_2. [DOI] [PubMed] [Google Scholar]
- 17.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 18.Ikawa T, Fujimoto S, Kawamoto H, Katsura Y, Yokota Y. Commitment to natural killer cells requires the helix-loop-helix inhibitor Id2. Proc Natl Acad Sci U S A. 2001;98:5164–5169. doi: 10.1073/pnas.091537598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. This paper demonstrates that Id2 is not essential for NK cell lineage commitment but is required at the iNK to mNK cell transition to suppress E-protein activity. The increased expression of Id3 in Id2−/− NKP raises the possibility that Id2 functions redundantly with Id3 to enforce commitment to the NK cell lineage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 21.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6 :1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- *22.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. This paper reports that the transcription factor E4PB4 is essential in early NK cell development and places the requirement for E4BP4 in the context of both IL-15 signaling and Id2. [DOI] [PubMed] [Google Scholar]
- 23.Di Santo JP. Functionally distinct NK-cell subsets: developmental origins and biological implications. Eur J Immunol. 2008;38:2948–2951. doi: 10.1002/eji.200838830. [DOI] [PubMed] [Google Scholar]
- 24.Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009;10:1103–1110. doi: 10.1038/embor.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97 :3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 26.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 27.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 28.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 29.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, Pequignot M, Casares N, Terme M, Flament C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 30.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1. 1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vosshenrich CA, Lesjean-Pottier S, Hasan M, Richard-Le Goff O, Corcuff E, Mandelboim O, Di Santo JP. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welner RS, Pelayo R, Garrett KP, Chen X, Perry SS, Sun XH, Kee BL, Kincade PW. Interferon-producing killer dendritic cells (IKDCs) arise via a unique differentiation pathway from primitive c-kitHiCD62L+ lymphoid progenitors. Blood. 2007;109:4825–4931. doi: 10.1182/blood-2006-08-043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29 :958–970. doi: 10.1016/j.immuni.2008.11.001. This study, along with references 34, 35, 36, and 37 are the first to describe NK22 cells. IL22 cells preferentially localized to mucosal tissues and commensal flora drives their development. [DOI] [PubMed] [Google Scholar]
- **34.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- *36.Luci C, Reynders A, Ivanov, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- *37.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 41.Mebius RE, Miyamoto T, Christensen J, Domen J, Cupedo T, Weissman IL, Akashi K. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3- cells, as well as macrophages. J Immunol. 2001;166:6593–6601. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- 42.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. The paper, along with references 43 and 44, are the first to describe novel adaptive immune features in NK cells including antigen specificity and memory. ** [DOI] [PubMed] [Google Scholar]
- **43.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanier LL, Sun JC. Do the terms innate and adaptive immunity create conceptual barriers? Nat Rev Immunol. 2009;9:302–303. doi: 10.1038/nri2547. [DOI] [PMC free article] [PubMed] [Google Scholar]