Abstract
AIM
To demonstrate the morphology and structure of in vitro reconstructed tissue-engineered human corneal epithelium (TE-HCEP) with seeder cells from an untransfected HCEP cell line.
METHODS
The TE-HCEPs were reconstructed in vitro with seeder cells from an untransfected HCEP cell line, and scaffold carriers of denuded amniotic membrane (dAM) in air-liquid interface culture for 3, 5, 7 and 9 days, respectively. The specimens were examined with hematoxylin-eosin (HE) staining of paraffin-section, immunocytochemical staining, scanning and transmission electron microscopy.
RESULTS
During in vitro reconstruction of TE-HCEP, HCEP cells formed a 3-4, 6-7 and 8-10 layers of an HCEP-like structure on dAMs in air-liquid interface culture for 3, 5 and 7 days, respectively. But the cells deceased to 5-6 layers and the structure of straified epithelium became loose at day 9. And the cells maintained positive expression of marker proteins (keratin 3 and keratin 12), cell-junction proteins (zonula occludens-1, E-cadherin, connexin 43 and integrin β1) and membrane transport protein of Na+-K+ ATPase. The HCEP cells in TE-HCEP were rich in microvilli on apical surface and established numerous cell-cell and cell-dAM junctions at day 5.
CONCLUSION
The morphology and structure of the reconstructed TE-HCEP were similar to those of HCEP in vivo. The HCEP cells in the reconstructed TE-HCEP maintained the properties of HCEP cells, including abilities of forming intercellular and cell-extracellular matrix junctions and abilities of performing membrane transportation. The untransfected HCEP cells and dAMs could promisingly be used in reconstruction HCEP equivalent for clinical corneal epithelium transplantation.
Keywords: tissue-engineered human corneal epithelium, in vitro reconstruction, untransfected human corneal epithelial cell, denuded amniotic membrane
INTRODUCTION
Human corneal epithelium (HCEP), with integrity and no blood vessels, is crucial for maintaining corneal transparency and normal physiological function[1]. The maintenance of a healthy corneal epithelium is provided by a unique subpopulation of limbal epithelial stem cells (LESCs)[2],[3]. Extensive microbial infection, chemical or thermal burns, contact lens wear, multiple surgeries, Stevens-Johnson syndrome, and so on often cause HCEP cell and/or LESC deficiency including conjunctivalization, epithelial defects, chronic inflammation, scarring and ulcerations which often result in edema and turbidity of cornea[4],[5].
Amniotic membrane (AM), with low or no immunogenicity, which is frequently used as a graft for ocular surface reconstruction and successful re-epithelialization, has been successfully used in ophthalmology[6],[7]. LESCs[8],[9]-[11] and AMs[6],[7],[12] have been clinically applied in treatment of LESC deficiency. However, autologous LESC transplantation is highly restricted to patients with unilateral LESC deficiency[13],[14]. An alternative approach by transplantation of cultivated allogeneic LESCs is also limited because of their short life span, rapid differentiation and limited availability of donor corneal tissues[15],[16]. At present, tissue-engineered HCEP (TE-HCEP) is now considered as an ideal HCEP equivalent for the therapy of HCEP cell deficiencies[17],[18]. Since an untransfected HCEP cell line (utHCEPC01), which is highly biocompatible with denuded AM (dAM), has been successfully established in our laboratory[16], reconstruction of TE-HCEPs can be performed in vitro with untransfected HCEP cells and dAMs. To obtain an ideal HCEP equivalent and lay foundation for its future clinical application, in vitro reconstruction and characterization of TE-HCEP were performed by using HCEP cells from utHCEPC01 cell line as seeder cells and dAMs as scarffold carriers in this study.
MATERIALS AND METHODS
Materials
Untransfected HCEP cells at passage 80 from the utHCEPC01 cell line, established previously in our laboratory with approved corneas donated from a 26 years old woman, was cultured in Dulbecco's modified Eagle medium/nutrient mixture F-12 (1:1, v:v)(DMEM/F12) medium containing 20% fetal bovine serum (FBS)(HyClone, Logan, Utah) (Invitrogen, Carlsbad, CA)(pH 7.2) at 37 °C in a 5% CO2 incubator as described previously[16]. Fresh AMs were obtained from Shandong Eye Institute of Shandong Medical Academy, Qingdao, China, and denuded by trypsinization with 0.02% EDTA-0.25% trypsin (Sigma-Aldrich, St. Louis, MO)(1:1, v:v) solution to obtain dAMs according to Fan et al[19].
