Abstract
Macrolide antibiotics possess several, beneficial, secondary properties which complement their primary antimicrobial activity. In addition to high levels of tissue penetration, which may counteract seemingly macrolide-resistant bacterial pathogens, these agents also possess anti-inflammatory properties, unrelated to their primary antimicrobial activity. Macrolides target cells of both the innate and adaptive immune systems, as well as structural cells, and are beneficial in controlling harmful inflammatory responses during acute and chronic bacterial infection. These secondary anti-inflammatory activities of macrolides appear to be particularly effective in attenuating neutrophil-mediated inflammation. This, in turn, may contribute to the usefulness of these agents in the treatment of acute and chronic inflammatory disorders of both microbial and nonmicrobial origin, predominantly of the airways. This paper is focused on the various mechanisms of macrolide-mediated anti-inflammatory activity which target both microbial pathogens and the cells of the innate and adaptive immune systems, with emphasis on their clinical relevance.
1. Introduction
Macrolides, which are primarily antibiotics, belong to the polyketide group of natural products [1]. They derive their name from their characteristic structural features, a macrocyclic lactone ring to which various deoxy sugars, most commonly cladinose and desosamine, are attached [1]. The most important macrolide antibiotics are 14-, 15-, and 16-membered compounds. The molecular structure of the 14-membered erythromycin, the prototype macrolide, is shown in Figure 1. Drug delivery problems resulting from acid instability prompted the design of newer macrolides. These compounds include (i) clarithromycin, roxithromycin, dirithromycin, and the ketolides and fluoroketolides, all of which have a 14-membered ring structure; (ii) the 15-membered azithromycin; and (iii) the 16-membered agents spiramycin, rokitamycin, and josamycin.
Figure 1.
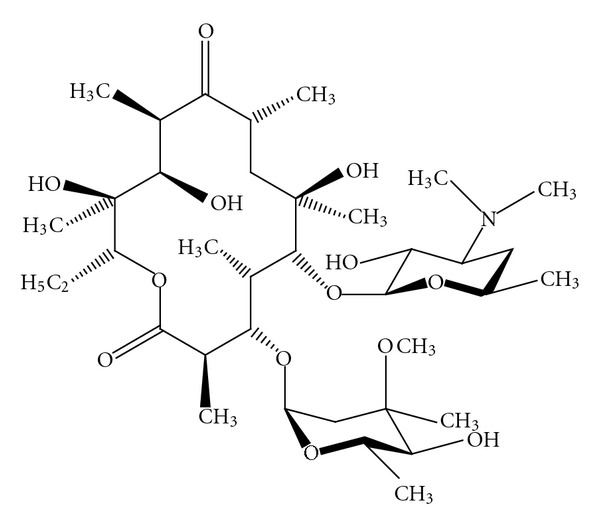
The molecular structure of erythromycin, the 14-membered prototype macrolide [1].
Macrolide antibiotics are generally used to treat respiratory and soft tissue infections caused by Gram-positive bacteria. They are also active against rickettsiae, chlamydiae, and Mycoplasma pneumoniae, as well as some Gram-negative bacterial pathogens, including Bacteroides fragilis, Bordetella pertussis, Campylobacter species, Haemophilus influenzae, Helicobacter pylori, Legionella pneumophila, Moxarella catarrhalis, and Neisseria species. The more advanced macrolides, azithromycin, and clarithromycin, as well as the ketolides/fluoroketolides, have several distinct advantages over erythromycin. These include extended spectrum of activity, improved pharmacokinetics, pharmacodynamics and tolerability, and once-daily administration [2]. Azithromycin and to a lesser extent clarithromycin are noted for their high and prolonged concentrations at sites of infection, reaching tissue levels of 10–100-fold and 2–20-fold greater than serum concentrations, respectively [3–5]. Both agents are also concentrated intracellularly by alveolar macrophages, attaining levels of approximately 400-fold (clarithromycin) and 800-fold (azithromycin) above serum concentrations [3]. The ketolide, telithromycin, also has excellent penetration into bronchopulmonary tissues and macrophages, while macrolides and macrolide-like agents are also accumulated by polymorphonuclear leukocytes (PMNL), which, in turn, effect the active delivery of these agents to sites of bacterial infection [3, 6].
With respect to their mechanism of antimicrobial action, macrolides are inhibitors of bacterial protein synthesis. This is achieved by reversible binding of these agents to the P site of the 50S subunit of the bacterial ribosome [1]. The macrolide/ribosome interaction has several apparent consequences, all of which result in inhibition of bacterial protein synthesis. These are (i) interference with peptidyltransferase, preventing polypeptide chain elongation; (ii) inhibition of ribosomal translocation; and (iii) untimely detachment of peptidyl-tRNA from the ribosome [1, 7, 8]. Macrolides, ketolides, and fluoroketolides possess 1, 2, and 3 ribosomal binding, sites respectively [1]. Although predominantly bacteriostatic, the high tissue and macrophage/PMNL concentrations attained by macrolides and macrolide-like agents may favour bactericidal activity in vivo.
Notwithstanding their primary antimicrobial activity, macrolides, unlike most other classes of antibiotic, also possess beneficial anti-inflammatory properties. These latter effects are achieved by two distinct mechanisms. Firstly, as a consequence of their primary ribosomal-targeted mechanism of antimicrobial action, they inhibit the production of proinflammatory microbial toxins and other virulence factors. Surprisingly, this pathogen-directed mechanism of anti-inflammatory activity has also been described for a number of ostensibly macrolide-resistant bacterial pathogens as described hereinafter. Secondly, macrolides have been reported to possess secondary anti-inflammatory activities which target cells of the innate and adaptive immune systems as well as structural cells.
The remainder of this paper is devoted to a consideration of the anti-inflammatory activities of macrolides and their therapeutic relevance.
2. Pathogen-Targeted Anti-Inflammatory Activities of Macrolides
Antibiotics cooperate with host defences to eradicate microbial pathogens. In this setting, the antibiotic-exposed pathogens are weakened, increasing their vulnerability to the cellular and humoral defences of the host. While these antibiotic/host defence interactions are clearly beneficial, some antibiotics may trigger over-exuberant inflammatory responses with potentially harmful consequences for the infected host. These include cell-wall-targeted, bactericidal antibiotics, especially, beta-lactams, as well as fluoroquinolones, which initiate the release of proinflammatory intracellular toxins and cell-wall components from damaged, disintegrating bacteria. Examples of these are the pneumococcal toxin, pneumolysin, as well as cell-wall-derived lipopolysaccharides and lipoteichoic acids. These initiate exaggerated inflammatory responses by several mechanisms, including (i) interactions with Toll-like receptors and nucleotide- oligomerization- (NOD-) like receptors on/in immune and inflammatory cells, as well as epithelial cells; and (ii) activation of complement cascades [9–11]. The harmful, proinflammatory activities of beta-lactams and fluoroquinolones have been demonstrated in a number of studies, either by measuring the release of intracellular toxins following exposure of susceptible bacteria to these antimicrobial agents in vitro [12–18], or in animal models of experimental infection in which survival is correlated with the antimicrobial and proinflammatory potencies of antibiotics [19–22].
