Abstract
AIM
To determine the location of c-jun protein, dynamic changes in c-jun mRNA and protein expression, and ultrastructure characteristics in the rd mouse retina, following a single dose of brain-derived neurotrophic factor (BDNF) in a short period of time.
METHODS
A single intravitreal injection of BDNF at two dosages (25µg/L or 50µg/L) was given to the right eye of the rd mouse at age 2 and 3 weeks respectively. Two weeks after injection, the location of c-jun protein in the retina was observed by immunofluorescence detection, c-jun mRNA and protein expression in retinas were detected by quantitative real time polymerase chain reaction (RT-PCR) and western immunoblotting analysis, ultrastructure characteristics of retinas were detected by transmission electron microscope (TEM) observation.
RESULTS
c-jun protein was expressed in the inner nuclear layer (INL) of retina. BDNF at two dosages (25µg/L and 50µg/L) increased c-jun mRNA expression at PN-4 weeks respectively (P1=0.019, P2=0.021). 50µg/L BDNF increased c-jun protein expression at PN-4 weeks (P =0.000). The retinal ultrastructure was improved.
CONCLUSION
The effects of BDNF exerts on the c-jun expression in the retina are dose-dependent and time-dependent, which may mediate photoreceptor rescue indirectly in the pathological process of retinitis pigmentosa (RP) at early stage.
Keywords: BDNF, c-jun, retina, photoreceptor
INTRODUCTION
Retinitis pigmentosa (RP) is one of the most common inherited retinal degenerative disorders, which are currently the leading cause of incurable blindness. It is a group of inherited retinal dystrophies characterized by progressive photoreceptor degeneration[1],[2]. Brain-derived neurotrophic factor (BDNF), an important neuroprotective factor that belongs to the neurotrophin family, exerts its survival-promoting effects on retinal photoreceptors and is critically involved in a variety of retinal degenerative animal models[3],[4]. The BDNF effects are known to be mediated by TrkB receptor-induced activation of two key signaling pathways including mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3-K)/Akt[5].
c-jun, an important member of the jun protein family[6], constitutes the activator protein (AP)-1 transcription factor complex with c-fos and is involved in many biological processes, including cell differentiation, proliferation, apoptosis, and survival[7]-[9]. Several studies have reported the correlation between BDNF and c-jun. BDNF and c-jun may be simultaneously induced in cortical impact brain injury[10] and spinal cord injury[11]. Recent studies have revealed that BDNF mediated extracellular signal-regulated kinase (ERK)1/2 phosphorylation and c-jun induction and then induced sulfiredoxin against 3-NP toxicity in primary rat cortical neurons, namely “BDNF → ERK1/2-Pi → c-jun → sulfiredoxin → 3-NP resistance”. This result established that c-jun mediated the BDNF-dependent neuroprotective effects directly[12]. These results raises the question that whether the BDNF-dependent neuroprotective effects are related to c-jun in the pathological process of RP.
In the present study, we observed the effects of BDNF on dynamic changes in c-jun mRNA and protein expression in the rd mouse retina.
