Abstract
The transplant of organs is one of the greatest therapeutic achievements of the twentieth century. In organ transplantation, the adaptive immunity is considered the main response exerted to the transplanted tissue, since the principal target of the immune response is the MHC (major histocompatibility complex) molecules expressed on the surface of donor cells. However, we should not forget that the innate and adaptive immunities are closely interrelated and should be viewed as complementary and cooperating. When a human transplant is performed, HLA (human leukocyte antigens) molecules from a donor are recognized by the recipient's immune system triggering an alloimmune response Matching of donor and recipient for MHC antigens has been shown to have a significant positive effect on graft acceptance. This paper will present MHC, the innate and adaptive immunities, and clinical HLA testing.
1. Introduction
The primary function of the immune system is to protect the host from infectious microbes in its environment. This system has evolved over millions of years, in response of coexistence with microorganisms. Basically, the system can be divided in two components, the innate and adaptive immunities.
2. Innate and Adaptive Immunities
The innate also called natural immunity refers to a nonspecific response that involves the recruitment of diverse components of the immune system such as macrophages, neutrophils, natural killer cells (NK cells), cytokines, several cellular receptors, complement components, cytokines, Toll-like receptors (TLRs), and antimicrobial peptides (AMPs). This response is phylogenetically older in comparison to the adaptive immunity, which involves recognition of specific antigen, conferring both specificity and a memory effect [1]. The main effectors of the adaptive immunity are the T and B cells. T cells recognize antigen in the form of peptide bound to major histocompatibility complex (MHC) molecules [2]. B cells have immunoglobulin receptors that recognize the antigenic portions of determined molecules [3].
In organ transplantation, the adaptive immunity is considered the main response exerted to the transplanted tissue, since the principal target of the immune response is the MHC molecules expressed on the surface of donor cells. However, we should not forget that the innate and adaptive immunities are divided only by educational purposes, since both are codependent. For example, T-cell activation leads to the production of cytokines and chemokines which in turn may recruit components of the innate immunity like NK cells or macrophages [4]. Furthermore, local tissue production of complement components seems to be essential for full T-cell activation [4], and some AMPs like defensins and cathelicidin have chemoattractant properties on T lymphocytes [5].
3. Discrimination of Self from Nonself
Because the immune system uses many different effector mechanisms to destroy the broad range of microbial cells and particles that it encounters, it is critical for the immune response to avoid unleashing these destructive mechanisms against its own tissues. This avoidance of destruction of self-tissues is referred to as self-tolerance. Mechanisms to avoid reaction against self-antigens are expressed in many parts of both the innate and the adaptive immune responses. Failure of self-tolerance underlies the broad class of autoimmune diseases [1]. Unfortunately, transplanted tissues from individuals of the same species (allogenic) or different species (xenogeneic) are recognized as nonself, causing graft rejection. The process by which the immune system recognizes pathogens, tumors, and transplantation antigens involves the same antigen recognition molecules.
4. Transplantation Antigens
The rejection response to grafted tissue is caused by cell surface molecules that induce an antigenic stimulus. A wide variety of transplantation antigens have been described, including the MHC molecules, minor histocompatibility antigens, ABO blood group antigens, and monocytes/endothelial cell antigens. The minor histocompatibility antigens are processed peptides derived from cellular antigens that are presented by MHC molecules but are not derived from the MHC [6]. ABO compatibility is of much less importance than MHC compatibility in graft survival. However, ABO incompatibility can result in hyperacute rejection of primarily vascularized grafts, such as kidney and heart [7]. As we mentioned before, the principal target of the transplantation immune response is the MHC molecules expressed on the surface of donor cells.