In contrast to beta-lactams and fluoroquinolones, antibiotics which inhibit bacterial protein synthesis, particularly macrolides and macrolide-like agents, prevent the release of proinflammatory protein toxins from both Gram-positive and Gram-negative bacteria, as well as the production of other virulence factors such as bacterial adhesins and biofilm. Consequently, the pathogen-targeted actions of macrolides have a much lesser propensity to trigger harmful inflammatory reactions than is the case with abruptly bactericidal agents, a contention which is supported by a considerable body of experimental evidence. This includes a number of in vitro studies which have demonstrated the inhibitory effects of macrolides and macrolide-like agents, often at subminimal inhibitory concentrations (MICs), on the production of proinflammatory/cytocidal bacterial toxins such as (i) pneumolysin by Streptococcus pneumoniae [23, 24], (ii) Panton-Valentine leukocidin and α-haemolysin by Staphylococcus aureus [12, 13], and (iii) shiga-like toxins by enterohaemorrhagic strains of Escherichia coli [14–18]. In contrast, exaggerated release of these toxins was observed when the bacteria were exposed to beta-lactams or fluoroquinolones [12–18, 25].
These findings have been confirmed in animal models of experimental infection. Spreer et al. in several studies using a rabbit model of experimental meningitis have reported that administration of the macrolide-like agent, clindamycin, as well as rifampicin, but not the beta-lactam, ceftriaxone, significantly reduced concentrations of pneumolysin in cerebrospinal fluid [19–21]. This was associated with an attenuated inflammatory response and decreased neuronal injury. More recently, others have investigated the effects of treatment with (i) ampicillin only, (ii) azithromycin or clindamycin only, or (iii) ampicillin in combination with either azithromycin or clindamycin on survival using a murine model of secondary, influenza-associated pneumococcal pneumonia [22]. The lowest survival rate in the antibiotic-treated animals was observed in mice treated with ampicillin only, while the highest rates were noted in those treated with azithromycin or clindamycin individually or in combination with ampicillin. Improved survival in the azithromycin/clindamycin-treated groups was associated with an attenuated inflammatory response in the airways characterized by decreases in both the numbers of inflammatory cells and concentrations of proinflammatory cytokines, as well as less severe histopathological changes [22].
In addition to the aforementioned effects of macrolides on dampening potentially harmful responses in the setting of acute bacterial infections caused by macrolide-susceptible pathogens, it is noteworthy that these agents have also been reported to inhibit the production of proinflammatory toxins by ostensibly macrolide-resistant pathogens. Notwithstanding the inhibitory effects of macrolides on the production of shiga toxins by E. coli mentioned previously, these agents have also been reported to inhibit the production of pneumolysin by macrolide-resistant strains of the pneumococcus both in vitro and in vivo. In an earlier study, Lagrou et al. reported that exposure of an ermAM-expressing, ribosomal methylase-producing, macrolide-resistant (MIC ≥ 256 μg/mL) strain of Streptococcus pneumoniae to a sub-MIC concentration of erythromycin prevented the adherence of the bacteria to human nasal respiratory epithelial cells [26]. Although the growth of the bacteria was unaffected, exposure to erythromycin almost completely attenuated the production of pneumolysin, which was the probable cause of interference with bacterial adherence [26]. These findings were confirmed in a later study in which Fukuda et al. reported that both azithromycin and clarithromycin at concentrations of 1–4 μg/mL inhibited the production of pneumolysin by ermB and mefE/A coexpressing, macrolide-resistant (MIC ≥ 256 μg/mL) strains of the pneumococcus in vitro [27]. Administration of these agents to mice (40–200 mg/kg) experimentally infected with macrolide-resistant pneumococci was found to result in prolonged survival, which was associated with decreased concentrations of pneumolysin in the airways. Similar findings have been described by Anderson et al., who reported that exposure of an ermB-expressing, macrolide-resistant strain of S. pneumoniae(MIC ≥ 256 μg/mL) to a range of macrolides and macrolide-like agents (0.5 μg · mL) resulted in significant attenuation of the production of pneumolysin, while amoxicillin, ceftriaxone, ciprofloxacin, doxycycline, and tobramycin were ineffective [23, 24].
More recently, Cockeran et al. have attempted to identify the molecular basis of the inhibitory effects of macrolides on the production of pneumolysin by macrolide-resistant strains of the pneumococcus [28]. They observed that exposure of 8 different ermB-expressing, macrolide-resistant strains (each with an MIC value of >256 μg/mL) to clarithromycin resulted in significant prolongation of the lag phase of bacterial growth (4.9–12.2 hours in comparison with 1.2–4.9 hours for non-exposed bacteria). Although rapid induction of the ermB gene was evident, according to a 4-fold increase in mRNA within 15 minutes of exposure to the antibiotic, synthesis of ribosomal methylase is probably hindered because of binding of clarithromycin to the peptide exit tunnel of the large ribosomal subunit, blocking peptide chain elongation [28]. The consequence is transient susceptibility due to slow acquisition of the full resistance phenotype.
Additional mechanisms which have been reported to underpin the efficacy of macrolides in murine models of experimental infection include high levels of intracellular accumulation of these agents by phagocytes and epithelial cells as well as their beneficial, secondary anti-inflammatory properties described hereinafter [29, 30].
2.1. Macrolides and Pseudomonas aeruginosa
Pseudomonas aeruginosa is a persistent opportunistic pathogen which colonizes the airways of immunocompromised individuals causing a chronic, ineffectual inflammatory response. This in turn results in inflammation-mediated tissue damage and pulmonary dysfunction and is particularly serious in patients with cystic fibrosis. Although macrolides do not affect the growth of P. aeruginosa, they are nevertheless protective by inhibiting the production of persistence-promoting and proinflammatory virulence factors. These include (i) proadhesive type IV pili, (ii) tissue-damaging pseudomonal elastase, (iii) proinflammatory rhamnolipid, and (iv) alginate and biofilm [31–34]. Alginate is an exopolysaccharide which functions as an antiphagocytic capsule, while biofilm is a self-generated, extracellular polymer matrix in which the pathogen is insulated against both antibiotics and the cellular and humoral defences of the host.
These P. aeruginosa-directed anti-infective, anti-inflammatory activities of macrolides, including erythromycin, clarithromycin, and azithromycin, appear to target quorum sensing in P. aeruginosa. Quorum sensing is a mechanism of microbial intercellular communication, utilising diffusible signalling molecules known as autoinducers, which enable bacteria to detect and regulate their population density and to upregulate virulence [35]. Gram-negative bacteria most commonly utilize type I family autoinducers known as N-acylated-L-homoserine lactones as their primary mediators of quorum sensing [35]. Both azithromycin and clarithromycin have been reported to inhibit the production of this class of autoinducers by P. aeruginosa [31, 36, 37]. Importantly, these effects were evident at sub-MIC concentrations of both macrolides, which in the case of azithromycin was 2 μg/mL [36]. In the case of biofilm formation, the quality of biofilm, as opposed to initiation of synthesis, appeared to be impaired by the macrolides, resulting in altered architecture, structure, and density, favouring the penetration of antibiotics [36, 37]. The pathogen-directed anti-inflammatory activities of macrolides are summarised in Table 1.
Table 1.
Targets of the pathogen-directed anti-inflammatory activities of macrolide antibiotics.
| (i) Synthesis and release of proinflammatory toxins and virulence factors | |
| (ii) Quorum sensing | |
| (iii) Biofilm formation |
As a strategy to counter P. aeruginosa in particular, the aforementioned antimicrobial/anti-inflammatory activities of macrolides are of proven benefit in the long-term therapy of cystic fibrosis [38], as well as the other chronic inflammatory disorders of the airways described hereinafter. However, the benefits of long-term administration of macrolides must be balanced against the potential risks, which include development of macrolide resistance, and, of particular concern, increased susceptibility to infection with nontuberculosus mycobacteria as a consequence of interference with lysosomal acidification [39].