MATERIALS AND METHODS
Materials
All the intravitreal injections were performed by one of the authors (NHVC). rd mice (at age 2 and 3 weeks, weighing 7-10g)) were anesthetized by 5% chloral hydrate (8µg/L). 2µL of BDNF recombinant protein [50µg/L or 100µg/L, diluted in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA),PeproTech, USA] (32 rd mice)or PBS (PBS controls, 16 rd mice) were injected into the vitreous chamber of the left eye using a 10µL Hamilton syringe adapted with a 29 gauge needle. Contralateral eyes or unoperated eyes served as intact controls (rd controls). The needle tip was inserted into the superior hemisphere of the eye, at the level of the pars plana, at a 45° angle through the sclera into the vitreous body. The injection was performed within 1 minute and the needle was kept in place for an additional period of 2 minutes, after which it was gently removed. Sixteen age-matched C57BL/6J mice with no intravitreal injections were used as blank controls. Two weeks after injection, eyes were enucleated for the following RT-PCR and western immunoblotting detection. All animals used in this study were cared and handled according to the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Methods
Immunohistochemistry
Eyes were embedded with optimal cutting temperature (OCT) compound and serial 7µm of section were produced. Sections were fixed with 4% paraffin for 10 minutes, rinsed with 0.1mol/L PBS ((pH 7.4, 3×5 minutes) and incubated in 5% BSA for 20 minutes. Rabbit polyclonal anti-c-jun antibody (1:100, Abcam, USA)incubation was performed in PBS overnight at 4°C. Slices were then incubated with fluoresceinisothiocyanate-conjugated secondary antibody for 45 min at 37°C, rinsed with 0.05mol/L PBS (3×5 minutes), Sections with PBS instead of primary antibody were included as negative controls. I and viewed with a Fluorescence images were acquired by fluorescence microscope (Leica DM4000B, Germany)and analyzed by Image Pro Plus 6.0 software (MC, USA).
RNA isolation and quantitative RT-PCR
Retinas were removed through a slit in the cornea and immediately frozen in liquid nitrogen. RNA was isolated using Trizol reagent (Invitrogen), and cDNA was synthesized with 2µg of total RNA using the Transcript First-Strand cDNA Synthesis Kit (Transgene Biotechnology Inc., Beijing, China). Quantitative real-time PCR was performed using the UltraSYBR mixture (Cwbiotech, China) in the Stratagene Mx3000P™ Real-Time PCR System (Agilent Technologies, CA). PCR primers for mouse c-jun were forward: 5′- TTCTACGACGATGCCCTCAAC -3′ and reverse: 5′'- CAGGTTCAAGGTCATGCTCTGTT -3′. PCR primers for mouse GAPDH were forward: 5′- CGACTTCAACAGCGACACTCAC -3′ and reverse: 5′- CCCTGTTGCTGTAGCCAAATTC -3′. The thermal cycling conditions were as follows: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturing at 95°C for 15 seconds, annealing at 60°C for 30 seconds and extension at 72°C for 30 seconds. Data were normalized according to copy number of GAPDH mRNA and were analyzed by SDS 2.2 software (ABI, USA). All experiments were performed in triplicate.
Western immunoblotting
Retinas were removed through a slit in the cornea and immediately frozen in liquid nitrogen. Retinal protein was extracted with lysis buffer (Beyotime, China) on ice for 30 minutes and was then centrifuged at 15,000r/min at 4°C for 15 minutes. Protein concentrations were determined using the BCA method. An aliquot of this total protein were boiled for 4 minutes, resolved by 10% SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Germany). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween 20 at room temperature for 1 hour. And then, they were incubated with mouse monoclonal anti-α-tubulin (1:1000, Santa Cruz, USA) or rabbit polyclonal anti-c-jun (1:1000, Abcam, USA) antibodies at 4°C overnight, followed by an incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies (1:10000, Cwbiotech, China) at room temperature for 1 hour. The chemiluminescence reaction was performed using ECL reagent (Thermo Scientific). Protein bands were scanned and evaluated by densitometry using Quantity One Analysis Software (Bio-Rad, USA), which was normalized for α-tubulin density. All experiments were performed in triplicate.
Electron microscopy
The enucleated eyes were dissected along the equators, and the retina was incubated for 4 hours in 2.5% glutaraldehyde (in 100mmol/L phosphate buffer, pH 7.4). The retina was postfixed in 1% buffered osmium tetroxide and dehydrated using graded ethanol solutions. Semi-thin sections (1µm) were prepared, stained with toluidine blue and examined by light microscopy. Ultrathin sections (0.5µm) of selected areas were then prepared and stained with uranyl acetate and lead citrate for transmission electron microscope (H 7650TEM; Hitachi, Tokyo, Japan).