5. The Major Histocompatibility Complex
According to their relative potencies in eliciting rejection, the major antigens in mammalian species are encoded by a closely linked series of genes called MHC. In humans, these genes reside in the short arm of chromosome 6 (Figure 1(A)). Organs transplanted between MHC identical individuals are readily accepted, whereas organs transplanted between MHC antigen-mismatched individuals are rejected in the absence of immunosuppressive therapy [8, 9]. Since the MHC was first defined in mice by Gorer and Snell [10, 11], the World Health Organization Nomenclature Committee has named HLA (human leukocyte antigen) to the human MHC [12].
Figure 1.
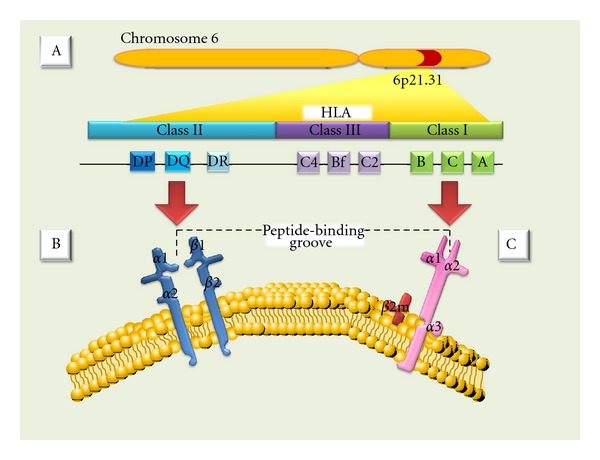
(A) MHC (major histocompatibility complex). (B) Class II antigens are expressed only on B lymphocytes, activated T lymphocytes, monocytes, macrophages, Langerhans cells, dendritic cells, endothelium, and epithelial cells. They are heterodimers composed of noncovalently associated α and β polypeptide chains chains encoded by genes of the HLA-D region. (C) Class I MHC antigens are present on all nucleated cells and are composed of a 45-kd transmembrane α heavy chain encoded by genes of the HLA-A, HLA-B, or HLA-C loci on chromosome 6.
The HLA complex genes and their protein products have been divided into three classes (I, II, and III) on the basis of their tissue distribution, structure, and function [13, 14]. MHC class I and II genes encode codominantly expressed HLA cell surface antigens, and class III genes encode several components of the complement system; all share important roles in immune function [12]. Class I MHC antigens are present on all nucleated cells and are composed of a 45-kd transmembrane α heavy chain encoded by genes of the HLA-A, HLA-B, or HLA-C loci on chromosome 6; the α heavy chains are associated noncovalently with a 12-kd protein, β2-microglobulin, encoded by a gene on chromosome 15 (Figure 1(C)) [13]. Additional (nonclassical) class I molecules, like those encoded by the HLA-E, -F, -G, -H loci, have been described and show limited variability and tissue distribution. The precise functions of these molecules are not yet clear, although they have been implied in presenting carbohydrate and peptide fragments to γδ T cells and mother's immunological tolerance of the fetus [14–17]. MHC class II antigens are expressed only on B lymphocytes, activated T lymphocytes, monocytes, macrophages, Langerhans cells, dendritic cells, endothelium, and epithelial cells [18]. Class II molecules are heterodimers composed of noncovalently associated α and β polypeptide chains chains encoded by genes of the HLA-D region (Figure 1(B)). There are 3 major class II proteins designated, HLA-DP, HLA-DQ, and HLA-DR. Class III genes are located between the HLA-B and HLA-D loci and determine the structure of three components of the complement system: C2, C4, and factor B [13, 19]. Class I MHC molecules present cytoplasm-derived peptides, or intracellular parasites, principally viruses; whereas MHC class II molecules bind peptides derived from extracellular proteins [1]. HLA class I and II molecules are recognized by CD8 and CD4 positive T cells, respectively [20–22]. Also, NK cells may recognize HLA classical and nonclassical type I molecules [23–25].