Methods
In vitro reconstruction of TE-HCEP
HCEP cells at logarithmic phase were collected using 0.25% trypsin (Sigma-Aldrich) as described previously[16]. After cell number counted with a Casy Model DT cell counter (Schärfe System, Reutlingen, German), the density of cell suspension was adjusted to 1.0×107/mL with 15% FBS-DMEM/F12 medium (pH 7.2). To each of a dAM-paved culture insert in a 24-well plate, 500 µL cell suspension was plated and cultured at the same conditions as described above for 12 hours. Then the culture inserts were transferred into a 6-well plate wells containing 0.8 mL 10% FBS-DMEM/F12 medium (pH 7.2) and cultured in air-liquid interface culture. The medium was refreshed daily, and the morphology and growth status of the HCEP cells were monitored with an Eclipse TS100 inverted microscope (Nikon, Tokyo, Japan).
Histological characterization of TE-HCEP
After air-liquid interface cultured for 3, 5, 7 and 9 days, the reconstructed TE-HCEP were harvested respectively. The histological property of the reconstructed TE-HCEP was examined with paraffin section and hematoxylin-eosin (HE) staining. The surface morphology of the reconstructed TE-HCEP was examined with a JSM2840 scanning electron microscope (SEM)(JEOL, Tokyo, Japan). And the multilayer structure and attachment status to dAM of the reconstructed TE-HCEP were examined with a H700 transmission electron microscope (TEM)(Hitachi, Tokyo, Japan).
Immunocytochemical characterization of TE-HCEP
The expression patterns of marker proteins (keratin 3, keratin 12) and function proteins including cell junction proteins (zonula occludens-1, E-cadherin, connexin 43, integrin β1) and membrane transport protein (Na+-K+ ATPase) of the reconstructed TE-HCEP were examined with freeze sections as described previously[16]. Each of freeze sections was incubated with monoclonal antibodies of mouse anti-human keratin 3, keratin 12, zonula occludens-1, E-cadherin, connexin 43, integrin β1 and Na+-K+ ATPase (Santa Cruz Biotechnology) at 4 °C overnight, respectively, according to manufacturer's instructions. After incubated with fluorescent isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (Biosynthesis Biotechnology, Beijing, China) at 37 °C for 1 hour, the wells were analyzed with a Ti-S inverted fluorescent microscope (Nikon). Omission of primary antibodies was used as controls.
Statistical Analysis
Data were expressed as mean±SD in triplicates and tested for statistical significance with ANOVA single factor.
RESULTS
In vitro Reconstruction of TE-HCEP
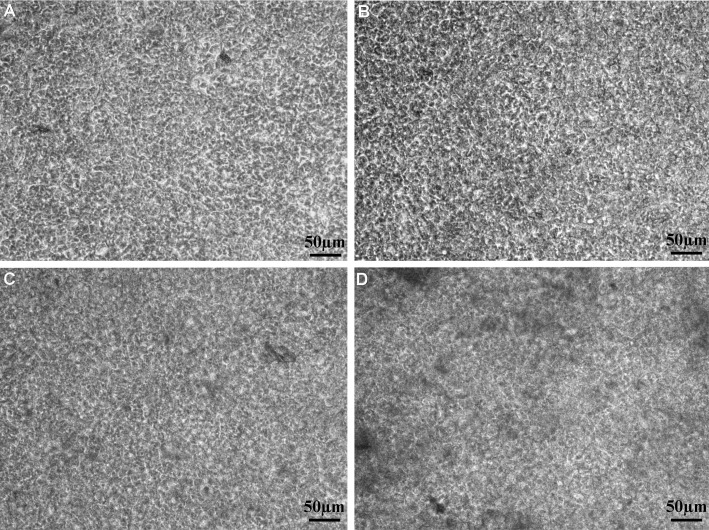
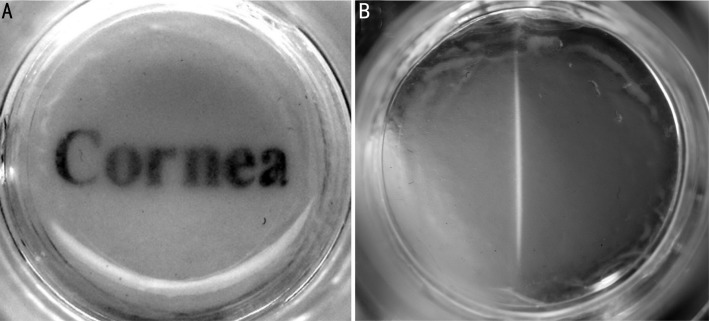
The HCEP cells grew well on dAM in cobblestone morphology during air-liquid interface culture (Figure 1). And the cells grew into multilayer and the boundary of cells became unclear with time. The reconstructed TE-HCEPs were highly transparent after air-liquid interface cultured for 5 days (Figure 2).