Statistical Analysis
Data were represented as mean±SD. Statistical analysis of the data was performed by SPSS18.0 using one-way analysis of variance (ANOVA) for planned comparisons among the various treatments at the same point and a least significant difference-t (LSD-t) test for planned comparisons between the same treatments at different time point. A P-value less than 0.05 was considered significant.
RESULTS
Immunohistochemical location of c-jun expression in the retina
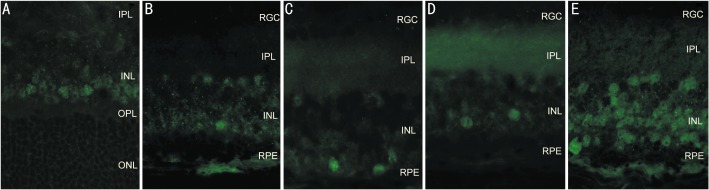
c-jun protein was only expressed in the INL at age 4 and 5 weeks. At age 4 weeks, c-jun protein levels increased in the 25µg/L BDNF group compared with the rd control group (P=0.212) and the PBS group (P=0.020), but decreased compared with the blank control group (P=0.001). 50µg/L BDNF treatment elevated c-jun protein expression compared with the rd control group (P=0.000), the PBS group (P=0.000), the blank control group (P=0.293), and the 25µg/L BDNF group (P=0.000, Figure 1).
Figure 1. Immunohistochemical location of c-jun expression in the retina at age 4 weeks(×400).
A: the Blank control group; B: the rd control group; C: the PBS control group; D: the 25µg/L BDNF group; E: the 50µg/L BDNF group. (RPE: retinal pigment epithelium; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer).
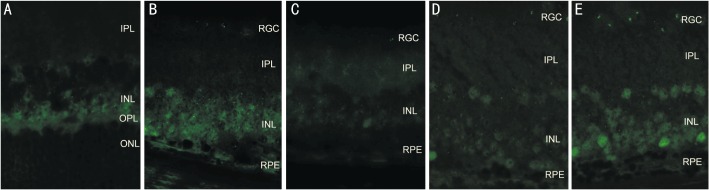
At age 5 weeks, c-jun protein levels increased in the 25µg/L BDNF group compared with the rd control group (P=0.571), but decreased compared with the PBS group (P=1.000) and the blank control group (P=0.000). In the 50µg/L BDNF group, c-jun protein expression increased compared with the rd control group (P=1.000) and the PBS group (P=0.177), but decreased compared with the blank control group (P=0.000), and the 25µg/L BDNF group (P=0. 695, Figure 2, Table 1).
Figure 2. Immunohistochemical location of c-jun expression in the retina at age 5 weeks(×400).
A: the Blank control group; B: the rd control group; C: the PBS control group; D: the 25µg/L BDNF group; E: the 50µg/L BDNF group.
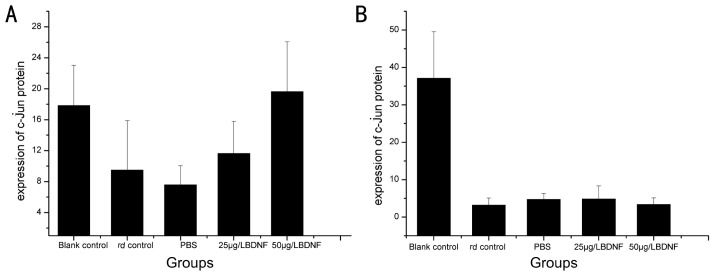
Table 1. c-jun protein expression in the retina by immunohistochemistry.
| C57BL/6 | rd | PBS | 25µg/L BDNF | 50µg/L BDNF | |
| PN-4w | 17.84±5.20 | 9.52±6.38 | 7.60±2.45 | 11.67±4.12 | 19.65±6.42 |
| PN-5w | 37.14±12.40 | 3.17±1.94 | 4.75±1.60 | 4.87±3.45 | 3.36±1.80 |
(mean±SD)
At age 5 weeks, c-jun protein expression decreased in the rd control group(P=0.000), the PBS group(P=0.000), and the BDNF group(P1=0.000, P2=0.000), but increased in the blank control group compared with the same group respectively at age 4 weeks(P=0.000, Figure 3).
