Abstract
AIM
To determine the involvement of the transforming growth factor (TGF)-β with the development of experimental subretinal fibrosis in a mouse model.
METHODS
Subretinal fibrosis was induced by subretinal injection of macrophage-rich peritoneal exudate cells (PECs) and the local expression of TGF-β isoforms was assessed by quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) at various time points. In addition, we investigated the effect of TFG-β-neutralizing antibodies (TGF-β NAb) on subretinal fibrosis development.
RESULTS
TGF-β1 and TGF-β2 mRNA level was significantly elevated at day 2 after subretinal fibrosis induction and increased further to 5 and 6.5-fold respectively at day 5, reaching the peak. TGF-β3 mRNA was not detected in the present study. The result of ELSIA showed that active TGF-β1 and TGF-β2 levels were upregulated to 10-fold approximately, while total TGF-β1 and TGF-β2 levels were even upregulated more than 10-fold and more than 20-fold respectively in subretinal fibrosis mice in comparison with naïve mice at day 5. TGF-β NAb resulted in a reduced subretinal fibrosis areas by 65% compared to animals from control group at day 7.
CONCLUSION
Our results indicate that TGF-β signaling may contribute to the pathogenesis of subretinal fibrogenesis and TGF-β inhibition may provide an effective, novel treatment of advanced and late-stage neovascular age-related macular degeneration.
Keywords: transforming growth factor-β, subretinal fibrosis, transforming growth factor-β neutralizing antibody
INTRODUCTION
Neovascular age-related macular degeneration (AMD) leads to severe deterioration of central vision in elderly individuals as the result of the development of choroidal neovascularization (CNV) in the macular region[1]. These new abnormal blood vessels first proliferate under the Bruch membrane and retinal pigment epithelium (RPE) and then invade the subretinal space, leading to subretinal hemorrhages, exudative lesions, serous retinal detachment, and disciform scars ultimately[2]. Local destruction of photoreceptors, RPE, and choroidal blood vessels leads to permanent reduction in macular function and vision.
Although molecular and cellular mechanisms underlying CNV are not fully elucidated, CNV is considered as a submacular wound healing process, requireing a continually evolving interaction among cells, cytokines and the extracellular matrix (ECM)[2],[3]. Angiogenesis is an essential component of this process and current clinical strategies for treating CNV are primarily aimed at inhibiting vascular endothelial growth factor (VEGF), the major promoter of angiogenesis[4],[5]. However, overall only 30%-40% of neovascular AMD patients gain three lines in visual acuity, and roughly every sixth patient continues losing visual acuity and progresses to legal blindness even under standard treatment with potent VEGF inhibitors[6]-[8]. Moreover, a recent study documented the development or progression of submacular fibrosis after anti-VEGF therapy in patients with neovascular AMD[9]. The greater fibrotic responses after anti-VEGF therapy are thought to be due to an imbalance between complex interaction of angiogenesis and tissue fibrosis during wound healing process. It therefore raises the prospect that CNV may be amenable to therapies other than just anti-angiogenesis approaches. Ultimately, it is the scarring response that irreversibly damages photoreceptors, so therapies that modify this response may help preserve or even rescue photoreceptors. Recently, Jo et al[10] successfully established a mouse model of subretinal fibrosis, resembling the fibrotic subretinal scarring observed in advanced and late-stage neovascular AMD, by introducing inflammatory macrophages into the subretinal space. This model is believed to prove a significant advance in investigating molecular mechanisms for neovascular AMD and establishing new therapy besides antiangiogenic approaches.
Transforming growth factor (TGF)-β is a multifunctional cytokine, regulating pivotal biological responses, such as differentiation, apoptosis, migration, immune cell function, and ECM synthesis[11]. TGF-β has three isoforms (TGF-β1, -β2, and -β3) and is secreted as a biologically inactive latent complex. Latent TGF-β is activated by various chemical or enzymatic treatments[12]-[14]. TGF-β has been implicated in various fibrous diseases, such as liver cirrhosis, pulmonary fibrosis, and systemic sclerosis[15]. In the eye, TGF-β is overexpressed in the vitreous of patients with proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR), and is also identified in proliferative membranes in these diseases[16]-[18]. Specifically, the concentration of TGF-β2 significantly correlates with the severity of PVR[16]. TGF-β is also detected in surgically excised human choroidal neovascular membranes and experimental CNV[19],[20]. Taken together, TGF-β is presumed to contribute to certain ocular wound healing processes, such as PDR, PVR and CNV.