3. Effects of Macrolides on Innate and Adaptive Immune Mechanisms
In addition to pathogen-directed anti-inflammatory activity, macrolides have also been reported to inhibit the proinflammatory activities of cells of both the innate and adaptive immune systems.
3.1. Innate Immunity
In the setting of innate immunity, the predominant anti-inflammatory activity of macrolides appears to be achieved via the modulation of the proinflammatory activities of neutrophils, in particular, inhibition of the production of the potent neutrophil activator and chemoattractant, IL-8 [40, 41]. Increased IL-8 in sputum and bronchoalveolar lavage is associated with severity of chronic inflammatory diseases such as cystic fibrosis (CF) and diffuse panbronchiolitis (DPB) [41–44]. Azithromycin, erythromycin, and clarithromycin have been shown to attenuate the production and secretion of IL-8 by airway smooth muscle cells, alveolar macrophages, and human gingival fibroblasts [40, 45, 46], as well as other cytokines such as (i) IL-1α and IL-2 by murine macrophages and splenocytes, respectively; (ii) IL-1β, GM-CSF, TNF-α, and MCP-1 by macrophages; and (iii) IL-1β, IL-6 and TNF-α from peripheral blood monocytes [47–53]. This is thought to result from the suppression of nuclear translocation of several transcription factors [54] by the macrolides, specifically nuclear factor- (NF-) κB, activator-protein- (AP-) 1, and specificity protein 1 in various types of inflammatory and structural cells [40, 54–60]. Inhibition of intracellular signalling via the extracellular signal-regulated kinase 1 and 2 (ERK 1/2) and p38 mitogen-activated protein kinase (MAPK) pathways are thought to mediate the downregulation of NF-κ-B, AP-1, and specificity protein 1 in response to clarithromycin [56, 57, 61–64]. In addition, azithromycin has been shown to attenuate the LPS/IFN-γ-mediated induction of IL-12p40, probably by the inhibition of the binding of AP-1, nuclear factor of activated T cells (NFAT), and interferon consensus sequence binding protein (ICSBP) to the DNA binding site of the IL-12p40 promoter [65]. This may also prove to be an important mechanism for regulating the anti-inflammatory effects of azithromycin in macrophages.
Interestingly, the ability of macrolide antibiotics to modulate cytokine expression by human neutrophils and their ability to decrease or increase cytokines is thought to depend on the presence or absence of bacteria [66, 67]. Clarithromycin was shown to inhibit the production of IL-6 and TNF-α by neutrophils primed with lipopolysaccharide (LPS), while increasing their expression when bacteria were present [67]. Shinkai et al. reported that clarithromycin initially increased IL-8 secretion by bronchial epithelial cells via ERK signalling but later inhibited ERK signalling leading to reduction (normalisation) in secretion of the chemokine. It is suggested that immunomodulation occurs, in part, by sequential cycles of ERK 1/2 inhibition and activation [60, 63]. This modulation of ERK 1/2 and transcription factors is consistent and unrelated to the antimicrobial properties of macrolides.
Notwithstanding interference with the production of IL-8 by monocytes/macrophages and various types of structural cells, several other mechanisms have been described by which macrolides inhibit neutrophil migration. These include (i) decreased synthesis and expression of the endothelial adhesion molecules ICAM-1 and VCAM-1, possibly as a consequence of decreased synthesis of IL-1β and TNF-α by tissue macrophages and other cell types [68, 69], (ii) interference with the expression of β2-integrins on activated neutrophils [69], (iii) decreased synthesis of leukotriene B4, a potent neutrophil chemoattractant, possibly as a secondary consequence of inhibitory effects on cytokines/chemokines [70], and (iv) interference with the synthesis and release of the matrix- metalloproteinases- (MMP-), 2, 7, and 9 from nasal polyp fibroblasts, as well as neutrophils, via antagonism of activation of NF-κB and AP-1 [71–73]. MMPs facilitate neutrophil migration.
In addition, macrolides may also interfere with signalling mechanisms initiated by activation of Toll-like receptors (TLRs). TLRs play a key role in innate host defence against viral and microbial pathogens by promoting the release of the neutrophil-mobilizing cytokines, IL-8, and TNF-α, from tissue macrophages and epithelial cells in particular. Treatment of monocyte-derived dendritic cells with erythromycin resulted in up-regulation of TLR2, down-regulation of TLR3, and no effect on expression of TLR4 [74]. However, clarithromycin has been reported to downregulate the expression of TLR4 on monocytes infected with Helicobacter pylori [75]. These results indicate that macrolides may selectively downregulate inflammatory responses which result from the interaction of viruses and Gram-negative bacteria with TLR3 and TLR4, respectively, while maintaining the interaction of Gram-positive bacteria with TLR2 [75].
Other anti-inflammatory interactions of macrolides with neutrophils include interference with the generation of reactive oxygen species (ROS) by these cells [76]. Although several mechanisms may exist, membrane-stabilizing activity has been proposed to underpin these effects by neutralizing the sensitizing actions of bioactive phospholipids such as lysophosphatidylcholine, platelet-activating factor (PAF), and lysoPAF on the membrane-associated, superoxide-generating complex of neutrophils, NADPH oxidase [77]. Macrolides have also been reported to induce phospholipidosis in eukaryotic cells, the magnitude of which appears to correlate with anti-inflammatory activity [78, 79]. Macrolides have also been reported to suppress the production of another type of ROS, nitric oxide, by activated macrophages, presumably by interfering with the induction of inducible nitric oxide synthase via antagonism of NF-κB [80, 81]. The anti-inflammatory interactions of macrolides with the cells of the innate immune system are summarised in Table 2.
Table 2.
Anti-inflammatory effects of macrolides on phagocytes and structural cells.
| Cellular target | Altered function | Mechanisms |
|---|---|---|
| Neutrophils | ↓ Migration | Interference with (i) production of IL-8 and TNF-α by macrophages and structural cells, (ii) decreased expression of adhesion molecules on vascular endothelium and neutrophils, and (iii)↓ production/release of MMPs by fibroblasts and neutrophils |
| ↓ production of ROS | Interference with NADPH oxidase, possibly by antagonizing the sensitizing actions of bioactive phospholipids | |
| Macrophages | ↓ cytokine production (IL-1β, IL-6, IL-8, TNF-α) | Interference with intracellular signalling mechanisms and transcription factor activation, resulting in suppression of gene expression |
| ↓ decreased NO production | As above, resulting in decreased expression of the gene encoding iNOS | |
| Airway epithelial cells, fibroblasts, smooth muscle cells | ↓ cytokine production (IL-8, TNF-α) | As above |
In addition to their effects on neutrophils and macrophages, macrolides, as alluded to what is mentioned before, can also downregulate the proinflammatory activities of structural cells, especially epithelial cells. Airway epithelial cells not only provide a mechanical barrier to inhaled micro-organisms but are also involved in the direct killing of microbial pathogens, as well as in activating other cells of the innate immune system [63]. The upper and lower respiratory tracts are lined by a highly specialised ciliated columnar epithelium which, together with the mucous layer covering these cells, constitute the mucociliary escalator which functions to keep the lower respiratory tract pathogen-free [82]. Macrolides have been shown to stimulate ciliary beat frequency and improve mucociliary clearance [83, 84]. Moreover, erythromycin, azithromycin, clarithromycin, and roxithromycin have been shown to inhibit chemotaxis and infiltration of neutrophils into the airways and subsequently suppress the synthesis and release of mucus by inhibiting muc5ac gene expression [68, 85–87]. Clarithromycin inhibits muc5ac gene expression, while azithromycin has been shown to inhibit muc5ac production in an ERK 1/2-dependent manner [68, 88]. Macrolides may also decrease sputum production by inhibiting chloride secretion [68]. In addition to these anti-inflammatory effects of macrolides on epithelial cells, these agents have also been reported to protect ciliated respiratory epithelium against the damaging effects of host-derived bioactive phospholipids [89].