HLA antigens are inherited in a Mendelian dominant manner. HLA genes are almost always inherited together, thus the antigens of the entire HLA region inherited from one parent collectively are called haplotype. Because chromosome 6 is an autosome (a chromosome with two pairs), all individuals have two HLA haplotypes (one for each chromosome) [12]. According to this, any sibling pair has a 25% chance of inheriting the same two parental haplotypes, a 50% chance of sharing one haplotype, and a 25% chance of having two completely different haplotypes. All children are haploidentical with each parent [6].
Since the biologic function of the HLA molecules is presenting endogenous and exogenous antigens, they manifest high structural polymorphism. Until 2010, 2558 HLA class I and II alleles have been recognized (Table 1) [26]. Mutations in microbial antigens might permit the microbe to avoid binding (and, consequently, recognition) by a few HLA alleles, but no mutations will permit the microbe to avoid recognition broadly throughout the population; assuring then, the continuity of species in the presence of pandemic infection [12].
Table 1.
List of all recognized serological and cellular HLA specificities.
| Locis | Class I | Class II | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | DR | DQ | DP | ||
| A1 | B5 | B50 (21) | Cw1 | DR1 | DQ1 | DPw1 | |
| A2 | B7 | B51 (5) | Cw2 | DR103 | DQ2 | DPw2 | |
| A203 | B703 | B5102 | Cw3 | DR2 | DQ3 | DPw3 | |
| A210 | B8 | B5103 | Cw4 | DR3 | DQ4 | DPw4 | |
| A3 | B12 | B52 (5) | Cw5 | DR4 | DQ5 (1) | DPw5 | |
| A9 | B13 | B53 | Cw6 | DR5 | DQ6 (1) | DPw6 | |
| A10 | B14 | B54 (22) | Cw7 | DR6 | DQ7 (3) | ||
| A11 | B15 | B55 (22) | Cw8 | DR7 | DQ8 (3) | ||
| A19 | B16 | B56 (22) | Cw9 (w3) | DR8 | DQ9 (3) | ||
| A23 (9) | B17 | B57 (17) | Cw10 (w3) | DR9 | |||
| A24 (9) | B18 | B58 (17) | DR10 | ||||
| A2403 | B21 | B59 | DR11 (5) | ||||
| A25 (10) | B22 | B60 (40) | DR12 (5) | ||||
| A26 (10) | B27 | B61 (40) | DR13 (6) | ||||
| Alleles | A28 | B2708 | B62 (15) | DR14 (6) | |||
| A29 (19) | B35 | B63 (15) | DR1403 | ||||
| A30 (19) | B37 | B64 (14) | DR1404 | ||||
| A31 (19) | B38 (16) | B65 (14) | DR15 (2) | ||||
| A32 (19) | B39 (16) | B67 | DR16 (2) | ||||
| A33 (19) | B3901 | B70 | DR17 (3) | ||||
| A34 (10) | B3902 | B71 (70) | DR18 (3) | ||||
| A36 | B40 | B72 (70) | DR51 | ||||
| A43 | B4005 | B73 | DR52 | ||||
| A66 (10) | B41 | B75 (15) | DR53 | ||||
| A68 (28) | B42 | B76 (15) | |||||
| A69 (28) | B44 (12) | B77 (15) | |||||
| A74 (19) | B45 (12) | B78 | |||||
| A80 | B46 | B81 | |||||
| B47 | B82 | ||||||
| B48 | Bw4 | ||||||
| B49 (21) | Bw6 | ||||||
In transplantation immunology, the major impact in graft loss comes from the effects of HLA-B and -DR antigens [27]. There also appears to be a temporal HLA mismatching effect. HLA-DR mismatch effect is the most important in the first 6 months after transplantation, the HLA-B effect emerges in the first 2 years, and HLA-A mismatches have a deleterious effect on long-term graft survival [28–32].