In this report we show, for the first time, that local expression of TGF-β in the subretinal space is up-regulated during subretinal fibrosis development. Administration of TFG-β-neutralizing antibodies (TGF-β NAb) potently attenuated subretinal fibrosis size. Thus, TGF-β inhibition may provide an effective, novel treatment of advanced and late-stage neovascular AMD.
MATERIALS AND METHODS
Materials
Female 7- to 10-week-old C57BL/6 (B6) mice were used in all experiments. Animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Methods
Induction and evaluation of subretinal fibrosis
Subretinal fibrosis was induced by subretinal injection of macrophage-rich peritoneal exudate cells (PECs), as previously described[10]. Briefly, B6 mice received an intraperitoneal injection of 2.5mL of thioglycolate and the PECs were isolated three days later. A concentration of PECs of 4×107/mL was prepared for subretinal injection. Laser photocoagulation (0.1 second, 200mW, 532nm diode laser, Iridex, Mountain View, CA) was performed in the posterior pole of the retina of B6 mice, and 0.5µL of prepared PECs were injected into the subretinal space with a 10-µL syringe (Hamilton Company, Reno, NV) with a blunt-tipped needle. Eyes in which there was bleeding or no focal retinal detachment were excluded.
Seven days later, the area of glial fibrillary acidic protein (GFAP) was measured to quantify the subretinal fibrosis on choroidal flat mounts, as GFAP has been suggested to detect and quantitate subretinal fibrosis effectively in this animal model[10]. For GFAP staining, polyclonal rabbit Anti-GFAP antibody (1:400, Dako, Glostrup, Denmark) and fluorescein isothiocyanate (FITC) - conjugated anti-rabbit IgG (Invitrogen, Carlsbad, CA) were used. The flat mounts were observed with a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan) and the area was measured with ImageJ software (National Institutes of Health, Bethesda, MD).
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen), from RPE-choroid complexes of B6 mice on day 1, 2, 3, 5 and 7 after induction of subretinal fibrosis. Aliquots containing 1µg total RNA were reverse transcribed with an RT-PCR kit (First-Strand cDNA Synthesis Kit; Roche Diagnostics GmbH, Mannheim, Germany) according to the anufacturer's instructions. Real-time PCR was conducted with Light Cycler (Roche Diagnostics GmbH) using SYBR green (TaKaRa Bio, Otsu, Shiga, Japan). The primers used were 5′-ACGCCTGAGTGGCTGTCTTTTGAC-3′ and 5′-GGGCTGATCCCGTTGATTTCCACG-3′ for TGF-β1, 5′-GGATGGAAATGGATCCATGAACCC-3′ and 5′-TGTTGTACAGGCTGAGGACTTTGG-3′ for TGF-β2, and 5′-GATGAGCACATAGCCAAGCA-3′ and 5′-ATTGGGCTGAAAGGTGTGAC-3′ for TGF-β3, 5′-GATGACCCAGATCATGTTTGA-3′ and 5′-GGAGAGCATAGCCCTCGTAG-3′ for β-actin. All estimated mRNA values were normalized to β-actin mRNA levels.
Enzyme-linked immunosorbent assay (ELISA)
RPE-choroid complexes were isolated from B6 mice at day 5 after induction of subretinal fibrosis and immersed in 200µL tissue protein extraction reagent (T-PER; Pierce, Rockford, IL), supplemented with protease inhibitor cocktail (Halt; Pierce). The mixture was homogenized (Polytron; Kinematica AG) and clarified by centrifuging at 10,000g for 5 minutes. TGF-β1 and TGF-β2 levels in the lysate were determined by a mouse TGF-β1 and TGF-β2 ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. TGF-β present in untreated samples were considered as mature (active) forms whereas those detected following acid activation were taken as the total (mature and latent) TGF-β.
