Abstract
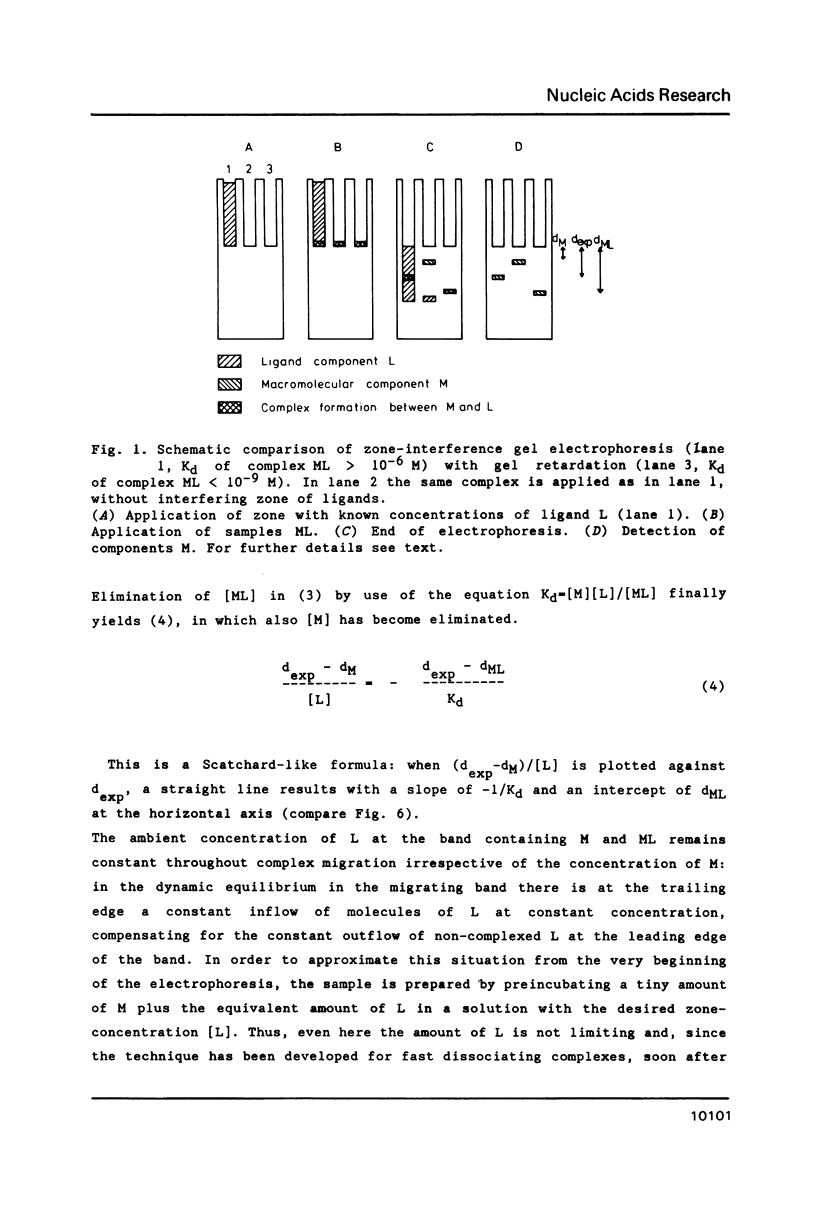
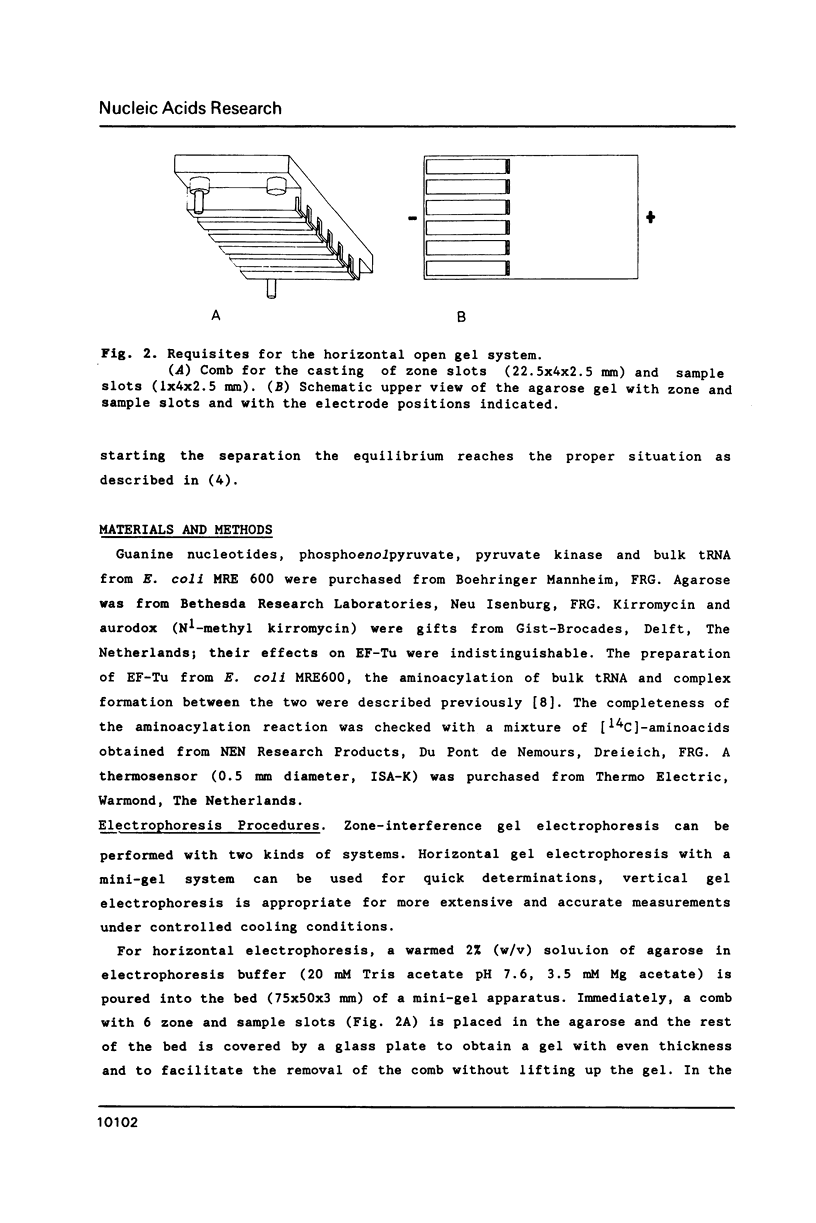
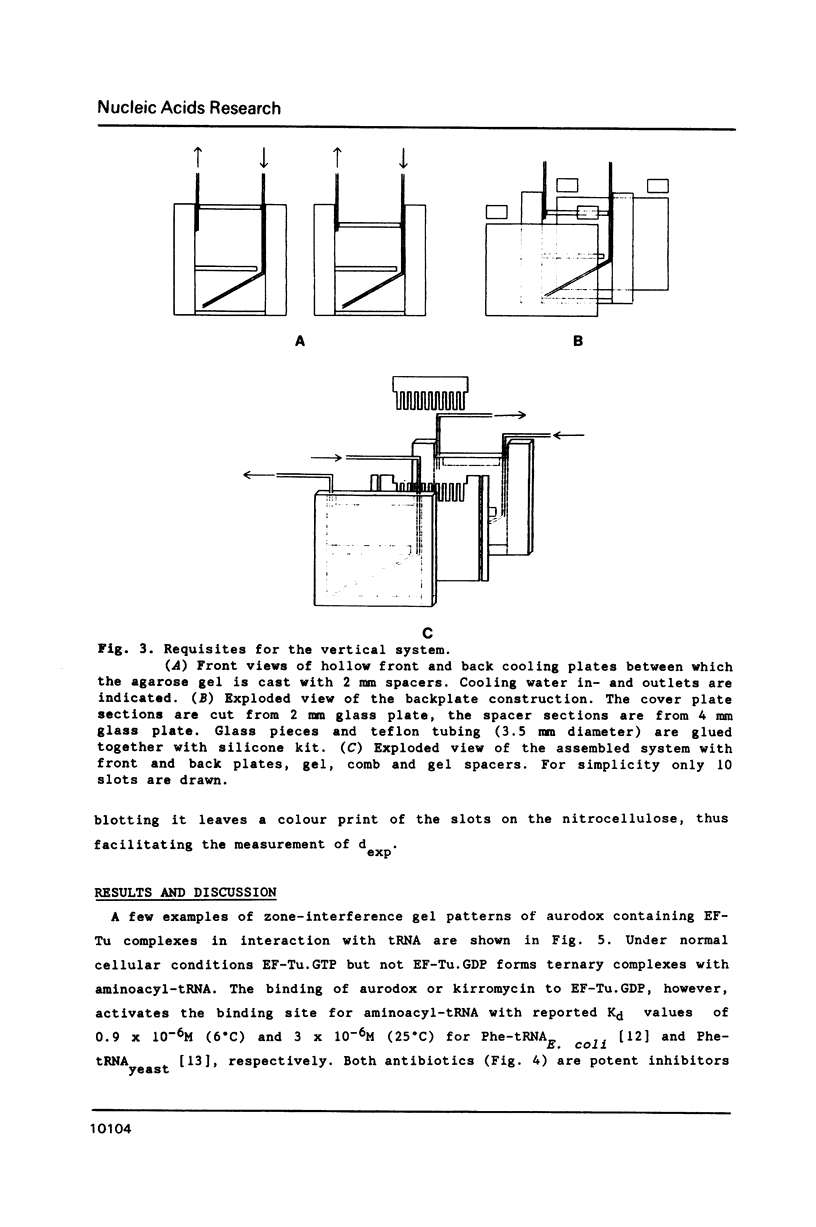
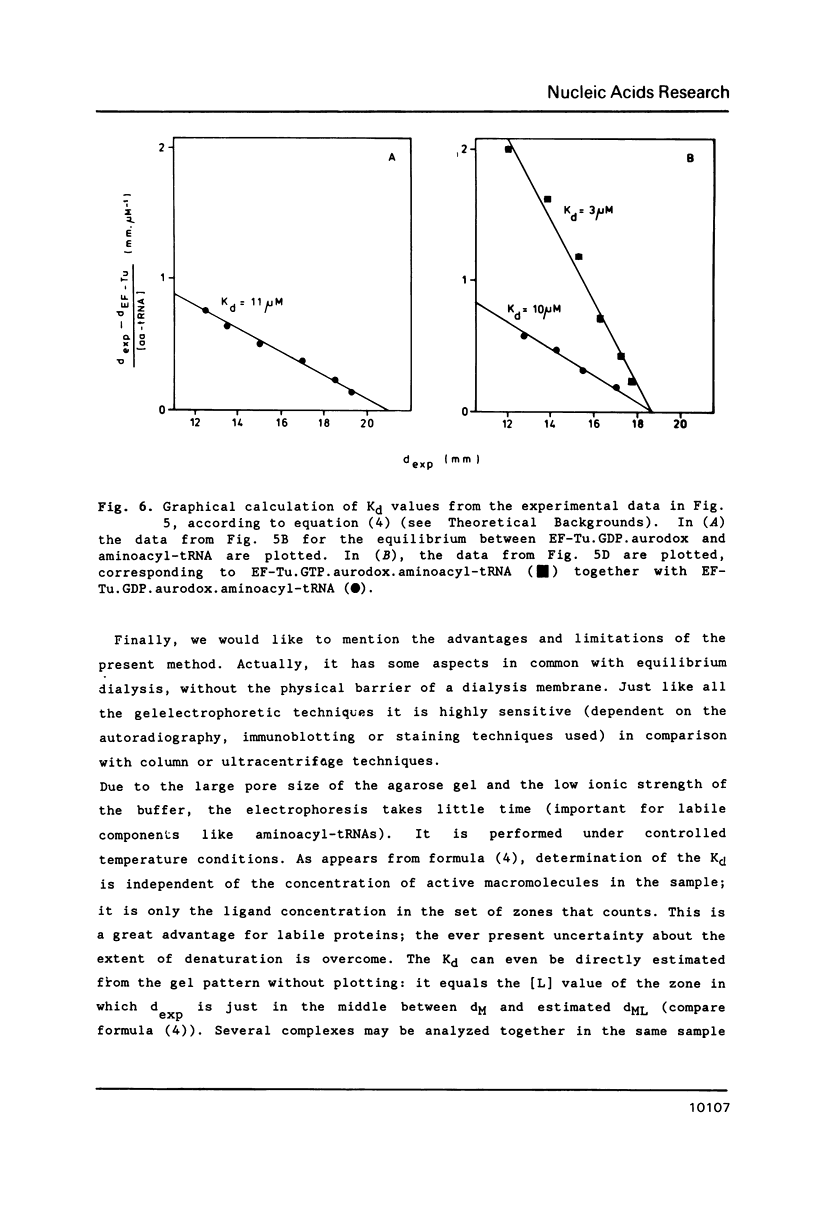
A new and general electrophoresis method is described for the determination of dissociation constants of weak macromolecular complexes in the range of 10(-6) to 10(-4) M. The method is based on the measurement of the migration distance of a macromolecular complex in rapid dynamic equilibrium as a function of the interacting ligand concentration in a surrounding zone. Special advantages of the method are: its high sensitivity (dependent on the autoradiography, immunoblotting or staining technique used), its speed (electrophoresis time 20 min), and the independence of the Kd determination on the