Abstract
Women drastically loose bone during and after menopause leading to osteoporosis, a disease characterized by low bone mass increasing the risk of fractures with minor trauma. Existing therapies mainly reduce bone resorption, however, all existing drugs have severe side effects. Recently, the focus is to identify alternative medicines that can prevent and treat osteoporosis with minimal or no side effects. We used Cissus quadrangularis (CQ), a medicinal herb, to determine its effects on bone loss after ovariectomy in C57BL/6 mice. Two-month old mice were either sham operated or ovariectomized and fed CQ diet. After eleven weeks, mice were sacrificed and the long bones scanned using pQCT and μCT. In the distal femoral metaphysis, femoral diaphysis, and proximal tibia, control mice had decreased cancellous and cortical bone, while CQ-fed mice showed no significant differences in the trabecular number, thickness, and connectivity density, between Sham and OVX mice, except for cortical bone mineral content in the proximal tibia. There were no changes in the bone at the tibio-fibular junction between groups. We conclude that CQ effectively inhibited bone loss in the cancellous and cortical bones of femur and proximal tibia in these mice.
1. Introduction
Osteoporosis is a disease associated with aging that causes fragility of bones making them susceptible to fractures with minor trauma. Bone is a dynamic organ that undergoes lifelong changes by bone remodeling using specialized cells and is the predominant process after attaining peak bone mass around the third decade. Remodeling is an essential process for maintaining the skeleton by repairing any damaged portions and removal of old bone as well as for discharging calcium and phosphorus from bone stores to maintain ionic homeostasis in the body. An imbalance in the process of bone remodeling where there is increased bone resorption alone or in combination with decreased bone formation results in net loss of bone. After attaining peak bone mass during the third decade, humans start losing bones at the rate of 0.6 to 1% every year for the rest of their lives. In case of women, during menopausal age, there is drastic loss of bone.
Treating and/or preventing bone loss can be focused on overall reduction of bone resorption and/or increasing bone formation. Currently, there are several different groups of agents that are used to treat and prevent osteoporosis. But the risks of side effects caused by these drugs are severe [1–4]. This has, recently, led to the search for alternative medicines to treat and prevent osteoporosis.
Different cultures around the world have used herbs for thousands of years to treat several health conditions. One of the herbs that have shown beneficial effects on bone belongs to the Cissus family of plants. Cissus quadrangularis (CQ) is a medicinal herb used in Siddha and Ayurvedic medicine since ancient times in Asia, as a general tonic and analgesic, especially for bone fracture healing [5]. Recently, CQ has been linked to several health benefits such as antiobesity [6], reduction of proinflammatory cytokines [7], anti-inflammatory [8], antioxidant [9], antiglucocorticoid [10], and antidiabetic properties [11].
As early as in the 1960's, CQ was used to determine its beneficial effects on bone fracture healing in young rats and it has been reported that CQ significantly enhances fracture healing process [12]. The study has further demonstrated that in the presence of CQ, bone mineralization takes place much earlier, when compared to that seen in its absence [13]. During bone mineralization, accumulation of mucopolysaccharides precedes the actual mineralization process and CQ increased mucopolysaccharides at the site of fracture [14]. Udupa and Prasad [15] have reported that CQ hastens fractures by reducing the total convalescent period by 33% in experimental animals when compared to those of the controls. CQ also increased calcium uptake and mechanical properties of bone in rats when compared to that of the controls [15]. More recently, Shirwaikar et al. [16] have demonstrated that the mechanical strength of bones in ovariectomized rats increased, significantly, in the long bones and lumbar vertebra. Petroleum ether extracts of CQ stimulated osteoblastogenesis and mineralization in bone marrow mesenchymal cells and murine osteoblastic cell lines [17, 18]. In the present study, we determined the effects of CQ on postmenopausal bone loss in the long bones of female mice. We tested the bones using peripheral quantitative computed tomography (pQCT) and microcomputed tomography (μCT). With pQCT, we determined the effects of CQ on the two envelopes (periosteal and endocortical) and the BMD and BMC measurements, while with μCT, we determined the microarchitecture of the cancellous bone in the distal femoral metaphysis. Both techniques give different measurements and they complement each other. We also tested some bone biochemical markers and proinflammatory cytokines in the serum.
2. Materials and Methods
2.1. Animals
Weanling C57BL/6 female mice were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained in our laboratory animal facility. When mice were eight weeks of age, they were either sham operated or ovariectomized. After one month, mice were divided into the following groups: Group (1) Lab chow sham (LC S), (n = 10); Group (2) Lab chow ovariectomy (LC O), (n = 11); Group (3) Cissus quadrangularis sham (CQ S), (n = 11); Group (4) CQ ovariectomy (CQ O), (n = 11) and fed the respective diets. Mice were maintained in the dietary regimens for eleven weeks and sacrificed. CQ was purchased from 1fast400 (Northborough, MA) in powder form and mixed with modified AIN-93 diet at a concentration of 500 mg/kg b wt. Mice were weighed regularly. Blood was collected by retro-orbital bleeding from anesthetized mice and tibia and femur were removed and stored for pQCT and μCT densitometry. All animal procedures were done according to the UT Health Science Center at San Antonio IACUC guidelines.
2.2. Measurement of Body and Organ Weights
Mice were weight matched at the beginning of the treatment using a CS 200 (Ohaus, Pine Brook, NJ, USA) balance. At the time of sacrifice, body weight was recorded. Uterus, peritoneal adipose tissue, liver, spleen, and kidneys were carefully dissected out and weighed using a Mettler Balance (Columbus, OH, USA).