3.2. Adaptive Immunity
Although lymphocytes are essential for adaptive immune responses to pathogens, they may also play a harmful role in inflammatory conditions such as autoimmunity and bronchial asthma. Several studies have described the anti-inflammatory effects of macrolides on lymphocytes, particularly T-lymphocytes. These include inhibition of proliferation of (i) Jurkat T cells treated with erythromycin and its non-antibacterial derivatives [90]; (ii) CD4 T cells, when clarithromycin- and roxithromycin-treated and untreated dendritic cells were used as antigen presenting cells [91]; (iii) peripheral blood mononuclear cells treated with azithromycin, clarithromycin, and roxithromycin and activated with concanavalin-A or toxic shock syndrome toxin-1 [92]; and (iv) T cells from house dust mite allergen-sensitive bronchial asthma patients treated with roxithromycin and stimulated with mite antigen [93]. In contrast, cystic fibrosis patients who were treated with clarithromycin (250 mg/day) and followed for a year showed a sustained increase in the ex vivo proliferative responses of peripheral blood lymphocytes activated with the T-cell mitogen, phytohemagglutinin [94], possibly reflecting transient inhibitory effects of the macrolides.
The effects of macrolides on cytokine production by T-lymphocytes have also been described in a number of studies. In their study, Pukhalsky et al. reported reversal of the serum IFN-γ/IL-4 ratio in cystic fibrosis patients treated with clarithromycin, compatible with a potentially beneficial elevation in the Th1/Th2 ratio [94]. Others also reported that roxithromycin and clarithromycin increased the Th1/Th2 ratio by decreasing production of IL-4 and IL-5, without affecting IL-2 and IFN-γ levels in several experimental systems, including (i) T cells isolated from the blood of healthy and allergic rhinitis subjects [95], (ii) house dust mite antigen-induced responses of peripheral blood lymphocytes of mite-sensitive bronchial asthma patients [93], and (iii) mononuclear leucocytes, isolated from the blood of healthy donors and stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin [96]. In contrast to these findings, Park et al. reported that patients with diffuse panbronchiolitis, receiving long-term treatment with erythromycin, showed decreased levels of IL-2 and IFN-γ, in the setting of increased levels of IL-4, IL-5, and IL-13 in the bronchoalveolar lavage fluid, suggesting a shift from Th1 to Th2 cytokine production following treatment with the macrolide [97]. Inhibition of the production of cytokines by T-lymphocytes by macrolides was also demonstrated in various other studies [91, 92, 98].
T-cell chemotaxis and apoptosis are also affected by treatment with macrolides. Th1, Th2, but not T regulatory cells, treated with roxithromycin, elicited reduced chemotactic responses to the chemokines IP10 (IFN-γ-inducible protein 10) and TARC (thymus- and activation-regulated chemokine) [99]. In addition, erythromycin, clarithromycin, azithromycin, and josamycin have been reported to induce apoptosis in lymphocytes, potentially reducing the number of lymphocytes in the lungs of patients with chronic respiratory tract diseases [90, 100–102].
Apart from effects on T cells, macrolides also appear to affect B-lymphocytes, specifically the expression of co-stimulatory molecules. Asano et al. reported that treatment of B-lymphocytes isolated from BALB/c mice spleens with roxithromycin (5.0 μg/mL) resulted in significant suppression of the expression of the costimulatory molecules, CD40, CD80, and CD86, induced by antigenic stimulation in vitro [103]. The anti-inflammatory interactions of macrolides with cells of the adaptive immune system are shown in Table 3.
Table 3.
The anti-inflammatory effects of macrolides on T- and B-lymphocytes.
| Cellular target | Altered function | Mechanisms |
|---|---|---|
| T-lymphocytes | ↓ Proliferation | Interference with (i) expression of NFκB,(ii) cellular JNK & ERK activity, and (iii) IFN-γ levels (enhancement may contribute to anti-proliferative activity) |
| T-lymphocytes | ↓ Cytokines of either Th1 (IL-2, TNF-α, IFN-γ), Th2 (IL-4, IL-5, IL-10, IL-13) or both cell types | Interference with cellular JNK and ERK activity |
| T-lymphocytes | ↓ Chemotaxis | Interference with F-actin polymerization and Ca2+ influx |
| T-lymphocytes | ↑ Apoptosis | Interference with (i) NF-κB activity,(ii) Bcl-xL expression, and (iii) Fas-Fas ligand pathway |
| B-lymphocytes | ↓ Costimulatory molecules (CD40, CD80, CD86) | — |
Abbreviations: NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; JNK: c-Jun N-terminal kinases; ERK: extracellular-signal-regulated kinases; Bcl-xL: B-cell lymphoma-extra large.
From a mechanistic perspective, these immunomodulatory activities of macrolides appear to be polymodal. Nonetheless, the weight of evidence favours inhibition of extracellular signal-regulated kinase 1/2 (ERK 1/2) phosphorylation and NF-κB activation as being the predominant mechanisms [104, 105].
4. Immunolides
The clinical efficacy of macrolides in the therapy of apparently nonmicrobial chronic inflammatory diseases of the airways has triggered the design and development of a novel class of macrolides, known as immunolides, which are attenuated with respect to antimicrobial activity in the setting of retention of anti-inflammatory properties [56, 106]. These include (i) 9- (S)-dihydroerythromycin derivatives which have been demonstrated to possess impressive anti-inflammatory activity in a murine model of phorbol ester-induced ear oedema [107], and (ii) more recently, the EM900 series of novel 12-membered, erythromycin-A-derived nonantibiotic macrolides [108]. EM900 was found to promote monocyte to macrophage differentiation, while suppressing activation of NF-κB and IL-1β, IL-8, and TNF-α gene expression in a human airway epithelial cell line (A549) activated with IL-1β, as well as mucin (muc5ac) gene expression by HM3-muc5ac cells [58]. Although promising, the development of immunolides remains in the preclinical stages. Nonetheless, it is our belief that it is the combination of antimicrobial and immunomodulatory properties, as described previously, that is most likely to confer optimum anti-inflammatory activity on the macrolide/azalide/ketolide group of antibiotics.
5. Clinical Conditions for Which Macrolides Are Used Primarily for Their Anti-Inflammatory, Immunomodulatory Properties
Many of the medical conditions for which macrolides are used primarily for their alternative properties, rather than their antimicrobial activity, are chronic disorders of the airway, of both the upper and lower respiratory tract, in which inflammation plays a major pathogenic role [109–112]. While in some of these disorders, such as DPB and CF, evidence for macrolide use is well accepted so that these agents have been included internationally as part of the standard of care, in other conditions, however, the evidence is somewhat less well established, and here these agents are used much more selectively, and particularly in cases that are not responding adequately to more standard therapy. The alternative mechanisms by which macrolides appear to have benefit mostly relate to the cytoprotective effects of these agents on human-ciliated epithelium, their anti-inflammatory, immunomodulatory activity, and their inhibitory activity against quorum sensing mechanisms of a number of important respiratory tract pathogens as mentioned previously [69, 104, 110, 111, 113–116]. Table 4 indicates some of the more common conditions for which macrolide use has been considered. Hereinafter are brief summaries of the evidence for the possible benefits and/or roles of macrolides in various medical conditions, based on an overview of appropriate scientific studies and reviews.
