Abstract
AIM
To investigate the early expression of surfactant proteins D(SP-D) in Fusarium solani infected rat cornea.
METHODS
Wistar rats were divided into group A, B and C randomly. The right eyes were chosen as the experiment one. Group A was control group. Group B was not inoculated with Fusarium solani. Group C was taken as fusarium solani keratitis model. Five rats in group B and C were executed randomly at 6, 12, 24, 48 and 96 hours respectively after the experimental model being established. The expression of SP-D was assessed through immunohistochemistry and reverse transcription polymerase chain reaction(RT-PCR).
RESULTS
RT-PCR detected that the SP-D mRNA expression was low in the corneal of normal rats and group B. The expression of fungal infected cornea increased gradually and reached the peak at 24 hours in group C. The synchronous expression of group B and C were in significant difference (P<0.01). Immunohistochemisty discovered the protein of SP-D expression was increased gradually from 12 hours and reached the peak at 48 hours in group C. The synchronous expression of group B and C were also in significant difference (P<0.01).
CONCLUSION
There exists SP-D in rat corneal tissue and the expression is significantly increased at the early period of fusarium solani infected cornea. SP-D may play a role in the early innate immunity response of the corneal resistance to Fusarium solani infection.
Keywords: keratitis, Fusarium solani, surfactant protein D, innate immune
INTRODUCTION
Fungal keratitis is one of the most serious disease to blindness in the clinic[1]. Current researches mainly focus on the treatment of fungal keratitis. The occurrence and development mechanism of the fungal keratitis are poorly understood, specially immunological features. As the first line of host defense microbial infection[2], Innate immunity activate and launch the immune defense response to identify and remove pathogens, through pattern-recognition receptors expressed by innate immune cells recognize pathogen associated molecular patterns, that highly conserved structure in pathogenic microorganism[3]. Surfactant protein D(SP-D) is a member of collectin family in C-type lectin super family and is important in innate immune defence mechanism[4]. SP-D is secreted primarily by alveolar II type cells and a important pattern recognition receptors to defense microbial infection in lung[5]. Recent studies have shown[6] that SP-D is also present in the lacrimal gland, tears, corneal epithelial cells and other parts and play an important role in the resistance to bacteria and viruses. Our previous studies have shown[7] that human corneal epithelial cells exists SP-D and it may play a significant role in pathogenesis of keratomycosis. Based on these, this study investigated the early expression of SP-D in Fusarium solani infected cornea and to explore the role of SP-D in innate immunity of fungal keratitis.
MATERIALS AND METHODS
Materials
Fusarium solani strains(NO3.4604) was bought from China General Microbiological Culture Collection Center; Sabouroud culture was purchased from American Sigma company; Rabbit anti-rats SP-D multi-clonal antibody, Histostain® PLUS kit and DAB kit were purchased from Beijing Biosynthesis Biotechnology Co., LTD; Trizol Reagent was purchased from Invitrigen; RNA PCR Kit(AIIv)Ver.3.0 was purchased from TaKaRa.
Animals
A total of 55 Wistar rats (both male and female) were purchased from Qingdao Institute of Drug Control (Qingdao, China). All rats weighed between 200-300g. Animals with corneal disease were excluded by slit-lamp examination. All animals were treated in accordance with the guide lines provided in the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research. Fifty-five Wistar rats were randomly divided into 3 groups: 5 for Group A, 25 for Group B and 25 for Group C. The right eyes were chosen as the experiment one. The method to establish model and the criteria to evaluate it was in accordance with Zhao et al[1]. Group A was control group. Group B completed the model but not inoculated with Fusarium solani. Group C were taken as fusarium solani keratitis model. Five rats in group B and C were executed randomly at 6, 12, 24, 48 and 96 hours respectively after the experimental model being established. The eyeball was removed under sterile conditions. The cornea was divided into two parts: one half was fixed with 40g/L formaldehyde solution for immunohistochemical observation, the other half was preserved into Epoxy (EP) tube that had been treated with diethypyrocarbonate (DEPC) water and high pressure sterilization, then stored in -80°C refrigerator for RT-PCR.