2.3. Collection of Blood Serum Biochemical Markers, Proinflammatory Cytokine Assays and Leptin
Blood was collected by retro-orbital bleeding from anesthetized mice and serum was obtained by centrifugation at 300 × g for 15 minutes at 4°C. Procollagen type 1 amino terminal propeptide (P1NP), Tartrate Resistant Acid Phosphatase (Trap5b) and ALP levels in the serum were measured using Rat/Mouse P1NP EIA kit (IDS, Fountain Hills, CA, USA), Mouse Trap EIA kit (IDS, Fountain Hills, CA, USA), and Quantichrome ALP assay kit (Bioassay systems, Hayward, CA, USA), respectively. Osteocalcin was measured using IRMA kit (Alpco Diagnostics, Salem, NH, USA). TNF-α, IL-1, and IL-6 were assayed using OptiEIA kits (BD Biosciences Pharmingen, San Diego, CA, USA). Serum leptin was measured using ELISA kit (Diagnostic systems laboratory, Webster, TX, USA).
2.4. Peripheral Quantitative Computerized Tomography Densitometry (pQCT)
Cortical and cancellous bones of distal femoral metaphysis (DFM), proximal tibial metaphysis (PTM), and pure cortical bone at the femoral diaphysis (FD) and tibia fibula junction (TF) were analyzed by pQCT densitometry, using an XCT research M system (Norland Stratec, Birkenfeld, Germany) as described previously [19, 20]. In the proximal tibial metaphysis (PTM) and distal femoral metaphysis (DFM), both cancellous and cortical bone surrounding the cancellous bone were scanned and analyzed. At the PTM and DFM, 5 slices were scanned including the growth plate and one slice, 1 mm distal to the knee joint (PTM) and 1 mm proximal to the knee joint (DFM) was analyzed. The following parameters were determined for both sites: cancellous bone mineral content (Cn BMC), cancellous bone mineral density (Cn BMD), cortical bone area (Ct Ar), cortical BMC (Ct BMC), cortical BMD (Ct BMD), cortical thickness (Ct Th), periosteal perimeter (Peri PM), and endocortical perimeter (Endo PM).
One slice was scanned at the FD (mid-diaphysis) and at the TF junction for measuring pure cortical bone and the following parameters determined: Ct B Ar, Ct BMC, Ct BMD, Ct Th, Peri PM, and Endo PM.
2.5. Micro Computerized Tomography (μCT)
Scans of the DFM was done using a high-resolution Xradia μCT 200 scanner (Xradia, Inc. Concord, CA) at 20 microns. All images were acquired using standard parameters, X-ray source of 90 KV, power of 8.0 W and current of 4.0 μA. Each scan consisted of 181 slices with an exposure time of 30 seconds per slice. The scans were analyzed using Tri/3D Bon (Ratoc System Engineering Co., Ltd., Tokyo, Japan) for the parameters including total volume (TV), bone volume (BV), BV/TV, trabecular number (Tb N), trabecular thickness (Tb Th), trabecular separation (Tb S) and connectivity density (Conn Den).
2.6. Statistical Analysis
Results are expressed as Mean ± SE. Data was analyzed with one-way ANOVA and unpaired t-test using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered to be significant. Newman-Keuls multiple comparison test was used to analyze the differences between groups for significance.
3. Results
3.1. Effects on Body Weights and Organ Weights
Effects of CQ —
Body weight (16%) and adipose tissue weight (148%) significantly increased in CQ S mice, when compared to that of LC S mice (Table 1). Uterus weight did not change between CQ S and LC S mice (Table 1). Body weight (8%) of CQ O mice increased significantly when compared to that of LC O mice. Liver weight increased in CQ S mice but this was not statistically significant when compared to that of LC S mice (Table 1). No significant changes were seen in the weights of the spleen and kidney (Table 1).
Table 1.
Effects of Cissus quadrangularis on the body weight and weight of different organs of female ovariectomized C57BL/6 mice.
| Parameters/Groups | LC Sham | LC OVX | % difference (LC Sham versus LC OVX) | CQ Sham | CQ OVX | % difference (CQ Sham versus CQ OVX) |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 19.17 ± 0.42 | 19.26 ± 0.41 | 19.15 ± 0.45 | 19.25 ± 0.34 | ||
| Final body weight (g) | 22.86 ± 0.86a | 27.42 ± 1.19 | 20↑ | 26.41 ± 0.65b | 29.73 ± 0.66a | 12↑ |
| Adipose tissue weight (g) | 0.45 ± 0.11a | 1.14 ± 0.18 | 153↑ | 1.12 ± 0.09b | 1.50 ± 0.10 | 34*↑ |
| Uterus weight (g) | 0.142 ± 0.011a | 0.072 ± 0.011 | 51↓ | 0.144 ± 0.015 | 0.043 ± 0.008c | 30*↓ |
| Liver weight (g) | 1.088 ± 0.043a | 1.318 ± 0.050 | 21↑ | 1.211 ± 0.043 | 1.330 ± 0.050 | |
| Spleen weight (g) | 0.086 ± 0.006a | 0.150 ± 0.018 | 74↑ | 0.116 ± 0.011 | 0.096 ± 0.005a | |
| Kidney weight (g) | 0.267 ± 0.012 | 0.303 ± 0.015 | 0.275 ± 0.014 | 0.253 ± 0.009 |
Data are Mean ± SE. aP < 0.05 versus LC O; bP < 0.05 versus LC S; cP < 0.05 versus CQ S; ∗= P < 0.05 versus LC; ↑ = increase; ↓ = decrease.