Figure 3. Immunohistochemical location of c-jun expression in the retina. A: Groups at the age 4 weeks; B: groups at age 5 weeks.
BDNF results in dynamic changes in c-jun mRNA expression in the retina
At age 4 weeks, BDNF treatment (25µg/L and 50µg/L) upregulated c-jun mRNA expression compared with the rd control group (P1=0.019, P2=0.021), the PBS group (P1=0. 550, P2=0. 596), the blank control group (P1=0.024, P2=0.027). c-jun mRNA expression was similar between the BDNF groups.
At age 5 weeks, BDNF treatment slightly upregulated c-jun mRNA expression compared with the rd control group, the PBS group, and the blank control group. c-jun mRNA expression in the 50µg/L BDNF group decreased compared with the 25µg/L BDNF group.(P=0.065, Table 2).
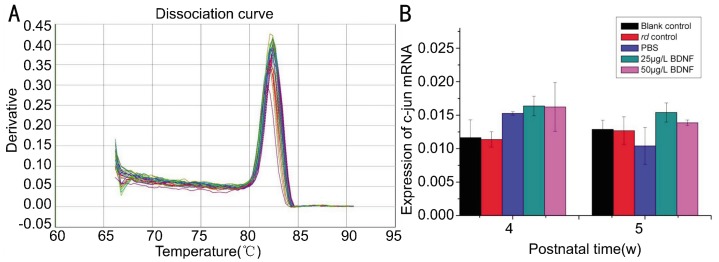
Table 2. c-jun mRNA expression in the retina.
| C57BL/6 | rd | PBS | 25µg/L BDNF | 50µg/L BDNF | |
| PN-4w | 0.0116±0.0026 | 0.0114±0.0011 | 0.0153±0.0003 | 0.0164±0.0015 | 0.0163±0.0037 |
| PN-5w | 0.0129±0.0014 | 0.0127±0.0021 | 0.0104±0.0028 | 0.0154±0.0014 | 0.0139±0.0004 |
(mean±SD)
At age 5 weeks, c-jun mRNA expression gradually increased in the rd control group (P=0.391) and the blank control group (P=0.507), but decreased in the PBS group (P=0.039) and the 50µg/L BDNF group (P=0.323) compared with the same group respectively at age 4 weeks. There were no obvious differences between the two time points in the 25µg/L BDNF group (P=0.458, Figure 4).
Figure 4. Dynamic changes in c-jun mRNA expression in the retina.
A: dissociation curve of c-jun produced by real-time polymerase chain reaction; B: altered c-jun mRNA expression
BDNF results in dynamic changes in c-jun protein expression in the retina
At age 4 and 5 weeks, BDNF treatment decreased c-jun protein expression compared with the rd control group and the PBS group, but similar to the blank control group. c-jun mRNA expression was similar between BDNF groups. c-jun protein levels in the 50µg/L BDNF group was lower than the 25µg/L BDNF at age 4 weeks and similar to the 25µg/L BDNF group at age 5 weeks (P1=0.134, P2=0.731).
At age 5 weeks, c-jun mRNA expression gradually decreased in the blank control group(P=0.571), the PBS group (P=0.41), and the 25µg/L BDNF group(P=0.737), but increased in the rd group (P=0.912) and the 50µg/L BDNF group(P=0.283) compared with the same group respectively at age 4 weeks (Figure 5, Table 3).