RT-PCR
Total RNA was extracted from samples using Trizol reagent according to the manufacturer's protocol. Five micrograms of total RNA template were used to make cDNA by using a PrimeScript RT-PCR Kit. A polymerase chain reaction (PCR) was performed using TaKaRa Ex Taq HS and the syn-thetic gene-specific primers for SP-D and β-actin used in the RT-PCR are listed as follow. SP-D target sequences were amplified for 38 cycles. Samples of each reaction product were separated by agarose gel electrophoresis, visualized by ethidium bromide staining, and photographed with 290-nm ultraviolet illumination. Each band was shot with gel shooting imager and analyzed by using software, then measured the integral absorbance (A) values of gene SP-D and internal reference β-actin and calculated the ratio of ASP-D/Aβ-actin, which is relative integral absorbance as the relative expression level of SP-D in each group.
| Gene | Primer sequence | Product size(bp) |
| SP-D | F: GAA TCA AAG GCG AAA GTG G | 285 |
| R: TGC TGT GGG CTG TGA CGA | ||
| β-actin | F: ATC ATG TTT GAG ACC TTC AAC | 317 |
| R: CAT CTC TTG CTC GAA GTC CA |
Immunocytochemistry
Corneal paraffin sections, the thickness were 2µm, were conventional dewaxed to water. We applied S-P method to stain SP-D in the corneal tissue. PBS buffer instead of primary antibody was used as negative control, lung biopsy immunohistochemical staining as positive control. Corneal tissue appeared brown particles for positive criteria. We randomly selected field of vision and save under 200 times view, analyzed the mean optical density of SP-D staining with the VIDAS-21 the computer color image analysis system.
Statistical Analysis
All data were presented as mean ± SD (n=5). The data were analyzed using SPSS11.5 statistical package. The differences were analyzed by T test and difference between group B and C were analyzed by Q test. P<0.05 was considered to indicate statistical significance of the test results.
RESULTS
Expression of SP-D mRNA
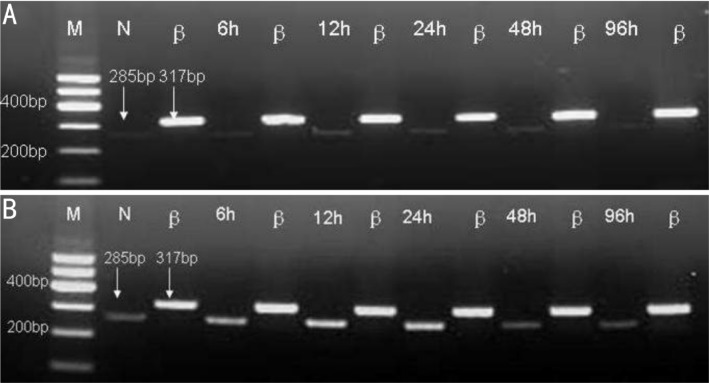
The SP-D mRNA expression (Figure 1) were low in normal cornea (Average expression 0.11232±0.0035) and group B. After infection, the SP-D mRNA expression increased gradually. It reached the peak at 24 hours. The synchronous expression of group B and C were in significant difference (P<0.01, Table 1).
Figure 1. RT-PCR results of SP-D.
A: Group B; B:Group C Ethidium bromide–stained agarose gels for visualization of PCR amplification products derived from the following groups (n=5 for each group). N:Control group; β:β-actin; 6h: 6 hours after the experimental model being established; 12h: 12 hours after the experimental model being established; 24h: 24 hours after the experimental model being established; 48h: 48 hours after the experimental model being established; 96h: 96 hours after the experimental model being established. To estimate quantitative correlation, a β-actin PCR control was performed for investigated tissue (right lanes). In accordance with the DNA marker (M), the distinct DNA bands are visible at 285bp for SP-D.
Table 1. The average expression of SP-D mRNA in group B and C.