Effects of Ovariectomy —
Lab chow groups. Body weight (20%) and adipose tissue weight (153%) increased significantly in LC O mice, while uterus weight (51%) decreased significantly in LC O mice, when compared to that of LC S mice (Table 1). Liver and spleen weights significantly increased in the LC O mice by 21% and 74% respectively, when compared to that of LC S mice (Table 1).
CQ groups. Body weight significantly increased in the CQ O group although no significant differences were observed in the adipose tissue weight between CQ S and CQ O mice (Table 1). Uterus weight (30%) decreased significantly in CQ O mice, when compared to that of CQ S mice (Table 1). No significant differences were observed in the liver, spleen and kidneys weights (Table 1). Uterus was carefully examined for any changes and no visible changes were seen.
3.2. Effects on Serum Bone Biochemical Parameters, Proinflammatory Cytokines, and Leptin
3.2.1. Bone Biochemical Markers
Effects of CQ —
Serum P1NP levels (45%) decreased significantly in CQ S mice, when compared to that of LC S mice (Table 2). No significant differences were observed between CQ and LC mice with respect to Trab5b and ALP levels (Table 2).
Table 2.
Effects of Cissus quadrangularis on the Biochemical markers of bone turnover, pro-inflammatory cytokines and leptin in the serum of female ovariectomized C57BL/6 mice.
| Parameters/Groups | LC Sham | LC OVX | % difference (LC Sham versus LC OVX) | CQ Sham | CQ OVX | % difference (CQ Sham versus CQ OVX) |
|---|---|---|---|---|---|---|
| P1NP (ng/mL) | 13.23 ± 1.17a | 10.44 ± 0.18 | 21↓ | 7.29 ± 0.85b | 6.55 ± 0.57 | 10↓ |
| Alkaline phosphatase (U/L) | 1.95 ± 0.24a | 0.93 ± 0.18 | 48↓ | 1.60 ± 0.09 | 2.09 ± 0.57 | 6 |
| Osteocalcin (ng/mL) | 45.78 ± 1.11 | 51.82 ± 7.74 | 40.45 ± 2.27 | 34.53 ± 4.49 | ||
| Trap5b (U/L) | 13.53 ± 0.87 | 12.39 ± 0.65 | 12.92 ± 0.56 | 13.23 ± 1.46 | ||
| IL-1β (pg/mL) | 418 ± 143 | 580 ± 127 | 39↑ | 66 ± 8b | 278 ± 30d | 322*↑ |
| IL-6 (pg/mL) | 818 ± 152 | 888 ± 202 | 9↑ | 387 ± 35b | 547 ± 77a | 41↑ |
| TNF-α(pg/mL) | 1.47 ± 0.43 | 2.12 ± 0.45 | 44↑ | 0.76 ± 0.21 | 0.69 ± 0.08a | 13↓ |
| Leptin (pg/mL) | 169 ± 27a | 1100 ± 85 | 550↑ | 621 ± 115b | 1321 ± 147c | 112↑ |
Data are Mean ± SE. aP < 0.05 versus LC O; bP < 0.05 versus LC S; cP < 0.05 versus CQ S; dP < 0.08 versus LC O; ∗= P < 0.05 versus LC; ↑: increase; ↓: decrease.
Effects of Ovariectomy —
Lab chow groups. P1NP (21%) and ALP (48%) levels decreased significantly in LC O mice, when compared to those of LC S mice (Table 2). Trap5b levels were not significantly different between LC S and LC O mice (Table 2).
CQ groups. No significant differences were observed in serum P1NP, ALP and Trap5b levels between CQ S and CQ O mice (Table 2).
3.2.2. Proinflammatory Cytokines
Effects of CQ —
TNF-α (48%), IL-1β (84%) and IL-6 (53%) levels decreased in the CQ S mice when compared to those of LC S mice (Table 2).
Effects of Ovariectomy —
Lab chow groups. Although there was an increase in the cytokines that were measured in the LC O mice these increases were not statistically significant between the different groups (Table 2).
CQ groups. No significant differences were observed between CQ S and CQ O mice with respect to pro-inflammatory cytokines (Table 2).
3.2.3. Leptin Levels
Effects of CQ —
Leptin levels (450%) increased significantly in CQ S mice when compared to that of LC S mice (Table 2).
Effects of Ovariectomy —
3.3. pQCT Densitometry
3.3.1. Distal Femoral Metaphysis (DFM)
Effects of CQ —
CQ S did not change any of the parameters studied when compared to those of LC S fed mice in the distal femoral metaphysis (Figure 1). But CQ O mice had significantly higher Cn BMC (8%), Cn BMD (34%), Ct BMC (32%), Ct BMD (8%), and Ct Th (30%) when compared to those of LC O mice (Figures 1(A)–1(D) and 2(A)). Endo PM (4%) decreased significantly in CQ O mice when compared to that of LC O mice (Figure 2(C)).
Figure 1.