6. The Allogeneic Immune Response
The phenomenon by which the recipient immune system reacts with donor antigens that are considered to be “non-self” is named allorecognition. The main and strongest responses to alloantigens are mediated by host T cells, which recognize peptide antigens presented in the context of MHC, by antigen-presenting cells (APCs). However, evidence that the innate alloimmunity has an important role in graft rejection has recently been proposed by Land and coworkers [33, 34]. They state in their “Injury Hypothesis” that initial allograft injury reflected by reactive oxygen species (ROS) during reperfusion is associated with generation of DAMPs (meaning damage-associated molecular patterns) such as heat shock proteins (HSP) and hyaluronan fragments (fHA) among others, all of which are recognized by TLR4 and/or TLR2. Subsequent TLR4- and TLR2-triggered signaling pathways utilize adaptor proteins including MyD88 (myeloid differentiation marker 88), which in turn initiate downstream signaling pathways that lead to activating the 3 master transcription factors NF-κB (nuclear factor-kappa B), AP-1 (activator protein-1), and IRF-3 (interferon regulatory factor 3). NF-κB seems mainly to be responsible for maturation of donor-derived and recipient-derived dendritic cells, which represents the bridge to development of an adaptive alloimmune response that results in rejection [35]. Certainly, further studies are needed to determine the extension and importance of this branch of the immune system in transplant rejection and/or tolerance.
In adaptive allogenic immune response, the foreign or donor antigen presentation to T cells may occur by three ways (Figure 2) [36]: (1) indirect recognition: donor's HLA molecules can be processed by APC (antigen presenting cells) from a receptor, then they are fractionated into peptides as well as other bacterial antigens and are presented according to the same route as the HLA in the receptor. This type of mechanism has a dominant role in chronic rejection [37–41]; (2) direct recognition: the donor's HLA molecules can be recognized directly on the donor-presenting cells, without requiring antigen processing by receptor. In these circumstances, it could be said that the receptor identifies the foreign HLA molecule as an own molecule with a foreign peptide. This mechanism determines a strong immune response in the acute rejection [37, 38, 42–50]; (3) a third mechanism could be mediated by immunoglobulin-like receptors of natural killer (NK) cells. In this mechanism, the activation of NK receptors promotes the inactivation of NK cells and cytotoxic T lymphocytes as well. These receptors recognize polymorphic sequences of HLA-C, -B, or -A in the target cells. The absence of these sequences in the cell would make them sensitive to cytolysis and therefore the loss of tolerance [51–56].
Figure 2.
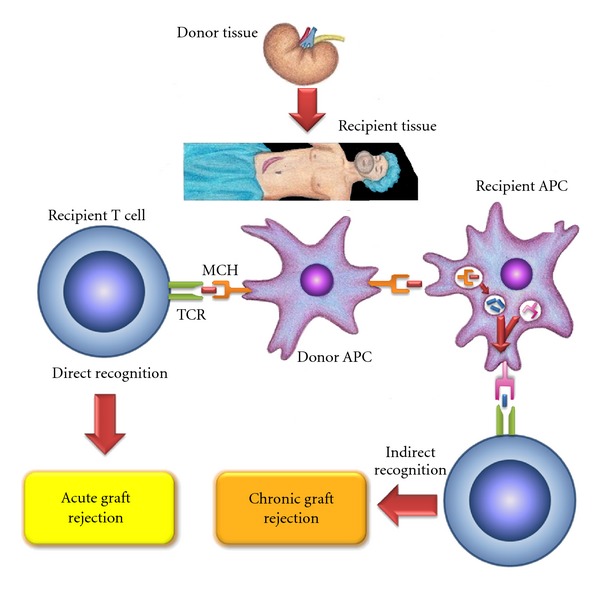
Allogeneic immune response: this could happen by three recognizing mechanisms: first, an indirect recognition: this type of mechanism has a dominant role in chronic rejection; second, a direct recognition: this mechanism determines a strong immune response in the acute rejection; third mechanism, a “semi-direct” recognition that could be mediated by immunoglobulin-like receptors of natural killer (NK) cells and can mediate potent acute rejection.