Figure 1. In vitro reconstruction of TE-HCEP from untransfected Passage 60 HCEP cells and dAMs in air-liquid interface culture.
A: Day 3; B: Day 5; C: Day 7; D: Day 9.
Figure 2. The transparency status of reconstructed TE-HCEP from untransfected Passage 60 HCEP cells and dAMs at day 5.
A: Macroscopic view; B: Slit-lamp microscopic view
Histological Characterization of TE-HCEP
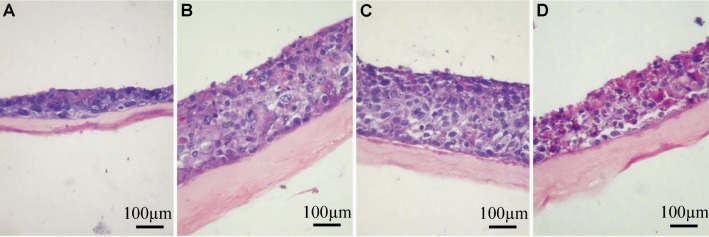
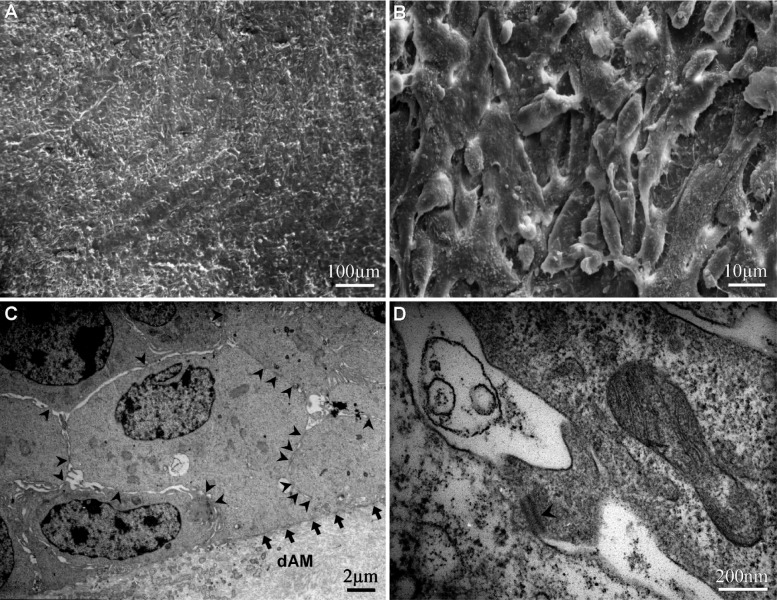
After air-liquid interface culture for 3 days, the HCEP cells formed a 3-4 layer epithelium-like structure (Figure 3A), and 6-7 and 8-10 layer epithelium-like structures was formed at day 5 and day 7, respectively (Figures 3B, 3C). But the number of cells began to decrease and the structure of only a 5-6 layer epithelium became loose and unstratified at day 9 (Figure 3D). The HCEP cells differentiated into flattened epidermal cells on its apical surface and cobblestone epithelial cells inside the multilayer epithelium. Under SEM and TEM, the HCEP cells were rich in microvilli on apical surface (Figures 4A, 4B), and constructed numerous intercellular cell junctions including desmosomes (arrowhead) and cell-dAM hemidesmosomes (arrow) at day 5 (Figures 4C, 4D). All these indicate that the reconstructed TE-HCEP has almost the same morphology and histological structure as that of innate HCEP.
Figure 3. HE staining of reconstructed TE-HCEP from untransfected HCEP cells and dAMs in air-liquid interface culture.
A: Day 3; B: Day 5; C: Day 7; D: Day 9.
Figure 4. Electron microscopic images of reconstructed TE-HCEP from untransfected HCEP cells and dAMs in air-liquid interface culture at day5.
A, B: SEM; C, D: TEM
Immunocytochemical Characterization of Marker Protein Expression in TE-HCEP
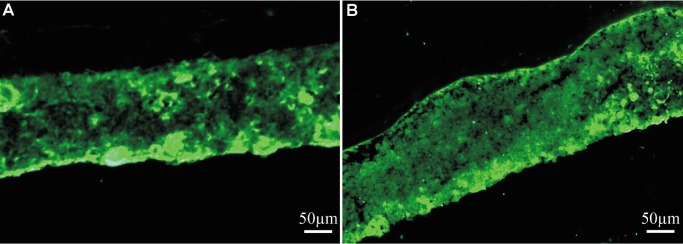
HCEP cells possessed positive expression of marker proteins including keratin 3 and keratin 12) in air-liquid interface culture on dAM at day 5 (Figure 5), indicating that they preserved the properties of HCEP cells.