TGF-β NAb treatment
B6 mice received TGF-β NAb (10mg/kg body weight in PBS) (R&D Systems) by intraperitoneal injection 1 hour before subretinal induction, and the treatments were continued daily until the end of the study. Control mice were treated with rabbit IgG (R&D Systems) at identical doses as those for TGF-β NAb. Seven days later, the area of GFAP was measured to quantify the subretinal fibrosis on choroidal flat mounts, to assess the effect of the TGF-β NAb on experimental subretinal fibrosis.
Statistical Analysis
Each result is representative of at least three independent experiments. All values are expressed as the mean±SD. Statistical analyses were made using Student t-test (SPSS, Chicago, IL) where appropriate. P<0.05 was considered statistically significant.
RESULTS
TGF-β Expression during Experimental Subretinal Fibrosis Development
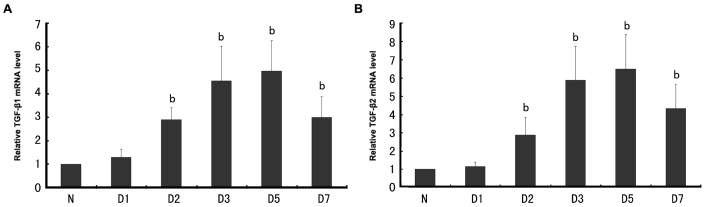
The expression of TGF-β isoforms during the development of experimental subretinal fibrosis was measured by quantitative real-time RT-PCR assay. TGF-β1 (Figure 1A) and TGF-β2 (Figure 1B) mRNA level was significantly elevated (P<0.01) at day 2 after subretinal fibrosis induction and increased further to 5 and 6.5-fold respectively at day 5 (P<0.01), reaching the peak. TGF-β3 mRNA was not detected either in naïve mice or during the development of experimental subretinal fibrosis (data not shown).
Figure 1. TGF-β mRNA expression in the course of subretinal fibrosis development.
A: TGF-β1; B: TGF-β2 bP<0.01 vs control group.
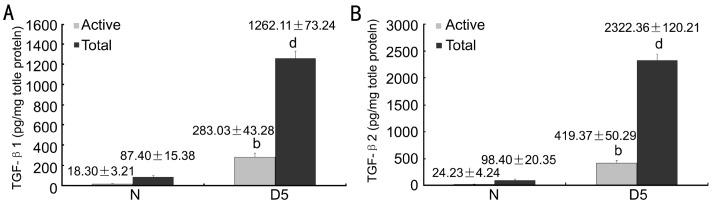
We next used ELISA analyses to detect active and total TGF-β1 and TGF-β2 protein in RPE-choroid complexes after subretinal fibrosis induction. At day 5 after subretinal fibrosis induction, in comparison with naïve mice, active TGF-β1 and TGF-β2 levels were upregulated to 10-fold approximately, while total TGF-β1 and TGF-β2 levels were even upregulated more than 10-fold and more than 20-fold respectively in subretinal fibrosis mice (Figure 2A, 2B).
Figure 2. TGF-β expression in the course of subretinal fibrosis development.
A: TGF-β1; B: TGF-β2). n=6-8. bP<0.01, dP<0.001 vs control group.
TGF-β NAb Suppresses Subretinal Fibrosis
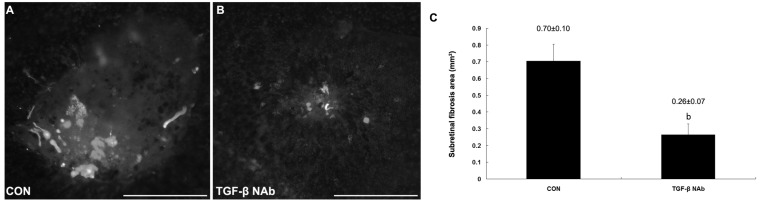
To address the roles of TGF-β signaling in this subretinal fibrosis model, we further investigated the effect of TGF-β NAb on subretinal fibrosis using intraperitoneal administration. TGF-β NAb resulted in a reduced subretinal fibrosis, and the GFAP-positive areas significantly decreased by 65% compared to animals from control group (Figure 3). No signs of systemic toxicity for intraperitoneal TGF-β NAb at this dosage (data not shown).
Figure 3. Suppression of subreitnal fibrosis by TGF-β blockage in murine model.