Recently, it was shown that both naïve and memory CD4+ and CD8+ T cells are frequently cross-reactive against allogeneic HLA molecules and that this allorecognition exhibits exquisite peptide and HLA specificity. Such advances in the understanding of the immunogenetics of allorecognition have led some researches to suggest a new model for allorecognition whereby the majority of T cell alloresponses may occur via direct recognition (cross-reactivity) by thymically educated naïve and memory T cells against allogeneic HLA molecules presenting self-peptides. According to this model, thymically educated T cells are commonly and specifically allo-HLA reactive and are activated by viral infection or vaccination to become alloreactive memory T cells which are a major barrier to successful tolerance [57].
7. Clinical HLA Testing
To support the transplant programs, several clinical laboratories perform various HLA tests, including HLA typing of the recipient and the donor, screening of HLA antibodies in the recipient, and detection of antibodies in the recipient that are reactive with lymphocytes of a prospective donor (cross-matching).
Historically, HLA typing was conducted by serologic testing by using antiserum in complement-dependent cytotoxic assays. Recently, more precise DNA-based HLA typing methods using molecular techniques, such as sequence-specific oligonucleotide probe hybridization, sequence-specific primer amplification, sequencing-based typing, and reference strand-based conformation analysis, have been developed and are frequently used [58].
There is a clear relationship between the degree of HLA matching and kidney graft survival in transplants from living-related donors. Simultaneous analysis of 5,262 one haplotype-matched living-related allografts, and 973 HLA identical allografts showed 10-year projected survival rates of 52% and 73% and graft half-lives of 11.9 and 23.6 years, respectively. Conversely, the influence of HLA matching on the survival of liver and thoracic organs is yet uncertain [59].
To avoid hyperacute rejection, it is very important to identify recipient anti-HLA antibodies to antigens expressed on donor with blood cells. The pioneer method to detect such antibodies, the complement-dependent cytotoxicity (CDC), has been gradually replaced by more-sensitive solid-phase assays, such as the enzyme-linked immunosorbent assay and the bead-based technology (i.e., flow cytometry: FlowPRA and Flow Analyzer: Luminex). However, the new techniques have been associated with decreased specificity, and some non-HLA antigens with no clinical relevance have been able to give a positive crossmatch [60]. These “false-positive” antibody results have as a consequence a decreased chance of the patient to receive an organ by way of exchange organizations, thus decreasing chances for the patient [61]. Thus, the experts recommend that the information these tests provide should complement that of the direct CDC assay.
8. Conclusions
Development of the field of organ and tissue transplantation has accelerated remarkably since the human major histocompatibility complex (MHC) was discovered in 1967. However, has been elusive avoid the graft rejection. This is due to that the transplantation immunobiology is very complex, because of the involvement of several components such as antibodies, antigen presenting cells, helper and cytotoxic T cell subsets, immune cell, surface molecules, signaling mechanisms, and cytokines, which play a role in innate and adaptive immunities.