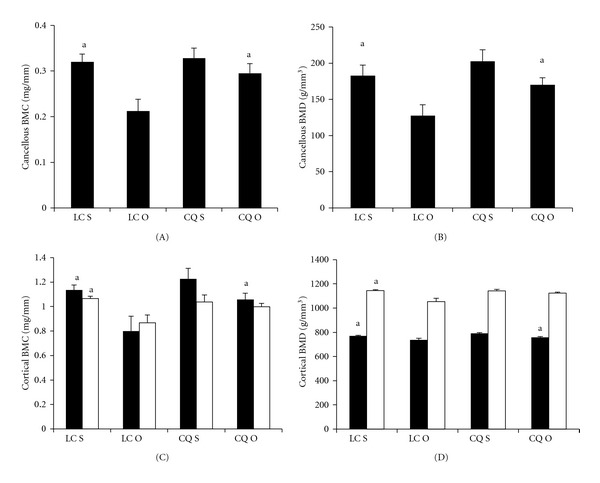
Effects of Cissus quadrangularis on the cancellous and cortical bone parameters of the distal femoral metaphysis and femoral diaphysis of C57Bl/6 mice after ovariectomy using pQCT. Data are Mean ± SE. (A) cancellous BMC; (B) cancellous BMD; (C) cortical BMC; (D); Cortical BMD. Black bars represent distal femoral metaphysis; white bars represent femoral diaphysis. LC S: lab chow sham; LC O: lab chow ovariectomy; CQ S: Cissus quadrangularis sham; CQ O: Cissus quadrangularis ovariectomy. aP < 0.05 versus LC O.
Figure 2.
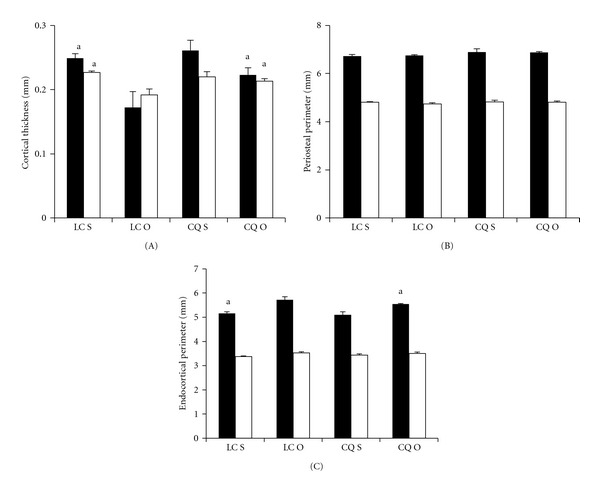
Effects of Cissus quadrangularis on the cortical bone thickness and perimeters of the distal femoral metaphysis and femoral diaphysis of C57Bl/6 mice after ovariectomy using pQCT. Data are Mean ± SE. (A) Cortical thickness; (B) periosteal perimeter; (C) Endocortical perimeter. Black bars represent distal femoral metaphysis; white bars represent femoral diaphysis. LC S: lab chow sham; LC O: lab chow ovariectomy; CQ S: Cissus quadrangularis sham; CQ O: Cissus quadrangularis ovariectomy. aP < 0.05 versus LC O.
Effects of Ovariectomy —
Lab chow groups. LC O mice had significantly less Cn BMC (34%), Cn BMD (36%), Ct BMC (36%), Ct BMD (4%), and Ct Th (26%), and increased Endo PM (6%), when compared to those of LC S mice (Figures 1(A)-1(D), 2(A) and 2(C)).
CQ groups. No significant differences were observed in Cn BMC, Cn BMD, Ct BMC, Ct BMD, and Ct Th levels between CQ S and CQ O mice (Figures 1(A)–1(D) and 2(A)). Endo PM increased in CQ O mice but this increase was not statistically significant (Figure 2(C)).
3.3.2. Femoral Diaphysis (FD)
Effects of CQ —
In FD, CQ did not change any of the parameters studied, when compared to those of LC S mice (Figures 1(C), 1(D) and 2(A)–2(C)). CQ O mice had significantly higher Ct Th, when compared to those of LC O mice (Figure 2(A)).
Effects of Ovariectomy —
Lab chow groups. LC O mice had significantly less Ct BMC (19%), Ct BMD (8%), Ct Th (15%), and increased Endo PM (5%), when compared to those of LC S mice (Figures 1(C), 1(D) and 2(A) and 2(C)).
CQ groups. No significant differences were observed in the Ct BMC, Ct BMD, and Ct Th levels between CQ S and CQ O mice (Figures 1(A)–1(D) and 2(A)). Endo PM increased in CQ O mice but this increase was not statistically significant (Figure 2(C)).
3.3.3. Proximal Tibial Metaphysis (PTM)
Effects of CQ —
CQ did not change any of the parameters studied when compared to those of LC S fed mice in the proximal tibial metaphysis (Figures 3(A)-3(D) and 4(A)–4(C)). CQ O mice had significantly higher Ct BMC (48%), Ct BMD (2%), and Ct Th (42%) when compared to those of LC O mice (Figures 3(C)–3(D) and 4(A)). Endo PM decreased in CQ O mice when compared to that of LC O mice, but this decrease was not statistically significant (Figure 4(C)).
Figure 3.