Figure 5. Expression pattern of marker proteins of reconstructed TE-HCEP from untransfected HCEP cells and dAMs in air-liquid interface culture at day 5.
A: Keratin 3; B: Keratin 12
Immunocytochemical Characterization of Function Protein Expression in TE-HCEP
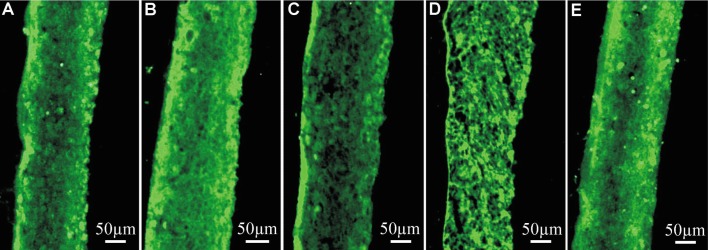
HCEP cells in reconstructed TE-HCEP expressed positively different cell-junction proteins (zonula occludens-1, E-cadherin, connexin 43, integrin β1) and membrane transport protein (Na+-K+ ATPase) in air-liquid interface culture on dAM at day 5 (Figure 6), indicating that they reserved the abilities of forming cell-cell and cell-extracellular matrix (ECM) junctions and abilities of membrane transportation.
Figure 6. Expression pattern of function proteins of reconstructed TE-HCEP from untransfected HCEP cells and dAMs in air-liquid interface culture at day 5.
A: Zonula occludens-1; B: E-cadherin; C: Connexin 43; D: Integrin β1; E: Na+-K+ ATPase
DISCUSSION
Due to the relative deficit of LESC donor and difficulty in LESCs culturing, untransfected HCEP cell lines can be used as cell banks of normal HCEP cells. The cell banks can provide a stable source of HCEP cells for TE-HCEP reconstruction and new therapies for HCEP diseases and damages. Since an untransfected HCEP cell line was successfully established in our laboratory[16], in vitro reconstruction and characterization of TE-HCEP was performed in this study.
After air-liquid interface cultured on dAM for more than 3 days, untransfected HCEP cells from the previously established untransfected HCEP cell line formed a multilayer layer epithelium-like structure with flattened cells on its apical surface and cobblestone cells underneath. And a 6-7 layer and 8-10 layer epithelium-like structure with a continuous layer of flattened apical cells was reconstructed at day 5 and day 7, respectively. The apical cells were flattened and rich in microvilli, which was similar in characteristics to those of squamous cells from HCEP both in vivo and in vitro[15],[16],[18]. After reconstructed for 5 days, the cells of TE-HCEP established numerous cell junctions including desmosomes between HCEP cells and hemidesmosomes between cells and dAM which can be visualized under TEM, and the ultrastructure of the HCEP cells was similar to that of HCEP cells in vivo[20]-[22]. All these indicate that the reconstructed TE-HCEP with integrity has almost the same morphology and structure as that of innate HCEP.
Besides, the cells of TE-HCEP maintained positive expression of marker proteins (keratin 3, keratin 12) and function proteins including cell junction proteins (zonula occludens-1, E-cadherin, connexin 43, integrin β1) and the membrane transport protein of Na+-K+ ATPase, indicating that cells reserved the properties of normal HCEP cells in vivo and abilities of forming cell-cell and cell-ECM junctions and abilities of membrane transportation. Combined with the ultrastructure of HCEP cells, it can be concluded that the ultrastructure, expression of cell-junction protein and membrane transport protein of reconstructed TE-HCEP is same as those of innate HCEP cells, which is better than that reported by Ban and his colleagues[23].
In conclusion, a novel TE-HCEP, with normal morphology and structure, has been successfully reconstructed in vitro in this study. It consists of 6-7 and 8-10 layers of HCEP cells, with positive expression of marker proteins and functional proteins, in air-liquid interface culture for 5 and 7 days, respectively. The reconstructed TE-HCEP, as an HCEP equivalent, provides a promising method in regenerative medicine for corneal epithelium reconstruction and corneal epithelial disorder therapy. TE-HCEP transplantation in LESC deficiency rabbit models is ongoing.