References
- 1.Chaplin DD. Overview of the human immune response. Journal of Allergy and Clinical Immunology. 2006;117(2):S430–S435. doi: 10.1016/j.jaci.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. Journal of the American Society of Nephrology. 2003;14(9):2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 3.Nemazee D. Receptor selection in B and T lymphocytes. Annual Review of Immunology. 2000;18:19–51. doi: 10.1146/annurev.immunol.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nature Medicine. 2002;8(6):582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 5.Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clinical Immunology. 2010;135(1):1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Chandraker A, Lacomini JJ. Transplantation immunobiology. In: Brenner BM, Leivne SA, editors. Brenner & Rector’s The kidney. 8th edition. Philadelphia, Pa, USA: Elsevier Saunders; 2007. pp. 2104–2111. [Google Scholar]
- 7.Mickelson EM, Fefer A, Storb R, Thomas ED. Correlation of the relative response index with marrow graft rejection in patients with aplastic anemia. Transplantation. 1976;22(3):294–302. doi: 10.1097/00007890-197609000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Guild WR, Harrison JH, Merrill JP, Murray J. Successful homotransplantation of the kidney in an identical twin. Transactions of the American Clinical and Climatological Association. 1955;67:167–173. [PMC free article] [PubMed] [Google Scholar]
- 9.Michon L, Hamburger J, Oeconomos N, et al. An attempted kidney transplantation in man: medical and biological aspects. La Presse Médicale. 1953;61(70):1419–1423. [PubMed] [Google Scholar]
- 10.Gorer PA. The genetic and antigenic basis of tumor transplantation. The Journal of Pathology and Bacteriology. 1937;44(3):691–697. [Google Scholar]
- 11.Snell GD. Methods for the study of histocompatibility genes. Journal of Genetics. 1948;49(2):87–108. doi: 10.1007/BF02986826. [DOI] [PubMed] [Google Scholar]
- 12.Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. Journal of Allergy and Clinical Immunology. 2010;125(2):S324–S335. doi: 10.1016/j.jaci.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein JAN, Sato A. The HLA system: first of two parts. The New England Journal of Medicine. 2000;343(10):702–709. doi: 10.1056/NEJM200009073431006. [DOI] [PubMed] [Google Scholar]
- 14.King A, Hiby SE, Gardner L, et al. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors—a review. Placenta. 2000;21(1):S81–S85. doi: 10.1053/plac.1999.0520. [DOI] [PubMed] [Google Scholar]
- 15.Garcia P, Llano M, de Heredia AB, et al. Human T cell receptor-mediated recognition of HLA-E. European Journal of Immunology. 2002;32(4):936–944. doi: 10.1002/1521-4141(200204)32:4<936::AID-IMMU936>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Le Bouteiller P, Solier C, Proll J, Aguerre-Girr M, Fournel S, Lenfant F. The major histocompatability complex in pregnancy: part II. Placental HLA-G protein expression in vivo: where and what for? Human Reproduction Update. 1999;5(3):223–233. doi: 10.1093/humupd/5.3.223. [DOI] [PubMed] [Google Scholar]
- 17.Le Bouteiller P, Lenfant F. Antigen-presenting function(s) of the non-classical HLA-E, -F and -G class I molecules: the beginning of a story. Research in Immunology. 1996;147(5):301–313. doi: 10.1016/0923-2494(96)89643-x. [DOI] [PubMed] [Google Scholar]
- 18.Klein J, Sato A. Advances in immunology: the HLA system (second of two parts) The New England Journal of Medicine. 2000;343(11):782–786. doi: 10.1056/NEJM200009143431106. [DOI] [PubMed] [Google Scholar]
- 19.Bodmer JG, Marsh SGE, Albert ED, et al. Nomenclature for factors of the HLA system, 1998. Tissue Antigens. 1999;53(4):407–446. doi: 10.1034/j.1399-0039.1999.530421.x. [DOI] [PubMed] [Google Scholar]