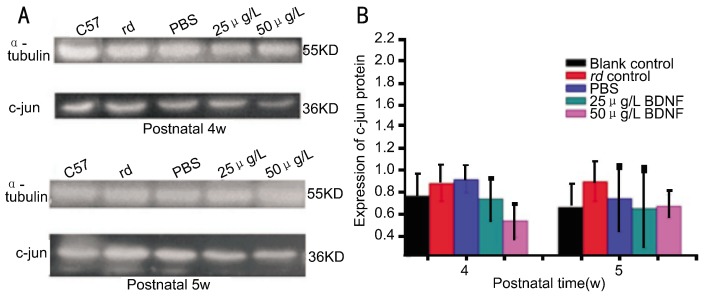
Figure 5. Dynamic changes in c-jun protein expression in the retina.
A: electrophoresis of c-jun protein expression; B: altered c-Jun protein expression.
Table 3. c-jun protein expression in the retina.
| C57BL/6 | rd | PBS | 25µg/L BDNF | 50µg/L BDNF | |
| PN-4w | 0.77±0.20 | 0.89±0.16 | 0.92±0.12 | 0.74±0.20 | 0.54±0.15 |
| PN-5w | 0.67±0.21 | 0.90±0.18 | 0.75±0.31 | 0.66±0.36 | 0.68±0.11 |
(mean±SD)
BDNF results in changes in retinal ultrastructure
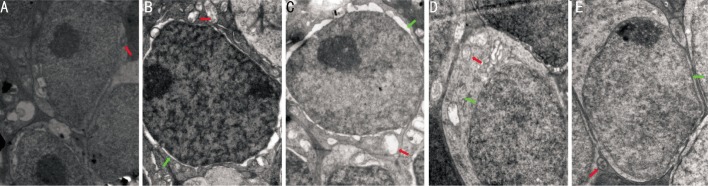
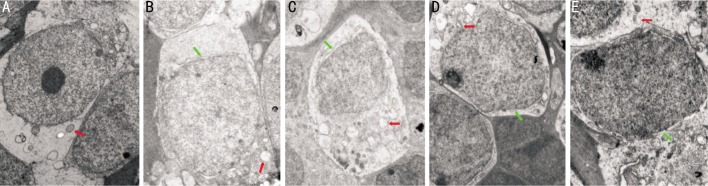
At age 4 and 5 weeks, BDNF treatment improved the retinal ultrastructure. Vacuolization and destruction of plasma and nuclear membranes were improved in the retinal pigment epithelium (RPE). In the outer nuclear layer (ONL), the number of nuclei increased and the swollen cell bodies and the damaged mitochondria were improved. The inner segments of cones were occasionly seen. The arrays of microtubules and synaptic structures were improved in the outer plexiform layer (OPL).There were more and larger mitochondria and ribosomes with fine morphology in the plasma and destruction of plasma and nuclear membranes were improved in the INL. There were no obvious changes in the inner plexiform layer and ganglion cell layer (Figures 6 and 7).
Figure 6. Changes in retinal ultrastructure in the INL at age 4 weeks.
A: the Blank control group(×15000); B: the rd control group (×25000); C: the PBS control group (×20000); D: the 25µg/L BDNF group (×25000); E: the 50µg/L BDNF group (×25000). (Red arrows: mitochondria, green arrows: nuclear membranes).
Figure 7. Changes in retinal ultrastructure in the INL at age 5 weeks.
A: the Blank control group (×15000); B: the rd control group (×15000); C: the PBS control group (×12000); D: the 25µg/L BDNF group (×15000); E: the 50µg/L BDNF group (×20000). (Red arrows: mitochondria, green arrows: nuclear membranes).
DISCUSSION
In the present study, a single dose of BDNF(25µg/L and 50µg/L) treatment prominently enhanced c-jun mRNA levels at age 4 weeks and the levels were greater in the 50µg/L BDNF group compared with the 25µg/L BDNF group. Corresponding c-jun protein changes at the same time point were observed following modification of c-jun gene expression shown by immunohistochemistry. Meanwhile, there were no obvious changes of c-jun mRNA and protein expression at age 5 weeks. These results suggested that The effects of BDNF exerts on the c-jun expression in the retina were dose-dependent and time-dependent and may be involved in the pathological process of RP at early stage.