Table 4.
Conditions for which macrolide use may be beneficial, primarily as a result of their anti-inflammatory, immunomodulatory activity.
| (i) Diffuse panbronchiolitis |
| (ii) Cystic fibrosis (CF) |
| (iii) Non-CF bronchiectasis |
| (iv) Bronchiolitis obliterans |
| (v) Chronic obstructive pulmonary disease |
| (vi) Asthma |
| (vii) Pneumonia |
5.1. Diffuse Panbronchiolitis (DPB)
DPB is a chronic inflammatory disorder of the airway occurring in many population groups, but being most common among individuals of Japanese origin [109–112]. The major presentation is with cough, sputum production, and progressive shortness of breath, and patients very frequently become colonised with pseudomonal isolates. Without any treatment the outcome of DPB is dismal. Chronic low-dose macrolide therapy is the treatment of choice and has had a major positive impact on the natural history of this condition [109–112, 117–130].
5.2. Cystic Fibrosis (CF)
CF is an autosomally recessive inherited disorder occurring predominantly in Caucasian populations in which abnormalities in epithelial cell ion transport occur as a consequence of defects in the CF transmembrane regulator, resulting in increased sputum viscosity, stasis of secretions, airway infection and inflammation, and progressive bronchiectasis. A myriad of studies has been conducted in the past 10 years evaluating the possible role of long-term macrolide therapy in this condition [94, 110–112, 131–153]. When evaluating these as a whole there is clear-cut evidence that long-term macrolide treatment has benefit with regard to clinically relevant end-points in patients with CF and macrolide therapy features prominently in guidelines for its management, particularly in those cases infected with Pseudomonas aeruginosa who have associated deterioration in lung function. It is interesting to note that the mechanisms of action of macrolides in such CF patients appear to relate not only to their antineutrophil, anti-inflammatory activities but also to their detrimental effects on the biology of P. aeruginosa, which have been well characterised [94, 110–112, 130–153].
5.3. Non-CF Bronchiectasis
Bronchiectasis is a condition most commonly occurring as a consequence of chronic airway infection and inflammation. In this disorder, airway obstruction mainly associated with bacterial infection, and its associated airway inflammation, leads to a “vicious circle” of chronic infection and inflammation with progressive damage to the ciliated epithelium lining the airways and subsequently its underlying structures. The condition is associated not only with airway disease punctuated by recurrent acute infective exacerbations but also with chronic systemic debility leading to considerable morbidity and even mortality. Since chronic airway inflammation is central to its pathogenesis and few other therapies have been shown to alter the natural course of the condition, it is not surprising that anti-inflammatory therapies of all sorts have been tried in this condition, of which the macrolides appear to be the most promising [36, 154–177]. Interest in macrolide use for non-CF bronchiectasis was developed following their successful use in patients with CF. Beneficial effects of long-term macrolide use for non-CF bronchiectasis have been found in small clinical trials. In most of these studies there was clear evidence of a decrease in sputum volume and, in some, a decrease in exacerbation frequency. Furthermore, in a small number in which this was tested there was an improvement in lung function parameters or a decrease in airway hyperreactivity. The common recommendation for this condition is to try macrolide therapy in selected cases for 3–6 months and to discontinue treatment if there is no clear evidence of benefit to the patient in terms of improvement in quality of life or reduction in exacerbation frequency.
5.4. Bronchiolitis Obliterans (BOs)
BO is one of the manifestations of chronic rejection following lung or bone marrow transplant and is a major cause of limited survival and death in lung transplant recipients. Although the exact pathogenesis has still to be unravelled, it appears to result as a consequence of repeated insults to the airways. More recently there has been considerable interest in using macrolides for this serious condition for which other therapies have been rather disappointing or are associated with considerable side-effects [178–189]. Studies have been undertaken to investigate not only the effects of macrolides as therapy for this condition but also, more recently, its prevention. In reviewing the various therapeutic studies, it has been said that there are differences in the clinical spectrum and macrolide response of patients with BO and that those cases associated with a predominantly neutrophilic pathogenesis are macrolide responsive, while those associated with a predominantly fibroproliferative response (so-called traditional BO) are not.
5.5. Chronic Obstructive Pulmonary Disease (COPD)
In more recent definitions of COPD, due recognition is given to the fact that in this condition there is an abnormal inflammatory process in the airways, which, although initially is most commonly associated with cigarette smoking, at some stage becomes self-perpetuating and contributes to the progressive deterioration that may be seen in patients with COPD, even in those that quit smoking. While macrolides may be used for the antibiotic management of acute exacerbations of COPD, studies have also been conducted wherein these agents are used for their anti-inflammatory, immunomodulatory activities and their effects on mucus secretion. In most of these studies a reduction in sputum production, as well as improvement in the quality of the sputum, has been noted, while in some an improvement in quality of life, various clinical end-points, and occasionally in lung function parameters has been seen. Importantly, some studies have suggested that macrolide therapy may alter the course of COPD by reducing both the number and the duration of acute exacerbations [68, 109, 190–199].
5.6. Asthma
It has been recognised for a number of years that asthma is a chronic inflammatory disorder of the airways, the inflammation being mediated by a variety of cells and mediators which are responsible for the manifestations including the symptoms, the lung function abnormalities, and the airway hyperresponsiveness. Therapy is therefore primarily with anti-inflammatory agents, particularly inhaled corticosteroids, but a number of the other drugs used in asthma treatment have also been recognised to have anti-inflammatory activity. While much of the airway inflammation may be driven by allergic/atopic responses, it has also been suggested that chronic lower respiratory tract infection with Mycoplasma pneumoniae and Chlamydia pneumoniae, both microorganisms that are responsive to macrolide therapy, may initiate airway inflammation and asthma and is therefore potentially amenable to macrolide therapy. All of these considerations provide the rationale for the use of macrolides in asthma, in the hope of achieving more effective asthma control. Although a number of studies have been undertaken over more recent years using different macrolides, with some showing modest benefits, the overall data suggests that there is no role for long-term macrolide therapy in asthma, although such treatment may be of benefit in some subgroups of patients, such as those described previously [200–214].
5.7. Pneumonia
Antibiotic therapy in patients with pneumonia is short course, aimed at treating the infection and eradicating the microorganism. However, there is still considerable ongoing debate as to what antibiotic regimen constitutes optimal therapy in hospitalised cases with community-acquired pneumonia (CAP), including those that require intensive care unit (ICU) admission. A myriad of studies in more severely ill-hospitalised patients with CAP has suggested that the outcome is improved by using combination antibiotic therapy, most commonly with the addition of a macrolide to standard beta-lactam therapy [215–226]. This understanding needs to be counterbalanced by additional studies suggesting that the outcome is similar when comparing fluoroquinolone monotherapy to the beta-lactam/macrolide combination in noncritically ill-hospitalised patients [227–229]. Thus for cases not in the ICU, most guidelines recommend either option, whereas in ICU patients, combination therapy is always recommended irrespective of which of these agents is used. Interestingly, in one study in intubated patients in the ICU, the outcome was better with the use of the macrolide rather than the fluoroquinolone combination [226]. The reason that combination therapy with macrolides is associated with an improved outcome in patients with CAP is uncertain and may be multifactorial; however, many believe that it may relate to the anti-inflammatory immunomodulatory effects of these agents [229]. Two recent studies appear to support this contention [230, 231]. In the first study, macrolide use was associated with decreased mortality in patients with CAP and severe sepsis even when the infection was due to macrolide-resistant pathogens. Furthermore, a placebo-controlled, randomised, clinical trial, undertaken to investigate whether patients with sepsis and ventilator-associated pneumonia (VAP), predominantly due to Gram-negative pathogens, had improved outcome when a macrolide was added to standard antibiotic therapy, demonstrated that clarithromycin accelerated the resolution of VAP and the weaning from mechanical ventilation and delayed death in those that ultimately died of sepsis. In addition, in a very recent review of the literature, Kovaleva, et al. concluded that macrolides appear to attenuate the inflammatory response during CAP [232]. In support of this contention, Walkey and Weiner have reported, also very recently, that patients with acute lung injury (ALI), predominantly associated with pneumonia, who were treated with macrolides, had a significantly lower 180-day mortality and shorter time to successful discontinuation of mechanical ventilation relative to those patients treated with fluoroquinolones or cephalosporins [233].