A:Control group; B: TGF-β NAb-treated group; C: Fluorescence-positive area bP<0.001 vs control group.
DISCUSSION
The present study reveals, for the first time to our knowledge, two important findings concerning the relationship of the TGF-β signaling with subretinal fibrosis. First, the TGF-β was present in experimental subretinal fibrosis and upregulated during subretinal fibrosis development. Second, subretinal fibrosis was suppressed by blocking TGF-β signaling using TGF-β NAb.
Peritoneal macrophages were injected into the subretinal space and successfully established an animal model of focal subretinal fibrosis, which mimics the fibrotic subretinal scarring observed in late-stage AMD. Ahead of PEC injection, high-power laser photocoagulation was performed to induce rupture of Bruch's membrane. The rupture of Bruch's membrane is known to induce CNV, which enabled easy accumulation of endogenous macrophages to the inflammatory site as an air bubble always forms in the subretinal space[10]. After injection, the air bubble sealed the retinal break, which prevented initial leakage of injected macrophages into the vitreous cavity. Macrophages can produce proinflammatory cytokines including IL-1β, TNF-α, and IL-6, which are known to promote myofibrosis[21],[22]. In addition, macrophages have the direct ability to induce α-smooth muscle actin (α- SMA) in co-cultured RPE cells[10]. These are considered as mechanisms of how injected macrophages mediate subretinal fibrosis.
TGF-β, a key molecule in various fibrotic disorders, can promote fibroblast proliferation, differentiation, migration, adhesion, and survival, induce cytokine secretion, and, most importantly, upregulate the synthesis of collagen and extracellular matrix, and is identified to have a fundamental role in pathological fibrogenesis[23]. The role of TGF-β in retinal function and in various ocular inflammatory, proliferative, and degenerative diseases is well documented[16], [24],[25]. Altered expression of TGF-β and its receptors in the vitreous, retina, and RPE has been closely correlated with the retinal fibrosis in proliferative vitreoretinal disorders and CNV associated with neovascular AMD[16]-[20],[26]. In the present study, the expression of TGF-β1 and TGF-β2, the predominant TGF-β isoforms in the posterior segment of the eye[20], [27], was upregulated during the development of experimental subretinal fibrosis. In the present study, TGF-β3 mRNA is not detected in the subretinal fibrosis or control group and this result is consistent with previous studies[20], [27]. Since only the mature form of the TGF-β can bind to the receptors and then transmit signals, we further examined active form, as well as total form of TGF-β1 and TGF-β2 protein using ELISA. Consistent with the results of quantitative RT-PCR, active and total TGF-β1 and TGF-β2 levels were significantly elevated at day 5 after subretinal fibrosis induction in comparison with control group. Our results indicated that TGF-β signaling may contribute to the pathogenesis of subretinal fibrogenesis.
Previous studies have revealed that TGF-β blockade can inhibit fibrosis responses seen in various diseases[28], [29]. In the present study, systemic blockade of TGF-β using TGF-β NAb led to significant suppression of subretinal fibrosis. Besides a well-known profibrotic role, several studies in human RPE cultures have reported that TGF-β significantly enhances VEGF secretion by promoting mRNA transcription, suggesting that TGF-β may work in concert with other angiogenic cytokines in VEGF upregulation[30],[31]. Recently, a study described that TGF-β inhibition can decrease CNV development in a laser-induced rat model, an effect associated with downregulation of VEGF, TGF-β, and platelet-derived growth factor (PDGF) cytokines[26]. As ideally treatment modalities for neovascular AMD would target the multiple mechanisms of AMD associated vision loss, including neovascularization and fibrosis, our results suggest TGF-β as an attractive molecular target in the treatment of neovascular AMD.
In summary, we report for the first time that TGF-β was upregulated during subretinal fibrosis development and TGF-β inhibition decreases subretinal fibrosis size. The future of neovascular AMD management may require combined therapies, with several drugs acting on different mediators of CNV and fibrosis, such as VEGF, complement system and TGF-β. Thus, in light of the current results, TGF-β_inhibition could be considered in this multivariate neovascular AMD treatment.