The effects of c-jun on neuronal cells are controversial. c-jun has been reported to be involved in neuronal survival and axon regeneration in adults[13]. c-jun has been hypothesized to act as a death signal in degenerating neurons and may play a role in photoreceptor apoptosis[14],[15].These contradictory results may be related to the various signaling pathways, which results in the mediation of distint biological actions[16],[17]. c-jun can be phosphorylated directly by activated c-jun N-terminal kinase (JNK), ERK1/2, and p38 cascades. p38 and JNK has been shown to be important signal transduction pathways contributing to glia-induced neuron death, but ERK pathway was not involved in neuron loss[18]. JNK/c-jun could directly inhibit expression of Nrl, which is essential for rod differentiation and function[19]. ERK pathway could be activated to product AP-1, which was a prosurvival signal in mediating photoreceptor cell death[20]. Recent studies have revealed that BDNF mediated ERK1/2 phosphorylation and c-jun induction and then induced sulfiredoxin against 3-NP toxicity in primary rat cortical neurons[12].
Studies have shown that photoreceptor cells did not express BDNF receptors[21]and BDNF had no direct effect on isolated photoreceptor cells[22]. BDNF binded to TrkB receptor in Müller cells and was involved in photoreceptor rescue indirectly[23]. After a diverse array of retinal injuries, Müller cell reactivity was thought to promote initially a survival response through the release of neurotrophic factors and antioxidants[24]-[26] and this early nonspecific responses involved ERK activation and upregulation of glial fibrillary astrocytic protein(GFAP) expression[27]. BDNF has been shown that it can modulate production and release of several neutrophins from Müller cells during light-induced retinal degeneration to influence photoreceptor survival indirectly[28]. Intraocular administration of BDNF can activate ERK pathway in Müller glial cells[29]. In the present study, c-jun protein was expressed in the INL, may be in Müller cells and the retinal ultrastructure was improved. It has been established that receptor isoforms of BDNF were expressed temporally and spatially on Müller cells during light-induced retinal degeneration[30]. In the present study, c-jun expression elevation mediated by BDNF was at the earlier time point. These results suggest that BDNF may bind to TrkB receptor in Müller cells and activate ERK pathway to produce c-jun, which mediates photoreceptor rescue indirectly in the pathological process of RP at early stage.
Footnotes
Foundation item: National Natural Science Foundation of China (No. 30973262)
REFERENCES
- 1.Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34(5):1659–1676. [PubMed] [Google Scholar]
- 2.Dryja TP, Berson EL. Retinitis pigmentosa and allied diseases: implications of genetic heterogeneity. Invest Ophthalmol Vis Sci. 1995;36(7):1197–1200. [PubMed] [Google Scholar]
- 3.Wenzel A, Grimm C, Samardzija M, Remé CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24(2):275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Tombran-Tink J, Barnstable CJ. Neuroprotective factors and retinal degenerations. Retinal Degenerations. 2007;(10):433–454. [Google Scholar]
- 5.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mechta-Grigoriou F, Gerald D, Yaniv M. The mammalian Jun proteins: redundancy and specificity. Oncogene. 2001;20(19):2378–2389. doi: 10.1038/sj.onc.1204381. [DOI] [PubMed] [Google Scholar]
- 7.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 8.Reddy SP, Mossman BT. Role and regulation of activator protein-1 in toxicant-induced responses of the lung. Am J Physiol. 2002;283(6):1161–1178. doi: 10.1152/ajplung.00140.2002. [DOI] [PubMed] [Google Scholar]