5.8. Upper Respiratory Tract Disorders
A number of studies have also been undertaken investigating the use of macrolides in upper airway conditions, such as chronic rhinosinusitis, and appear to show promise [234–244]. Such studies clearly suffer from the methodological issues discussed hereinafter and need to be repeated in appropriate fashion before conclusions can be drawn about the value of macrolides and their use in upper airway diseases, although recommendations for macrolide use do appear in many of the international guidelines on rhinosinusitis management, in certain circumstances. As in many of the conditions already discussed, these potential benefits are thought to relate to the anti-inflammatory, immunomodulatory activity of macrolides and their effects on the virulence of and tissue damage caused by the chronic colonising bacteria [234–244].
6. Conclusions
It is clear from the various studies that macrolides have a clear-cut role in conditions such as DPB and CF, and possibly additional beneficial effects on morbidity, and possibly even mortality, in various other airway disorders. Furthermore, additional studies have also uncovered potential beneficial effects in various disorders unrelated to the airway. Many of these studies suffer from the fact that they are limited in terms of size, patient numbers, and length of treatment and follow-up. It is therefore clear that in many of these conditions further studies are needed in order to clarify such questions as in which patients these agents should be used, which macrolide drugs is/are best, what dosing schedules are appropriate, for how long should treatment be continued, and what are the long-term side-effects?
References
- 1.Wikipedia, the free encyclopedia. Macrolide, 2011, http://en.wikipedia.org/wiki/Macrolide.
- 2.Zuckerman JM, Qamar F, Bono BR. Review of macrolides (azithromycin, clarithromycin), ketolides (telithromycin) and glycylcyclines (tigecycline) Medical Clinics of North America. 2011;95(4):761–791. doi: 10.1016/j.mcna.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. Journal of Antimicrobial Chemotherapy. 1990;25(supplement):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 4.Fraschini F, Scaglione F, Pintucci G, Maccarinelli G, Dugnani S, Demartini G. The diffusion of clarithromycin and roxithromycin into nasal mucosa, tonsil and lung in humans. Journal of Antimicrobial Chemotherapy. 1991;27:61–65. doi: 10.1093/jac/27.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman JM. Macrolides and ketolides: azithromycin, clarithromycin, telithromycin. Infectious Disease Clinics of North America. 2004;18(3):621–649. doi: 10.1016/j.idc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Mandell GL. Delivery of antibiotics by phagocytes. Clinical Infectious Diseases. 1994;19(5):922–925. doi: 10.1093/clinids/19.5.922. [DOI] [PubMed] [Google Scholar]
- 7.Tenson T, Lovmar M, Ehrenberg M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. Journal of Molecular Biology. 2003;330(5):1005–1014. doi: 10.1016/s0022-2836(03)00662-4. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser G. Protein synthesis inhibitors: macrolides mechanism of action animation. Classification of agents Pharmamotion. The Community College of Baltimore County, 2011, http://en.wikipedia.org/wiki/Protein_synthesis_inhibitor.
- 9.Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Current Molecular Medicine. 2008;8(6):497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 10.Tsujimoto H, Ono S, Efron PA, Scumpia PO, Moldawer LL, Mochizuki H. Role of toll-like receptors in the development of sepsis. Shock. 2008;29(3):315–321. doi: 10.1097/SHK.0b013e318157ee55. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Kimmitt PT, Harwood CR, Barer MR. Induction of type 2 Shiga toxin synthesis in Escherichia coli 0157 by 4-quinolones. The Lancet. 1999;353(9164):1588–1589. doi: 10.1016/s0140-6736(99)00621-2. [DOI] [PubMed] [Google Scholar]
- 13.Murakami J, Kishi K, Hirai K, Hiramatsu K, Yamasaki T, Nasu M. Macrolides and clindamycin suppress the release of Shiga-like toxins from Escherichia coli O157:H7 in vitro . International Journal of Antimicrobial Agents. 2000;15(2):103–109. doi: 10.1016/s0924-8579(00)00126-6. [DOI] [PubMed] [Google Scholar]
- 14.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. New England Journal of Medicine. 2000;342(26):1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus . Journal of Infectious Diseases. 2007;195(2):202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 16.Dumitrescu O, Badiou C, Bes M, et al. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clinical Microbiology and Infection. 2008;14(4):384–388. doi: 10.1111/j.1469-0691.2007.01947.x. [DOI] [PubMed] [Google Scholar]
- 17.Serna A, IV, Boedeker EC. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Current Opinion in Gastroenterology. 2008;24(1):38–47. doi: 10.1097/MOG.0b013e3282f2dfb8. [DOI] [PubMed] [Google Scholar]
- 18.McGannon CM, Fuller CA, Weiss AA. Different classes of antibiotics differentially influence shiga toxin production. Antimicrobial Agents and Chemotherapy. 2010;54(9):3790–3798. doi: 10.1128/AAC.01783-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spreer A, Kerstan H, Böttcher T, et al. Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrobial Agents and Chemotherapy. 2003;47(8):2649–2654. doi: 10.1128/AAC.47.8.2649-2654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Böttcher T, Ren H, Goiny M, et al. Clindamycin is neuroprotective in experimental Streptococcus pneumoniae meningitis compared with ceftriaxone. Journal of Neurochemistry. 2004;91(6):1450–1460. doi: 10.1111/j.1471-4159.2004.02837.x. [DOI] [PubMed] [Google Scholar]
- 21.Karlström A, Boyd KL, English BK, McCullers JA. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. Journal of Infectious Diseases. 2009;199(3):311–319. doi: 10.1086/596051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spreer A, Lugert R, Stoltefaut V, Hoecht A, Eiffert H, Nau R. Short-term rifampicin pretreatment reduces inflammation and neuronal cell death in a rabbit model of bacterial meningitis. Critical Care Medicine. 2009;37(7):2253–2258. doi: 10.1097/CCM.0b013e3181a036c0. [DOI] [PubMed] [Google Scholar]
- 23.Anderson R, Steel HC, Cockeran R, et al. Clarithromycin alone and in combination with ceftriaxone inhibits the production of pneumolysin by both macrolide-susceptible and macrolide-resistant strains of Streptococcus pneumoniae . Journal of Antimicrobial Chemotherapy. 2007;59(2):224–229. doi: 10.1093/jac/dkl479. [DOI] [PubMed] [Google Scholar]
- 24.Anderson R, Steel HC, Cockeran R, et al. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro . Journal of Antimicrobial Chemotherapy. 2007;60(5):1155–1158. doi: 10.1093/jac/dkm338. [DOI] [PubMed] [Google Scholar]