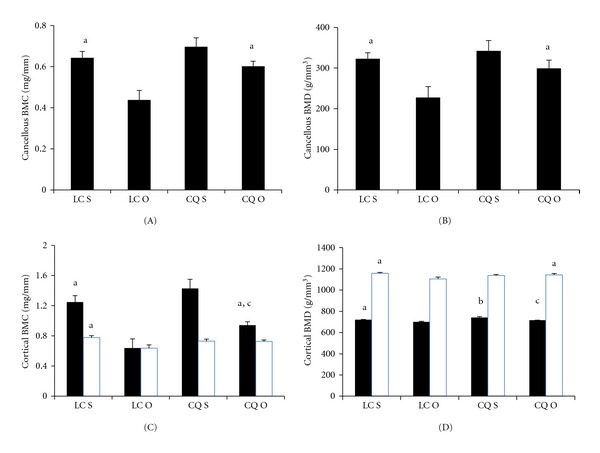
Effects of Cissus quadrangularis on the cancellous and cortical bone parameters of the proximal tibial metaphysis and tibia fibula junction of C57Bl/6 mice after ovariectomy using pQCT. Data are Mean ± SE. (A) cancellous BMC; (B) cancellous BMD; (C) cortical BMC; (D) cortical BMD. Black bars represent distal femoral metaphysis; White bars represent femoral diaphysis. LC S: lab chow sham; LC O: lab chow ovariectomy; CQ S: Cissus quadrangularis sham; CQ O: Cissus quadrangularis ovariectomy. aP < 0.05 versus LC O; bP < 0.05 versus CQ S; cP < 0.05 versus LC S.
Figure 4.

Effects of Cissus quadrangularis on the cortical bone thickness and perimeters of the proximal tibial metaphysis and tibia fibula junction of C57Bl/6 mice after ovariectomy using pQCT. Data are Mean ± SE. (A) Cortical thickness; (B) periosteal perimeter; (C) Endocortical perimeter. Black bars represent proximal tibial metaphysis; white bars represent tibia fibula junction. LC S: lab chow sham; LC O: lab chow ovariectomy; CQ S: Cissus quadrangularis sham; CQ O: Cissus quadrangularis ovariectomy. aP < 0.05 versus LC O; bP < 0.05 versus LC S.
Effects of Ovariectomy —
Lab chow groups. LC O mice had significantly less Cn BMC (32%), Cn BMD (21%), Ct BMC (59%), Ct BMD (2%), Ct Th (65%), Peri PM (3%) and increased Endo PM (7%), when compared to those of LC S mice (Figures 3(A)–3(D) and 4(A)–4(C).
CQ groups. Although Cn BMC and Cn BMD decreased in CQ O mice, when compared to those of CQ S mice, these decreases were not statistically significant (Figures 3(A) and 3(B)). Ct BMC (34%), Ct BMD (3%), and Ct Th (30%) levels significantly decreased in CQ O mice when compared to those of CQ S mice (Figures 3(C) and 3(D) and Figure 4(a)). Endo PM increased in CQ O mice but this increase was not statistically significant, when compared to that of LC O (Figure 4(C)).
3.3.4. Tibia Fibular Junction (TF)
Effects of CQ —
CQ did not change any of the parameters studied when compared to those of LC fed mice in the tibia fibular junction of sham and ovariectomized mice, except in the Endo PM which significantly decreased in CQ S, when compared to that of LC S mice (Figures 3(C), 3(D), and 4(A)–4(C)).
Effects of Ovariectomy —
Lab chow groups. LC O mice had significantly less Ct BMC (13%), Ct BMD (6%) and Ct Th (11%) when compared to those of LC S mice (Figures 3(C), 3(D), and 4(A)). LC O mice showed increased Endo PM (−2%), but this increase was not statistically significant (Figure 4(C)).
CQ groups. No significant differences were observed in the Ct BMC, Ct BMD, and Ct Th levels between CQ S and CQ O mice (Figures 3(C), 3(D), and 4(A)). Endo PM increased in CQ O mice but this increase was not statistically significant (Figure 4(C)).
3.4. μCT Densitometry
Effects of CQ —
CQ treatment did not significantly change the trabecular number, trabecular thickness, connectivity density, and BV/TV (Figure 5) but trabecular separation (65%) significantly decreased in CQ S mice when compared to that of LC S mice (Figure 5). There was 45% increase in Tb N and 28% increase in connectivity density in CQ S mice but these increases were not statistically significant, when compared to those of LC S mice (Figure 5). CQ O mice had significantly higher trabecular number (353%) and connectivity density (363%) and lower trabecular separation (28%), when compared to those of LC O mice (Figure 5).
Figure 5.
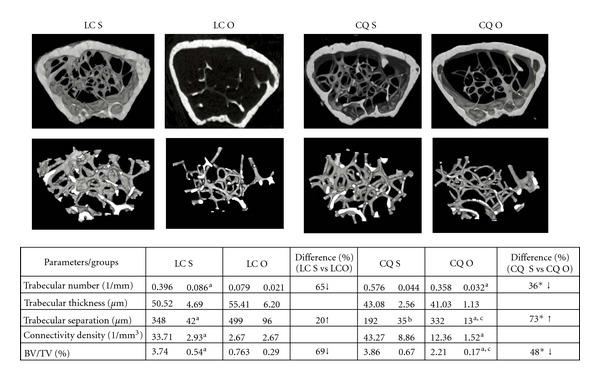
Effects of Cissus quadrangularis on the static histomorphometry parameters of the distal femoral metaphysis of C57Bl/6 mice after ovariectomy using CT. Data are Mean ± SE. LC S: lab chow sham; LC O: lab chow ovariectomy; CQ S: Cissus quadrangularis sham; CQ O: Cissus quadrangularis ovariectomy. aP < 0.05 versus LC O; bP < 0.05 versus LC S; cP < 0.05 versus CQ S, *P < 0.05 versus LC. The figures are representative of the mean values that were obtained.