- 9.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9(2):240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 10.Kobori N, Clifton GL, Dash P. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Res Mol Brain Res. 2002;104(2):148–158. doi: 10.1016/s0169-328x(02)00331-5. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000;17(3):203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- 12.Wu CL, Yin JH, Hwang CS, Chen SD, Yang DY, Yang DI. c-jun-dependent sulfiredoxin induction mediates BDNF protection against mitochondrial inhibition in rat cortical neurons. Neurobiol Dis. 2012;46(2):450–462. doi: 10.1016/j.nbd.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Herdegen T, Skene P, Bähr M. The c-jun transcription factor-bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20(5):227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 14.Chiarini LB, de Freitas FG, Leal-Ferreira ML, Tolkovsky A. Cytoplasmic c-jun N-terminal immunoreactivity: a hallmark of retinal apoptosis. Cell Mol Neurobiol. 2002;22(5-6):711–726. doi: 10.1023/A:1021857007976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katai N, Yanagidaira T, Senda N, Murata T, Yoshimura N. Expression of c-jun and Bcl-2 family proteins in apoptotic photoreceptors of RCS rats. Jpn J Ophthalmol. 2006;50(2):121–127. doi: 10.1007/s10384-005-0296-7. [DOI] [PubMed] [Google Scholar]
- 16.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 17.Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31(2):151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45(2):170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- 19.Merienne K, Friedman J, Akimoto M, Abou-Sleymane G, Weber C, Swaroop A, Trottier Y. Preventing polyglutamine-induced activation of c-jun delays neuronal dysfunction in a mouse model of SCA7 retinopathy. Neurobiol Dis. 2007;25(3):571–581. doi: 10.1016/j.nbd.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu D, Beltran WA, Li Z, Acland GM, Aguirre GD. Clinical light exposure, photoreceptor degeneration, and AP-1 activation: a cell death or cell survival signal in the rhodopsin mutant retina? Invest Ophthalmol Vis Sci. 2007;48(11):4907–4918. doi: 10.1167/iovs.07-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ugolini G, Cremisi F, Maffei L. TrkA, TrkB and p75 mRNA expression is developmentally regulated in the rat retina. Brain Res. 1995;704(1):121–124. doi: 10.1016/0006-8993(95)01191-9. [DOI] [PubMed] [Google Scholar]
- 22.Carwile ME, Culbert RB, Sturdivant RL, Kraft TW. Rod outer segment maintenance is enhanced in the presence of bFGF, CNTF and GDNF. Exp Eye Res. 1998;66(6):791–805. doi: 10.1006/exer.1998.0488. [DOI] [PubMed] [Google Scholar]
- 23.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7(2):148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 24.Oku H, Ikeda T, Honma Y, Sotozono C, Nishida K, Nakamura Y, Kida T, Kinoshita S. Gene expression of neurotrophins and their high-affinity Trk receptors in cultured human Muller cells. Ophthalmic Res. 2002;34(1):38–42. doi: 10.1159/000048323. [DOI] [PubMed] [Google Scholar]
- 25.Honjo M, Tanihara H, Kido N, Inatani M, Okazaki K, Honda Y. Expression of ciliary neurotrophic factor activated by retinal Müller cells in eyes with NMDA- and kainic acid-induced neuronal death. Invest Ophthalmol Vis Sci. 2000;41(2):552–560. [PubMed] [Google Scholar]
- 26.Schutte M, Werner P. Redistribution of glutathione in the ischemic rat retina. Neurosci Lett. 1998;246(1):53–56. doi: 10.1016/s0304-3940(98)00229-8. [DOI] [PubMed] [Google Scholar]
- 27.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K. Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22(21):9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Müller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41(3):927–936. [PubMed] [Google Scholar]
- 30.Asai N, Abe T, Saito T, Sato H, Ishiguro S, Nishida K. Temporal and spatial differences in expression of TrkB isoforms in rat retina during constant light exposure. Exp Eye Res. 2007;85(3):346–355. doi: 10.1016/j.exer.2007.05.010. [DOI] [PubMed] [Google Scholar]