| Parameters | n | Group C | Group B | t | P |
| 6h | 5 | 0.32521±0.0041 | 0.12012±0.0039 | 81.0476 | <0.01 |
| 12h | 5 | 0.74135±0.0032 | 0.12115±0.0040 | 270.7290 | <0.01 |
| 24h | 5 | 0.83478±0.0035 | 0.11993±0.0038 | 309.3822 | <0.01 |
| 48h | 5 | 0.34353±0.0049 | 0.12105±0.0053 | 68.9281 | <0.01 |
| 96h | 5 | 0.20252±0.0037 | 0.12096±0.0046 | 30.9082 | <0.01 |
Immunocytochemistry Results of SP-D
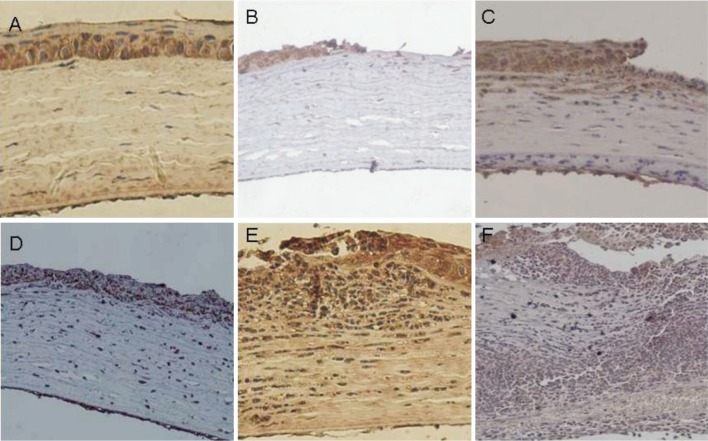
Brown staining indicates the expression of SP-D (Figure 2). Normal cornea (Figure 2A) only found faint brown staining in the corneal epithelium, which mainly expressed in the cytoplasm of epithelial cells. The expression in matrix layer and endothelium layer were not obvious. The staining in group B was similar to group A. The result of 6 hours after fungal infection showed that SP-D positive staining were found in corneal epithelial cell layer (Figure 2B). 12 hours showed that positive staining were increased and found in shallow stromal layer (Figure 2C). 24 hours (Figure 2D), 48 hours (Figure 2E) showed that the expression of SP-D continued to increase and appeared in cortex layer and endothelium layer. Brown staining were weaker in 96 hours (Figure 2F). By analyzing the mean optical density of SP-D staining in cornea of each group with the VIDAS-21 computer color image analysis system, we obtained the data (Table 2). There was low SP-D protein expression in group A(Average A values 0.215±0.023) and B. The expression was increased gradually from 12 hours and reached the peak at 48 hours in group C. The synchronous expression of group B and C were in significant difference (P<0.01).
Figure 2. SP-D expression evaluated by immunohistochemical staining (200×).
A: Normal cornea; B: Corneal epithelial cell layer; C: Shallow stromal layer; D: 24 hours; E: 48 hours; F: 96 hours.
Table 2. The average A values of SP-D in group B and C.
| n | Group C | Group B | t | P | |
| 6h | 5 | 0.232±0.037 | 0.228±0.042 | 0.1598 | >0.05 |
| 12h | 5 | 0.419±0.034 | 0.271±0.028 | 7.5136 | <0.01 |
| 24h | 5 | 0.554±0.024 | 0.256±0.037 | 15.1092 | <0.01 |
| 48h | 5 | 0.637±0.051 | 0.269±0.041 | 12.5750 | <0.01 |
| 96h | 5 | 0.328±0.030 | 0.254±0.039 | 3.3629 | <0.01 |
DISCUSSION
Surfactant protein D is a member of the collectin family of C-type lectins that includes a number of molecules which known host defense functions. SP-D can decrease the cytokines such as IL- 2, IL- 4 and IL- 5 in spleen and increase IFN-γ to make immunocytes change from Th2 to Th1 type in IPA models[8]. The Th2 type immune response of SP- D gene-knockoff mice alleged by aspergillus fumigatus was stronger than wild type mice. Many studies[5],[9] showed that SP-D can inhibit inflammatory responses by down regulating the expression of cytokines such as TNF-α, macrophage inflammatory protein-1α, monocyte chemoattractant protein -1 and the IL-2, IL-8, IL-6. The ability of SP-D to bi-directional regulate inflammation is important for the development of infectious diseases. This may be an important part in pathogenesis of infectious diseases.
Earlier studies have shown that early innate immune response occurred within the 4-96 hours after infection while acquired immune response occurred at 96h after infection. Therefore we sacrificed animals at 5 different time spot as 6, 12, 24, 48, 96 hours after infection to discuss the function of SP-D in the innate immune response in fungal keratitis. Our research discovered that SP-D have the trace expression in the cornea of normal rats, mainly in the cytoblastema of epithelia corneal cell and no expression in the stroma and the cornea endothelium. This result is similar to the finding of Bräuer et al[6] saying that SP-D mainly expressed in the shallow layer of human corneal epithelium. Simultaneously it is similar to Ni's[10] study about C57BL/6mice. Our results showed that the expression of SP-D protein and mRNA of group B didn't increased significantly suggesting that injury without infection wouldn't lead to increase of expression of SP-D. The expression of SP-D in fungal keratitis group showed that the expression increased 6 hours after infection. The results of immunohistochemical staining showed that 12 hours after infection neutral granular cells began to infiltrate into the stroma of cornea and the expression of SP-D increased in corneal epithelium, stroma cells and the endothelium cells. These results shown that, after Fusarium solani infected rats' cornea, the expression of SP-D in gene and protein levels were increased. This may be associated with fungal invasion. The polymorphonuclear neutral granular cells are the main immune cells for host's resistance to fungal infections in early stage which mainly through the way of swallowing fungus hypha and producing free radical to prevent infection of fungus[11]. Earlier experiments in vitro[12] have found that co-culturing SP-D with candida albicans can recognize, combine and aggregate candida albicans as to inhibit the growth of candida albicans and its changing into hypha, and this function doesn't rely on macrophage cells. Other studies showed[13] that SP-D could combine Aspergillus fumigatus conidia and strengthen the swallow function of neutrophill and macrophage cells to eliminate the pathogen. This protective function of SP-D can be related with reducing clone unit forming, suppressing the produce of hypha as well as to increasing the level of protective cytokine TNF-α and IFN-γ. However, we need further experiment to prove the relationship between SP-D and the accumulation of neutrophils to the lesions in fungal keratitis.