- 25.Hirst RA, Mohammed BJ, Mitchell TJ, Andrew PW, O’Callaghan C. Streptococcus pneumoniae-induced inhibition of rat ependymal cilia is attenuated by antipneumolysin antibody. Infection and Immunity. 2004;72(11):6694–6698. doi: 10.1128/IAI.72.11.6694-6698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagrou K, Peetermans WE, Jorissen M, Verhaegen J, Van Damme J, Van Eldere J. Subinhibitory concentrations of erythromycin reduce pneumococcal adherence to respiratory epithelial cells in vitro . Journal of Antimicrobial Chemotherapy. 2000;46(5):717–723. doi: 10.1093/jac/46.5.717. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda Y, Yanagihara K, Higashiyama Y, et al. Effects of macrolides on pneumolysin of macrolide-resistant Streptococcus pneumoniae . European Respiratory Journal. 2006;27(5):1020–1025. doi: 10.1183/09031936.06.00116805. [DOI] [PubMed] [Google Scholar]
- 28.Cockeran R, Steel HC, Wolter N, et al. Effects of clarithromycin at sub-minimum inhibitory concentrations on early ermB gene expression, metabolic activity and growth of an ermB-expressing, macrolide-resistant strain of Streptococcus pneumoniae . Open Journal of Respiratory Diseases. 2012;2:1–8. [Google Scholar]
- 29.Yasuda Y, Kasahara K, Mizuno F, Nishi K, Mikasa K, Kita E. Roxithromycin favorably modifies the initial phase of resistance against infection with macrolide-resistant Streptococcus pneumoniae in a murine pneumonia model. Antimicrobial Agents and Chemotherapy. 2007;51(5):1741–1752. doi: 10.1128/AAC.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura S, Yanagihara K, Araki N, et al. Efficacy of clarithromycin against experimentally induced pneumonia caused by clarithromycin-resistant Haemophilus influenzae in mice. Antimicrobial Agents and Chemotherapy. 2010;54(2):757–762. doi: 10.1128/AAC.00524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bui KQ, Banevicius MA, Nightingale CH, Quintiliani R, Nicolau DP. In vitro and in vivo influence of adjunct clarithromycin on the treatment of mucoid Pseudomonas aeruginosa . Journal of Antimicrobial Chemotherapy. 2000;45(1):57–62. doi: 10.1093/jac/45.1.57. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka G, Shigeta M, Komatsuzawa H, Sugai M, Suginaka H, Usui T. Effect of clarithromycin on Pseudomonas aeruginosa biofilms. Chemotherapy. 2000;46(1):36–42. doi: 10.1159/000007254. [DOI] [PubMed] [Google Scholar]
- 33.Tateda K, Standiford TJ, Pechere JC, Yamaguchi K. Regulatory effects of macrolides on bacterial virulence: potential role as quorum-sensing inhibitors. Current Pharmaceutical Design. 2004;10(25):3055–3065. doi: 10.2174/1381612043383377. [DOI] [PubMed] [Google Scholar]
- 34.Wozniak DJ, Keyser R. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa . Chest. 2004;125(2, supplement 12):62S–69S. doi: 10.1378/chest.125.2_suppl.62s. [DOI] [PubMed] [Google Scholar]
- 35.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cellular Microbiology. 2009;11(7):1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 36.Nalca Y, Jänsch L, Bredenbruch F, Geffers R, Buer J, Häussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrobial Agents and Chemotherapy. 2006;50(5):1680–1688. doi: 10.1128/AAC.50.5.1680-1688.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bala A, Kumar R, Harjai K. Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. Journal of Medical Microbiology. 2011;60(3):300–306. doi: 10.1099/jmm.0.025387-0. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y, Chai D, Wang R, Bai N, Liang BB, Liu Y. Effectiveness and safety of macrolides in cystic fibrosis patients: a meta-analysis and systematic review. Journal of Antimicrobial Chemotherapy. 2011;66(5):968–978. doi: 10.1093/jac/dkr040. [DOI] [PubMed] [Google Scholar]
- 39.Renna M, Schaffner C, Brown K, et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. Journal of Clinical Investigation. 2011;121(9):3554–3563. doi: 10.1172/JCI46095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oishi K, Sonoda F, Kobayashi S, et al. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infectious Immunology. 1994;62(10):4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanaudenaerde BM, Wuyts WA, Geudens N, et al. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. American Journal of Transplantation. 2007;7(1):76–82. doi: 10.1111/j.1600-6143.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 42.Brennan S, Cooper D, Sly PD. Directed neutrophil migration to IL-8 is increased in cystic fibrosis: a study of the effect of erythromycin. Thorax. 2001;56(1):62–64. doi: 10.1136/thorax.56.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaryo T, Oishi K, Yoshimine H, Tsuchihashi Y, Matsushima K, Nagatake T. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrobial Agents and Chemotherapy. 2003;47(1):48–53. doi: 10.1128/AAC.47.1.48-53.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodge S, Hodge G, Jersmann H, et al. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2008;178(2):139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 45.Kita E, Sawaki M, Nishikawa F, et al. Enhanced interleukin production after long-term administration of erythromycin stearate. Pharmacology. 1990;41(4):177–183. doi: 10.1159/000138716. [DOI] [PubMed] [Google Scholar]
- 46.Kamemoto A, Ara T, Hattori T, Fujinami Y, Imamura Y, Wang PL. Macrolide antibiotics like azithromycin increase lipopolysaccharide-induced IL-8 production by human gingival fibroblasts. European Journal of Medical Research. 2009;14(7):309–314. doi: 10.1186/2047-783X-14-7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailly S, Pocidalo JJ, Fay M, Gougerot-Pocidalo MA. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrobial Agents and Chemotherapy. 1991;35(10):2016–2019. doi: 10.1128/aac.35.10.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konno SI, Adachi M, Asano K, Kawazoe T, Okamoto KI, Takahashi T. Influences of roxithromycin on cell-mediated immune responses. Life Sciences. 1992;51(10):PL107–PL112. doi: 10.1016/0024-3205(92)90493-9. [DOI] [PubMed] [Google Scholar]
- 49.Suzaki H, Asano K, Ohki S, Kanai K, Mizutani T, Hisamitsu T. Suppressive activity of a macrolide antibiotic, roxithromycin, on pro- inflammatory cytokine production in vitro and in vivo. Mediators of Inflammation. 1999;8(4-5):199–204. doi: 10.1080/09629359990351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamasawa H, Oshikawa K, Ohno S, Sugiyama Y. Macrolides inhibit epithelial cell-mediated neutrophil survival by modulating granulocyte macrophage colony-stimulating factor release. American Journal of Respiratory Cell and Molecular Biology. 2004;30(4):569–575. doi: 10.1165/rcmb.2003-0105OC. [DOI] [PubMed] [Google Scholar]
- 51.Bosnar M, Bošnjak B, Ćužić S, et al. Azithromycin and clarithromycin inhibit lipopolysaccharide-induced murine pulmonary neutrophilia mainly through effects on macrophage-derived granulocyte-macrophage colony-stimulating factor and interleukin-1 beta. Journal of Pharmacology Experimental Therapy. 2009;331(1):104–113. doi: 10.1124/jpet.109.155838. [DOI] [PubMed] [Google Scholar]
- 52.Meyer M, Huaux F, Gavilanes X, et al. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2009;41(5):590–602. doi: 10.1165/rcmb.2008-0155OC. [DOI] [PubMed] [Google Scholar]
- 53.Abe S, Nakamura H, Inoue S, et al. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2000;22(1):51–60. doi: 10.1165/ajrcmb.22.1.3400. [DOI] [PubMed] [Google Scholar]