Effects of Ovariectomy —
Lab Chow Groups. In LC O mice, Tb N (65%); connectivity density (92%) and BV/TV (69%) decreased significantly, when compared to those of LC S mice (Figure 5). Although Tb Th decreased by 20%, this decreases was not statistically significant.
CQ groups. In CQ O mice, Tb Sp (73%) significantly increased, and BV/TV (48%) significantly decreased when compared to those of CQ S mice (Figure 5).
4. Discussion
Cissus quadrangularis belongs to the vitaceae family and is found in South East Asia where it is edible and is used as a vegetable. This plant has been used from ancient times to enhance fracture healing and has several other health benefits including antiinflammatory [8], antiglucocorticoid [10], antidiabetic [11] antibacterial [5, 21], and antioxidant properties [9]. This plant has triterpenoids [22, 23], steroids [22, 24] stilbenes [25], flavanoids [13], lipids [13], and several catalpols [13]. Slowly, interest in natural products for the treatment and prevention of disease is growing in the quest to minimize severe side effects that existing drugs can cause and WHO has endorsed the safe and effective use of such medicines [26]. We studied the effects of CQ-dried powder (stems and leaves) in an animal model for postmenopausal bone loss. Although CQ by itself did not increase bone mass we observed that it decreased bone loss in the distal femoral metaphysis and proximal tibial metaphysis regions of the long bones that have both cancellous and cortical bones. Loss of cancellous bone from these regions is typical after ovariectomy and menopause, mainly because endocortical resorption is stimulated. Bone protection in the distal femur and proximal tibia is mainly due to decreased bone resorption at the endocortical bone surface and preservation of trabecular microarchitecture. Cancellous bone at the femur also showed higher trabecular number, connectivity density and BV/TV in CQ O mice suggesting that bone resorption is decreased considerably. This is supported by the well preserved trabecular morphology in the CQ O mice (Figure 5) and data is in line with reports using CQ ethanol extracts. When young Wistar rats were fed CQ ethanol extracts, after ovariectomy for three months, there was restoration of architecture and increased biomechanical properties in the femur [16]. However, this is the first report to show the effects of CQ on bone using densitometric morphometric analyses including actual BMD and BMC values for the different bone sites. Moreover, we have tested several bone sites (femoral and tibial metaphysis as well as femoral and tibial diaphysis) as it is well known that different bone sites do not react to treatment regimens in the same manner [27].
We measured levels of several serum biochemical markers to determine the influence of CQ on the state of bone turnover in these mice. P1NP and ALP were decreased in LC O mice as expected. With CQ treatment P1NP was decreased in both sham and OVX mice which suggests that CQ maybe altering the processing of procollagen to collagen. However, there was no difference in P1NP levels between CQ S and CQ O groups. ALP that was measured is the total ALP which is an indirect marker for increased osteoblast activity, and with CQ treatment especially in the OVX group, there was increased activity which is in line with reports that show increased ALP activity in bone marrow cells in rats treated with CQ [18]. We also measured osteocalcin as a direct marker for osteoblast activity and Trap5b as a marker for osteoclast activity. We observed that there were no statistical differences between the levels, of either of these markers in any of the groups studied; therefore, CQ does not alter the levels of these markers. Based on these results, we suggest that CQ does not change the bone turnover rate in these mice.
The mechanism(s) by which CQ inhibits bone loss is yet to be fully studied. As a preliminary investigation, we measured a few proinflammatory cytokines. Certain proinflammatory cytokines like TNF-α, IL-1, IL-6, and IL-11 play a critical role in the bone remodeling process [28, 29] mainly by activation of osteoclasts and increasing bone resorption [30–32]. While IL-1 activates NF-κB and MAPKs through TRAF-6, it may also induce PGE2 and the expression of RANKL in osteoblast [28]. IL-6 is produced by osteoblasts and stimulate the formation of osteoclasts [33]. Interestingly, IL-6 knockout mice do not lose cancellous bone after ovariectomy [33]. In our study, significant decreases in the serum levels of IL-1 (84%), IL-6 (53%), and TNF-α (40%) were observed with CQ diet. Even in CQ O mice the levels of IL-6 and TNF-α were significantly lower than those of the LC O mice and IL-1 levels decreased by 52%. Our results clearly show that CQ alters proinflammatory cytokines and maybe one of the major pathways used to reduce bone resorption—the characteristic response to ovariectomy.
Based on the literature, CQ was also used to induce weight loss [6, 34], but in our mice, we noticed that there was increased body weight and peritoneal fat. Both the studies that reported weight reducing benefits of CQ were in obese humans. The major difference between these reports and our study is that they were using already obese patients while the mice in our study were not obese to begin with. So, it may be that a stimulus (obesity-linked proteins) is required for CQ to reduce fat mass or the increase in fat mass is specific to mice and may not affect humans. We measured the levels of leptin, a hormone derived from the adipose tissue which plays a key role in regulating energy intake and expenditure and also influences bone formation as well as bone resorption [35]. At lower than physiological concentrations leptin stimulates bone formation and probably can induce apoptosis of osteoclasts [36]. We were not surprised to find increased circulating levels of leptin and there was bone loss in the LC O mice, but CQ could protect bone in the long bones suggesting that CQ blocks the bone resorption action of leptin. It will be interesting to see if CQ has any influence on the adipocytes found in the bone marrow. If CQ reduces the adipocytes in the bone marrow then the bone resorbing properties of leptin will be reduced as local leptin concentrations produced by increased adipocytes in the bone marrow will increase bone loss [37]. Therefore, leptin CQ interaction needs to be further studied to determine the mechanism by which CQ beneficially influences bone through leptin.