REFERENCES
- 1.Fine SL, Berger JW, Maguire MG, Ho AC. Age-related macular degeneration. N Engl J Med. 2000;342(7):483–492. doi: 10.1056/NEJM200002173420707. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117(3):576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent D, Sheridan C. Choroidal neovascularization: a wound healing perspective. Mol Vis. 2003;9:747–755. [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–530. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 7.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 8.Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, Ambati J. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460(7252):225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang JC, Del Priore LV, Freund KB, Chang S, Iranmanesh R. Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2011;42(1):6–11. doi: 10.3928/15428877-20100924-01. [DOI] [PubMed] [Google Scholar]
- 10.Jo YJ, Sonoda KH, Oshima Y, Takeda A, Kohno R, Yamada J, Hamuro J, Yang Y, Notomi S, Hisatomi T, Ishibashi T. Establishment of a new animal model of focal subretinal fibrosis that resembles disciform lesion in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(9):6089–6095. doi: 10.1167/iovs.10-5189. [DOI] [PubMed] [Google Scholar]
- 11.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 12.Brown PD, Wakefield LM, Levinson AD, Sporn MB. Physicochemical activation of recombinant latent transforming growth factor-beta's 1, 2, and 3. Growth Factors. 1990;3(1):35–43. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- 13.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110(4):1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazono K, Heldin CH. Role for carbohydrate structures in TGF-beta 1 latency. Nature 1989. 1989;338(6211):158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- 15.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 16.Connor TB, Jr, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, de Bustros S, Enger C, Kato H, Lansing M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83(5):1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kita T, Hata Y, Kano K, Miura M, Nakao S, Noda Y, Shimokawa H, Ishibashi T. Transforming growth factor-beta2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes. 2007;56(1):231–238. doi: 10.2337/db06-0581. [DOI] [PubMed] [Google Scholar]
- 18.Bochaton-Piallat ML, Kapetanios AD, Donati G, Redard M, Gabbiani G, Pournaras CJ. TGF-beta1, TGF-beta receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Invest Ophthalmol Vis Sci. 2000;41(8):2336–2342. [PubMed] [Google Scholar]
- 19.Amin R, Puklin JE, Frank RN. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994;35(8):3178–3188. [PubMed] [Google Scholar]
- 20.Ogata N, Yamamoto C, Miyashiro M, Yamada H, Matsushima M, Uyama M. Expression of transforming growth factor-beta mRNA in experimental choroidal neovascularization. Curr Eye Res. 1997;16(1):9–18. doi: 10.1076/ceyr.16.1.9.5121. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Katayama I, Nishioka K. Fibroblast proliferation by bleomycin stimulated peripheral blood mononuclear cell factors. J Rheumatol. 1999;26(3):609–615. [PubMed] [Google Scholar]
- 22.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 24.Forrester JV, Lumsden L, Liversidge J, Kuppener M, Mesri M. Immunoregulation of uveoretinal inflammation. Prog Retin Eye Res. 1995;14(2):393–412. [Google Scholar]
- 25.Wiedemann P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol. 1992;36(5):373–384. doi: 10.1016/0039-6257(92)90115-a. [DOI] [PubMed] [Google Scholar]
- 26.Recalde S, Zarranz-Ventura J, Fernandez-Robredo P, Garcia-Gomez PJ, Salinas-Alaman A, Borras-Cuesta F, Dotor J, Garcia-Layana A. Transforming growth factor-beta inhibition decreases diode laser-induced choroidal neovascularization development in rats: P17 and P144 peptides. Invest Ophthalmol Vis Sci. 2011;52(10):7090–7097. doi: 10.1167/iovs.11-7300. [DOI] [PubMed] [Google Scholar]
- 27.Pasquale LR, Dorman-Pease ME, Lutty GA, Quigley HA, Jampel HD. Immunolocalization of TGF-beta 1, TGF-beta 2, and TGF-beta 3 in the anterior segment of the human eye. Invest Ophthalmol Vis Sci. 1993;34(1):23–30. [PubMed] [Google Scholar]
- 28.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5(4):200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 30.Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J Cell Physiol. 2003;197(3):453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- 31.Bian ZM, Elner SG, Elner VM. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res. 2007;84(5):812–822. doi: 10.1016/j.exer.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]