- 20.Luz JG, Huang M, Garcia KC, et al. Structural comparison of allogeneic and syngeneic T cell receptor-peptide-major histocompatibility complex complexes: a buried alloreactive mutation subtly alters peptide presentation substantially increasing Vβ interactions. Journal of Experimental Medicine. 2002;195(9):1175–1186. doi: 10.1084/jem.20011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76(2):287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 22.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annual Review of Immunology. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annual Review of Immunology. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama WM, Daniels BF, Seaman WE, Hunziker R, Margulies DH, Smith HRC. A family of murine NK cell receptors specific for target cell MHC class I molecules. Seminars in Immunology. 1995;7(2):89–101. doi: 10.1006/smim.1995.0013. [DOI] [PubMed] [Google Scholar]
- 25.Hoglund P, Sundback J, Olsson-Alheim MY, et al. Host MHC class I gene control of NK-cell specificity in the mouse. Immunological Reviews. 1997;155:11–28. doi: 10.1111/j.1600-065x.1997.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 26.Marsh SGE, Albert ED, Bodmer WF, et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75(4):291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opelz G, Mytilineos J, Scherer S, et al. Survival of DNA HLA-DR typed and matched cadaver kidney transplants. The Lancet. 1991;338(8765):461–463. doi: 10.1016/0140-6736(91)90540-6. [DOI] [PubMed] [Google Scholar]
- 28.Opelz G, Wujciak T, Dohler B, Scherer S, Mytilineos J. HLA compatibility and organ transplant survival. Collaborative transplant study. Reviews in Immunogenetics. 1999;1(3):334–342. [PubMed] [Google Scholar]
- 29.Morris PJ, Johnson RJ, Fuggle SV, et al. Analysis of factors that affect outcome of primary cadaveric renal transplantation in the UK. HLA Task Force of the Kidney Advisory Group of the United Kingdom Transplant Support Service Authority (UKTSSA) The Lancet. 1999;354(9185):1147–1152. doi: 10.1016/s0140-6736(99)01104-6. [DOI] [PubMed] [Google Scholar]
- 30.Takemoto SK, Terasaki PI, Gjertson DW, Cecka JM. Twelve years’ experience with national sharing of HLA-matched cadaveric kidneys for transplantation. The New England Journal of Medicine. 2000;343(15):1078–1084. doi: 10.1056/NEJM200010123431504. [DOI] [PubMed] [Google Scholar]
- 31.Opelz G, Mytilineos J, Wujciak T, Schwarz V, Back D. Current status of HLA matching in renal transplantation. Clinical Investigator. 1992;70(9):767–772. doi: 10.1007/BF00180746. [DOI] [PubMed] [Google Scholar]
- 32.Zantvoort FA, D’Amaro J, Persijn GG, et al. The impact of HLA-A matching on long-term survival of renal allografts. Transplantation. 1996;61(5):841–844. doi: 10.1097/00007890-199603150-00030. [DOI] [PubMed] [Google Scholar]
- 33.Land W. The potential impact of the reperfusion injury on acute and chronic rejection events following organ transplantation. Transplantation Proceedings. 1994;26(6):3169–3171. [PubMed] [Google Scholar]
- 34.Land W, Messmer K. The impact of ischemia/reperfusion injury on specific and non-specific, early and late chronic events after organ transplantation. Transplantation Reviews. 1996;10(4):236–253. [Google Scholar]
- 35.Land WG. Innate alloimmunity: history and current knowledge. Experimental and Clinical Transplantation. 2007;5(1):575–584. [PubMed] [Google Scholar]
- 36.Bharat A, Mohanakumar T. Allopeptides and the alloimmune response. Cellular Immunology. 2007;248(1):31–43. doi: 10.1016/j.cellimm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transplant Immunology. 2002;10(2-3):101–108. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 38.Portoles P, Rojo JM, Janeway CA., Jr. Asymmetry in the recognition of antigen: self class II MHC and non-self class II MHC molecules by the same T-cell receptor. Journal of Molecular and Cellular Immunology. 1988;4(3):129–137. [PubMed] [Google Scholar]
- 39.Kourilsky P, Chaouat G, Rabourdin-Combe C, Claverie JM. Working principles in the immune system implied by the “peptidic self” model. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(10):3400–3404. doi: 10.1073/pnas.84.10.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman LA, Chattopadhyay S. The molecular basis of allorecognition. Annual Review of Immunology. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 41.Matzinger P, Bevan MJ. Hypothesis. Why do so many lymphocytes respond to major histocompatibility antigens. Cellular Immunology. 1977;29(1):1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- 42.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. American Journal of Transplantation. 2003;3(5):525–533. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 43.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. Journal of Immunology. 1999;162(1):352–358. [PubMed] [Google Scholar]