sample concentration of macromolecules. The latter is of great value for labile macromolecules: unknown partial inactivation does not influence the measurement. Studying the interactions between elongation factor EF-Tu and tRNA from E. coli we found for EF-Tu.GTP.aurodox.aminocyl-tRNA a Kd of 3 microM and for EF-Tu.GDP.aurodox.aminoacyl-tRNA a Kd of 11 microM at 9 degrees C.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carey J. Gel retardation at low pH resolves trp repressor-DNA complexes for quantitative study. Proc Natl Acad Sci U S A. 1988 Feb;85(4):975–979. doi: 10.1073/pnas.85.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S., Hayashi H., Wada A. Affinity chromatography without immobilized ligands; a new method for studying macromolecular interactions using high-performance liquid chromatography. Anal Biochem. 1982 Aug;124(2):372–379. doi: 10.1016/0003-2697(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Swart G. W. Mechanism of action of kirromycin-like antibiotics. Annu Rev Microbiol. 1985;39:557–577. doi: 10.1146/annurev.mi.39.100185.003013. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Block W., Wittinghofer A., Wolf H., Fischer E. The elongation factor Tu binds aminoacyl-tRNA in the presence of GDP. J Biol Chem. 1982 Oct 10;257(19):11261–11267. [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Wolf H., Maass G. The binding of kirromycin to elongation factor Tu. Structural alterations are responsible for the inhibitory action. Eur J Biochem. 1978 May;86(1):153–157. doi: 10.1111/j.1432-1033.1978.tb12294.x. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Van Noort J. M., Kraal B., Bosch L. A second tRNA binding site on elongation factor Tu is induced while the factor is bound to the ribosome. Proc Natl Acad Sci U S A. 1985 May;82(10):3212–3216. doi: 10.1073/pnas.82.10.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noort J. M., Kraal B., Bosch L. GTPase center of elongation factor Tu is activated by occupation of the second tRNA binding site. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4617–4621. doi: 10.1073/pnas.83.13.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. On the specificity of the binding of the estradiol receptor protein to deoxyribonucleic acid. J Biol Chem. 1974 Nov 25;249(22):7076–7086. [PubMed] [Google Scholar]