CQ may primarily attenuate bone resorption in OVX mice through the downregulation of proinflammatory cytokines but it does not rule out the possibility that it may also act through other pathways. There are reports that CQ also enhances bone mineralization by accumulating mucopolysaccharides at the site of bone formation [14]. Moreover, CQ is reported to increase calcium uptake and mechanical properties of bone in rats [15]. Phytochemical analyses of CQ show the presence of high levels of calcium, vitamin C, β-carotene [38, 39], and flavanoids [25] some of these substances have established beneficial properties on bone. In vitro studies have shown that ethanolic extracts of CQ increased mRNA and proteins related to the bone formation pathway and IGF-I, IGF-II, and IGF binding protein [40, 41]. More investigations are necessary to elucidate the mechanism(s) by which CQ influences bone metabolism. However, it is very encouraging to note that in studies done with CQ using very high doses (5000 mg/kg body weight) [9] have not reported any toxic side effects. In the present study, we have used only 500 mg/kg body weight of CQ and observed that the liver, spleen, and kidney weights were not altered significantly, suggesting that CQ may not have any severe side-effects.
5. Conclusions
We conclude that CQ can reduce OVX induced bone loss and it does this in the long bones in a site-specific manner with more effects on the cancellous bone of femur followed by tibia. CQ probably reduces bone resorption primarily by downregulating proinflammatory cytokines that are often increased after ovariectomy. The beneficial effects of CQ are probably due to the flavanoids present. Although the mechanism(s) by which CQ attenuates ovariectomy-induced bone loss has to be studied, CQ being an edible plant and with a history of medicinal effects, especially in healing bone fractures, may be a good supplement to existing medication for the reversal of postmenopausal bone loss.
Conflict of Interests
None of the authors have competing interests to declare.
Acknowledgment
The authors wish to acknowledge the NIH grant AT005232-04.
References
- 1.Foidart JM, Desreux J, Pintiaux A, Gompel A. Hormone therapy and breast cancer risk. Climacteric. 2007;10(2):54–61. doi: 10.1080/13697130701598324. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE. Clinical concerns of alendronate use. Journal of Oral and Maxillofacial Surgery. 2008;66(6):p. 1322. doi: 10.1016/j.joms.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Bisphosphonate-induced osteonecrosis of the jaws: a challenge, a responsibility, and an opportunity. International Journal of Periodontics and Restorative Dentistry. 2008;28(1):5–6. [PubMed] [Google Scholar]
- 4.Valverde P. Pharmacotherapies to manage bone loss-associated diseases: a quest for the perfect benefit-to-risk ratio. Current Medicinal Chemistry. 2008;15(3):284–304. doi: 10.2174/092986708783497274. [DOI] [PubMed] [Google Scholar]
- 5.Selvarajan B. Ayurvedic Drugs and Their Plant Sources. Oxford and India Book House; 1994. [Google Scholar]
- 6.Oben JE, Enyegue DM, Fomekong GI, Soukontoua YB, Agbor GA. The effect of Cissus quadrangularis (CQR-300) and a Cissus formulation (CORE) on obesity and obesity-induced oxidative stress. Lipids in Health and Disease. 2007;6, article 4 doi: 10.1186/1476-511X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jainu M, Devi CSS. Attenuation of neutrophil infiltration and proinflammatory cytokines by Cissus quadrangularis: a possible prevention against gastric ulcerogenesis. Journal of Herbal Pharmacotherapy. 2005;5(3):33–42. [PubMed] [Google Scholar]
- 8.Panthong A, Norkaew P, Kanjanapothi D, Taesotikul T, Anantachoke N, Reutrakul V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. Journal of Ethnopharmacology. 2007;111(2):335–340. doi: 10.1016/j.jep.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Jainu M, Devi CSS. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chemico-Biological Interactions. 2006;161(3):262–270. doi: 10.1016/j.cbi.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Prasad GC, Udupa KN. Effect of Cissus quadrangularis on the healing of cortisone treated fractures. Indian Journal of Medical Research. 1963;51:667–676. [PubMed] [Google Scholar]
- 11.Onyechi UA, Judd PA, Ellis PR. African plant foods rich in non-starch polysaccharides reduce postprandial blood glucose and insulin concentrations in healthy human subjects. British Journal of Nutrition. 1998;80(5):419–428. [PubMed] [Google Scholar]
- 12.Udupa KN, Arnikar HJ, Singh LM. Experimental studies of the use of “Cissus quadrangularis” in healing of fractures. II. Indian Journal of Medical Sciences. 1961;15:551–557. [PubMed] [Google Scholar]
- 13.Singh LM, Udupa KN. Studies on “Cissus quadrangularis” in fracture by using phosphorus 32. III. Indian Journal of Medical Sciences. 1962;16:926–931. [PubMed] [Google Scholar]
- 14.Irving JT. Theories of mineralization of bone. Clinical Orthopaedics and Related Research. 1973;97:225–236. doi: 10.1097/00003086-197311000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Udupa KN, Prasad GC. Biomechanical and calcium-45 studies on the effect of Cissus quadrangularis in fracture repair. Indian Journal of Medical Research. 1964;52:480–487. [PubMed] [Google Scholar]