- 44.Sawyer GJ, Dalchau R, Fabre JW. Indirect T cell allorecognition: a cyclosporin A resistant pathway for T cell help for antibody production to donor MHC antigens. Transplant Immunology. 1993;1(1):77–81. doi: 10.1016/0966-3274(93)90063-e. [DOI] [PubMed] [Google Scholar]
- 45.Susskind B, Iannotti MR, Shornick MD, Steward NS, Gorka J, Mohanakumar T. Indirect allorecognition of HLA class I peptides by CD4+ cytolytic T lymphocytes. Human Immunology. 1996;46(1):1–9. doi: 10.1016/0198-8859(95)00215-4. [DOI] [PubMed] [Google Scholar]
- 46.Chen BP, Madrigal A, Parham P. Cytotoxic T cell recognition of an endogenous class I HLA peptide presented by a class II HLA molecule. Journal of Experimental Medicine. 1990;172(3):779–788. doi: 10.1084/jem.172.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Essaket S, Fabron J, de Preval C, Thomsen M. Corecognition of HLA-A1 and HLA-DPw3 by a human CD4+ alloreactive T lymphocyte clone. Journal of Experimental Medicine. 1990;172(1):387–390. doi: 10.1084/jem.172.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vella JP, Spadafora-Ferreira M, Murphy B, et al. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64(6):795–800. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Colovai AI, Tugulea S, et al. Indirect recognition of donor HLA-DR peptides in organ allograft rejection. Journal of Clinical Investigation. 1996;98(5):1150–1157. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 51.Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Current Opinion in Immunology. 2004;16(5):550–557. doi: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Andre F, Chaput N, Schartz NEC, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. Journal of Immunology. 2004;172(4):2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 53.Bedford P, Garner K, Knight SC. MHC class II molecules transferred between allogeneic dendritic cells stimulate primary mixed leukocyte reactions. International Immunology. 1999;11(11):1739–1744. doi: 10.1093/intimm/11.11.1739. [DOI] [PubMed] [Google Scholar]
- 54.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature Immunology. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 55.Boyington JC, Brooks AG, Sun PD. Structure of killer cell immunoglobulin-like receptors and their recognition of the class I MHC molecules. Immunological Reviews. 2001;181:66–78. doi: 10.1034/j.1600-065x.2001.1810105.x. [DOI] [PubMed] [Google Scholar]
- 56.Kroemer A, Xiao X, Degauque N, et al. The innate NK cells, allograft rejection, and a key role for IL-15. Journal of Immunology. 2008;180(12):7818–7826. doi: 10.4049/jimmunol.180.12.7818. [DOI] [PubMed] [Google Scholar]
- 57.D’Orsogna LJ, Roelen DL, Doxiadis II, Claas FH. TCR cross-reactivity and allorecognition: new insights into the immunogenetics of allorecognition. Immunogenetics. 2012;64(2):77–85. doi: 10.1007/s00251-011-0590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung HL. Shedding a new light on the HLA matching. The Korean Journal of Hematology. 2011;46(1):1–2. doi: 10.5045/kjh.2011.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qureshi BH. Consensus and controversies on HLA matching and crossmatching in transplantation. Saudi Journal of Kidney Diseases and Transplantation. 1997;8(2):138–144. [PubMed] [Google Scholar]
- 60.Gloor J, Stegall MD. Sensitized renal transplant recipients: current protocols and future directions. Nature Reviews Nephrology. 2010;6(5):297–306. doi: 10.1038/nrneph.2010.34. [DOI] [PubMed] [Google Scholar]
- 61.Minucci PB, Grimaldi V, Casamassimi A, et al. Methodologies for anti-HLA antibody screening in patients awaiting kidney transplant: a comparative study. Experimental and Clinical Transplantation. 2011;9(6):381–386. [PubMed] [Google Scholar]