In conclusion, the recognition of fungus by innate immune response is the foundation of eliminating fungus infection. Through our research we found that SP-D exists in rat corneal tissue and the expression is significantly increased at the early period of fusarium solani infected cornea. SP-D may play a role in the early innate immunity response of the corneal resistance to Fusarium solani infection.
REFERENCES
- 1.Zhao GQ, Wang X, Hu LT, Lin J, Che CY, Jiang N. Expression of substance P in experimental fungal keratitis. Zhonghua Yanke Zazhi. 2011;47(5):443–450. [PubMed] [Google Scholar]
- 2.Li N, Che CY, Hu LT, Lin J, Wang Q, Zhao GQ. Effects of COX-2 inhibitor NS-398 on IL-10 expression in rat fungal keratitis. Int J Ophthalmol. 2011;4(2):116–120. doi: 10.3980/j.issn.2222-3959.2011.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Zhao GQ. Mechanism of the immune response to keratomycosis. Zhonghua Yanke Zazhi. 2011;47(4):378–381. [PubMed] [Google Scholar]
- 4.Zhang L, Ikegami M, Korfhagen TR, McCormack FX, Yoshida M, Senior RM, Shipley JM, Shapiro SD, Whitsett JA. Neither SP-A nor NH2-terminal domains of SP-A can substitute for SP-D in regulation of alveolar homeostasis. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L181–190. doi: 10.1152/ajplung.00015.2006. [DOI] [PubMed] [Google Scholar]
- 5.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109(6):707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bräuer L, Kindler C, Jäger K, Sel S, Nölle B, Pleyer U, Ochs M, Paulsen FP. Detection of surfactant proteins A and D in human tear fluid and the human lacrimal system. Invest Ophthalmol Vis Sci. 2007;48(9):3945–3953. doi: 10.1167/iovs.07-0201. [DOI] [PubMed] [Google Scholar]
- 7.Che CY, Jia WY, Xu Q, Li N, Hu LT, Jiang N, Lin J, Wang Q, Zhao GQ. The roles of surfactant protein D during Aspergillus fumigatus infection in human corneal epithelial cells. Int J Ophthalmol. 2012;5(1):23–27. doi: 10.3980/j.issn.2222-3959.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemons KV, Calich VL, Burger E, Filler SG, Grazziutti M, Murphy J, Roilides E, Campa A, Dias MR, Edwards JE, Jr, Fu Y, Fernandes-Bordignon G, Ibrahim A, Katsifa H, Lamaignere CG, Meloni-Bruneri LH, Rex J, Savary CA, Xidieh C. Pathogenesis I: interactions of host cells and fungi. Med Mycol. 2000;38(1):99–111. [PubMed] [Google Scholar]
- 9.Knudsen L, Wucherpfennig K, Mackay RM, Townsend P, Muhlfeld C, Richter J, Hawgood S, Reid K, Clark H, Ochs M. A recombinant fragment of human surfactant protein D lacking the short collagen-like stalk fails to correct morphological alterations in lungs of SP-D deficient mice. Anat Rec (Hoboken) 2009;292(2):183–189. doi: 10.1002/ar.20830. [DOI] [PubMed] [Google Scholar]
- 10.Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SM. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by pseudomonas aeruginosa. Infect Immun. 2005;73(4):2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6(7):e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rozendaal BA, van Spriel AB, van De Winkel JG, Haagsman HP. Role of pulmonary surfactant protein D in innate defense against Candida albicans. J Infect Dis. 2000;182(3):917–922. doi: 10.1086/315799. [DOI] [PubMed] [Google Scholar]
- 13.Madan T, Eggleton P, Kishore U, Strong P, Aggrawal SS, Sarma PU, Reid KB. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997;65(8):3171–3179. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]