- 16.Shirwaikar A, Khan S, Malini S. Antiosteoporotic effect of ethanol extract of Cissus quadrangularis Linn. on ovariectomized rat. Journal of Ethnopharmacology. 2003;89(2-3):245–250. doi: 10.1016/j.jep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Parisuthiman D, Singhatanadgit W, Dechatiwongse T, Koontongkaew S. Cissus quadrangularis extract enhances biomineralization through up-regulation of MAPK-dependent alkaline phosphatase activity in osteoblasts. In Vitro Cellular & Developmental Biology—Animal. 2009;45(3-4):194–200. doi: 10.1007/s11626-008-9158-1. [DOI] [PubMed] [Google Scholar]
- 18.Potu BK, Bhat KMR, Rao MS, et al. Petroleum ether extract of Cissus quadrangularis (linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics. 2009;64(10):993–998. doi: 10.1590/S1807-59322009001000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banu MJ, Orhii PB, Mejia W, et al. Analysis of the effects of growth hormone, voluntary exercise, and food restriction on diaphyseal bone in female F344 rats. Bone. 1999;25(4):469–480. doi: 10.1016/s8756-3282(99)00195-7. [DOI] [PubMed] [Google Scholar]
- 20.Banu J, Wang L, Kalu DN. Effects of increased muscle mass on bone in male mice overexpressing IGF-I in skeletal muscles. Calcified Tissue International. 2003;73(2):196–201. doi: 10.1007/s00223-002-1072-z. [DOI] [PubMed] [Google Scholar]
- 21.Udupa KN, Chaturvedi GN, Tripathi SN. Advances in Research in Indian Medicine. Banaras Hindu University; 1970. [Google Scholar]
- 22.Bhutani KK, Kapoor R, Atal CK. Two unsymmetric tetracyclic triterpenoids from Cissus quadrangularis. Phytochemistry. 1984;23(2):407–410. [Google Scholar]
- 23.Gupta MM, Verma RK. Unsymmetric tetracyclic triterpenoid from Cissus quadrangularis. Phytochemistry. 1990;29(1):336–337. [Google Scholar]
- 24.Sen SP. Studies on active constituents of Cissus quadrangularis .2. Current Science. 1966;35(12):p. 317. [Google Scholar]
- 25.Adesanya SA, Nia R, Martin MT, Boukamcha N, Montagnac A, Païs M. Stilbene derivatives from Cissus quadrangularis. Journal of Natural Products. 1999;62(12):1694–1695. [Google Scholar]
- 26.Research Guidlines for Evaluating the safety and Efficacy of Herbal Medicines. World Health Organization, Regional Office for the Wetern Pacific; 1993. [Google Scholar]
- 27.Banu J, Kalu DN. Site-specific effects of cerivastatin on bone in male Sprague-Dawley rats. Bone. 2004;34(3):432–442. doi: 10.1016/j.bone.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling—emerging insights into the pathophysiology of osteoporosis. The New England Journal of Medicine. 1995;332(5):305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz MC. Cytokines and estrogen in bone: anti-osteoporotic effects. Science. 1993;260(5108):626–627. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]
- 30.Ershler WB, Harman SM, Keller ET. Immunologic aspects of osteoporosis. Developmental and Comparative Immunology. 1997;21(6):487–499. doi: 10.1016/s0145-305x(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 31.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 32.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocrine Reviews. 1999;20(3):345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 33.Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. The EMBO Journal. 1994;13(5):1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oben JE, Ngondi JL, Momo CN, Agbor GA, Sobgui CSM. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double-blind placebo-controlled study. Lipids in Health and Disease. 2008;7, article 12 doi: 10.1186/1476-511X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas T. The complex effects of leptin on bone metabolism through multiple pathways. Current Opinion in Pharmacology. 2004;4(3):295–300. doi: 10.1016/j.coph.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochemical Journal. 2006;393(1):7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporosis International. 2008;19(7):905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 38.Mehta M, Kaur N, Bhutani KK. Determination of marker constituents fron Cissus quadrangularis Linn. and their quantitation by HPTLC and HPLC. Phytochemical Analysis. 2001;12(2):91–95. doi: 10.1002/pca.569. [DOI] [PubMed] [Google Scholar]
- 39.Chidambara Murthy KN, Vanitha A, Swamy MM, Ravishankar GA. Antioxidant and antimicrobial activity of Cissus quadrangularis L. Journal of Medicinal Food. 2003;6(2):99–105. doi: 10.1089/109662003322233495. [DOI] [PubMed] [Google Scholar]
- 40.Muthusami S, Ramachandran I, Krishnamoorthy S, Govindan R, Narasimhan S. Cissus quadrangularis augments IGF system components in human osteoblast like SaOS-2 cells. Growth Hormone & IGF Research. 2011;21(6):343–348. doi: 10.1016/j.ghir.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Muthusami S, Senthilkumar K, Vignesh C, et al. Effects of Cissus quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 cells. Journal of Cellular Biochemistry. 2011;112(4):1035–1045. doi: 10.1002/jcb.23016. [DOI] [PubMed] [Google Scholar]