Footnotes
Foundation item: Supported by National High Technology Research and Development Program (“863” Program) of China (No. 2006AA02A132)
References
- 1.Kinoshita S, Adachi W, Sotozono C, Nishida K, Yokoi N, Quantock AJ, Okubo K. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20(5):639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 2.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89(5):529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stepp MA, Zieske JD. The corneal epithelial stem cell niche. Ocul Surf. 2005;3(1):15–26. doi: 10.1016/s1542-0124(12)70119-2. [DOI] [PubMed] [Google Scholar]
- 4.Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48(2):83–92. [PubMed] [Google Scholar]
- 5.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44(5):415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 6.Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16(4):233–240. doi: 10.1097/01.icu.0000172827.31985.3a. [DOI] [PubMed] [Google Scholar]
- 7.Andri KR, Roger WB, Laurence SL, Jodhbir SM. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biom. 2010;31(2):216–225. doi: 10.1016/j.biomaterials.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Levis H, Daniels JT. New technologies in limbal epithelial stem cell transplantation. Curr Opin Biotechnol. 2009;20(5):593–597. doi: 10.1016/j.copbio.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Shortt AJ, Secker GA, Notara MD, Limb GA, Khaw PT, Tuft SJ, Daniels JT. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52(5):483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Sangwan VS, Matalia HP, Vemuganti GK, Fatima A, Ifthekar G, Singh S, Nutheti R, Rao GN. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54(1):29–34. doi: 10.4103/0301-4738.21611. [DOI] [PubMed] [Google Scholar]
- 11.Kolli S, Ahmad S, Lako M, Figueiredo F. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells. 2010;28(3):597–610. doi: 10.1002/stem.276. [DOI] [PubMed] [Google Scholar]
- 12.Kruse FE, Joussen AM, Rohrschneider K, You L, Sinn B, Baumann J, Völcker HE. Cryoperserved human amniotic membrane for ocular surface reconstruction. Graefe's Arch Clin Exp Ophthalmol. 2000;238(1):68–75. doi: 10.1007/s004170050012. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Sotozono C, Bentley AJ, Mano S, Inatomi T, Koizumi N, Fullwood NJ, Kinoshita S. Long-term phenotypic study after allogeneic cultivated corneal limbal epithelial transplantation for severe ocular surface diseases. Ophthalmology. 2010;117(12):2247–2254. doi: 10.1016/j.ophtha.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Chew HF. Limbal stem cell disease: Treatment and advances in technology. Saudi J Ophthalmol. 2011;25(3):213–218. doi: 10.1016/j.sjopt.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Song G, Wang Z, Huang B, Gao Q, Liu B, Xu Y, Liang X, Ma P, Gao N, Ge J. Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp Eye Res. 2007;84(3):599–609. doi: 10.1016/j.exer.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Fan TJ, Xu B, Zhao J, Yang Hs, Wang RX, Hu XZ. Establishment of an untransfected human corneal epithelial cell line and its biocompatibility with denuded amniotic membrane. Int J Ophthalmol. 2011;4(3):228–234. doi: 10.3980/j.issn.2222-3959.2011.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan TJ, Yang HS, Hu XZ, Zhao J. Research advances on in vitro reconstruction of tissue-engineered human corneal epithelium. J Shandong Univ Med. 2010;48(7):7–13. [Google Scholar]
- 18.Federico CM. Corneal epithelial cell cultures as a tool for research, drug screening and testing. Exp Eye Res. 2008;86(3):459–469. doi: 10.1016/j.exer.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Fan T, Zhao J, Ma X, Xu X, Zhao W, Xu B. Establishment of a continuous untransfected human corneal endothelial cell line and its biocompatibility to denuded amniotic membrane. Mol Vis. 2011;17:469–480. [PMC free article] [PubMed] [Google Scholar]
- 20.Riau AK, Beuerman RW, Lim LS, Mehta JS. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31(2):216–225. doi: 10.1016/j.biomaterials.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Shortt AJ, Secker GA, Rajan MS, Meligonis G, Dart JK, Tuft SJ, Daniels JT. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008;115(11):1989–1997. doi: 10.1016/j.ophtha.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Zakaria N, Koppen C, Van Tendeloo V, Berneman Z, Hopkinson A, Tassignon MJ. Standardized Limbal Epithelial Stem Cell Graft Generation and Transplantation. Tissue Eng Part C Methods. 2010;16(5):921–927. doi: 10.1089/ten.TEC.2009.0634. [DOI] [PubMed] [Google Scholar]
- 23.Ban Y, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Koizumi N, Dota A, Mochida C, Kinoshita S. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003;76(6):735–743. doi: 10.1016/s0014-4835(03)00033-2. [DOI] [PubMed] [Google Scholar]