- 54.Kikuchi T, Hagiwara K, Honda Y, et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. Journal of Antimicrobial Chemotherapy. 2002;49(5):745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 55.Desaki M, Okazaki H, Sunazuka T, Omura S, Yamamoto K, Takizawa H. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-kappaB activation. Antimicrobial Agents and Chemotherapy. 2004;48(5):1581–1585. doi: 10.1128/AAC.48.5.1581-1585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinkai M, Foster GH, Rubin BK. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. American Journal of Physiology. 2006;290(1):L75–L85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- 57.Shinkai M, Tamaoki J, Kobayashi H, et al. Clarithromycin delays progression of bronchial epithelial cells from G 1 phase to S phase and delays cell growth via extracellular signal-regulated protein kinase suppression. Antimicrobial Agents and Chemotherapy. 2006;50(5):1738–1744. doi: 10.1128/AAC.50.5.1738-1744.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cigana C, Assael BM, Melotti P. Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrobial Agents and Chemotherapy. 2007;51(3):975–981. doi: 10.1128/AAC.01142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bosnar M, Čužić S, Bošnjak B, et al. Azithromycin inhibits macrophage interleukin-1β production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. International Immunopharmacology. 2011;11(4):424–434. doi: 10.1016/j.intimp.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Otsu K, Ishinaga H, Suzuki S, et al. Effects of a novel nonantibiotic macrolide, EM900, on cytokine and mucin gene expression in a human airway epithelial cell line. Pharmacology. 2009;88(5-6):3272–332. doi: 10.1159/000334339. [DOI] [PubMed] [Google Scholar]
- 61.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. Journal of Leukocyte Biology. 2002;72(5):847–855. [PubMed] [Google Scholar]
- 62.Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochemical and Biophysical Research Communications. 2006;350(4):977–982. doi: 10.1016/j.bbrc.2006.09.132. [DOI] [PubMed] [Google Scholar]
- 63.Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacology and Therapeutics. 2008;117(3):393–405. doi: 10.1016/j.pharmthera.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Ikegaya S, Inai K, Iwasaki H, Naiki H, Ueda T. Azithromycin reduces tumor necrosis factor-alpha production in lipopolysaccharide-stimulated THP-1 monocytic cells by modification of stress response and p38 MAPK pathway. Journal of Chemotherapy. 2009;21(4):396–402. doi: 10.1179/joc.2009.21.4.396. [DOI] [PubMed] [Google Scholar]
- 65.Yamauchi K, Shibata Y, Kimura T, et al. Azithromycin suppresses interleukin-12p40 expression in lipopolysaccharide and interferon-γ stimulated macrophages. International Journal of Biological Sciences. 2009;5(7):667–678. doi: 10.7150/ijbs.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reato G, Cuffini AM, Tullio V, et al. Immunomodulating effect of antimicrobial agents on cytokine production by human polymorphonuclear neutrophils. International Journal of Antimicrobial Agents. 2004;23(2):150–154. doi: 10.1016/j.ijantimicag.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Parnham MJ, Culić O, Eraković V, et al. Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short-term azithromycin treatment. European Journal of Pharmacology. 2005;517(1-2):132–143. doi: 10.1016/j.ejphar.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 68.Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. The American Journal of Medicine. 2004;117(supplement 9):5S–11S. doi: 10.1016/j.amjmed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 69.Feldman C, Anderson R. The cytoprotective interactions of antibiotics with human ciliated airway epithelium. In: Rubin BK, Tamaoki J, editors. Antibiotics as Anti-Inflammatory and Immunomodulatory Agents. Basel, Switzerland: Birkhauser; 2005. pp. 49–63. [Google Scholar]
- 70.Oda H, Kadota JI, Kohno S, Hara K. Leukotriene B4 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Chest. 1995;108(1):116–122. doi: 10.1378/chest.108.1.116. [DOI] [PubMed] [Google Scholar]
- 71.Hashimoto N, Kawabe T, Hara T, et al. Effect of erythromycin on matrix metalloproteinase-9 and cell migration. Journal of Laboratory and Clinical Medicine. 2001;137(3):176–183. doi: 10.1067/mlc.2001.112726. [DOI] [PubMed] [Google Scholar]
- 72.Kanai K, Asano K, Hisamitsu T, Suzaki H. Suppresion in matrix metalloproteinase production from nasal fibroblasts by macrolide antibiotics in vitro . European Respiratory Journal. 2004;23(5):671–678. doi: 10.1183/09031936.04.00057104. [DOI] [PubMed] [Google Scholar]
- 73.Kanai KI, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase-9 production from neutrophils by a macrolide antibiotic, roxithromycin, in vitro . Mediators of Inflammation. 2004;13(5-6):313–319. doi: 10.1155/S0962935104000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yasutomi M, Ohshima Y, Omata N, et al. Erythromycin differentially inhibits lipopolysaccharide- or poly(I:C)-induced but not peptidoglycan-induced activation of human monocyte-derived dendritic cells. Journal of Immunology. 2005;175(12):8069–8076. doi: 10.4049/jimmunol.175.12.8069. [DOI] [PubMed] [Google Scholar]
- 75.Park JY, Kim YH, Ja YL, et al. Macrolide-affected Toll-like receptor 4 expression from Helicobacter pylori-infected monocytes does not modify interleukin-8 production. FEMS Immunology and Medical Microbiology. 2005;44(2):171–176. doi: 10.1016/j.femsim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Mitsuyama T, Tanaka T, Hidaka K, Abe M, Hara N. Inhibition by erythromycin of superoxide anion production by human polymorphonuclear leukocytes through the action of cyclic AMP-dependent protein kinase. Respiration. 1995;62(5):269–273. doi: 10.1159/000196461. [DOI] [PubMed] [Google Scholar]
- 77.Anderson R, Theron AJ, Feldman C. Membrane-stabilizing, anti-inflammatory interactions of macrolides with human neutrophils. Inflammation. 1996;20(6):693–705. doi: 10.1007/BF01488805. [DOI] [PubMed] [Google Scholar]
- 78.Montenez JP, Van Bambeke F, Piret J, Brasseur R, Tulkens PM, Mingeot-Leclercq MP. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine [dirithromycin], and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicology and Applied Pharmacology. 1999;156(2):129–140. doi: 10.1006/taap.1999.8632. [DOI] [PubMed] [Google Scholar]
- 79.Munić V, Banjanac M, Koštrun S, et al. Intensity of macrolide anti-inflammatory activity in J774A.1 cells positively correlates with cellular accumulation and phospholipidosis. Pharmacological Research. 2011;64(3):298–307. doi: 10.1016/j.phrs.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 80.Tamaoki J, Kondo M, Kohri K, Aoshiba K, Tagaya E, Nagai A. Macrolide antibiotics protect against immune complex-induced lung injury in rats: role of nitric oxide from alveolar macrophages. Journal of Immunology. 1999;163(5):2909–2915. [PubMed] [Google Scholar]
- 81.Asano K, Kamakazu K, Hisamitsu T, Suzaki H. Suppressive activity of macrolide antibiotics on nitric oxide production from nasal polyp fibroblasts in vitro . Acta Oto-Laryngologica. 2003;123(9):1064–1069. doi: 10.1080/00016480310002519. [DOI] [PubMed] [Google Scholar]
- 82.Anderson R, Tintinger G, Cockeran R, Potjo M, Feldman C. Beneficial and harmful interactions of antibiotics with microbial pathogens and the host innate immune system. Pharmaceuticals. 2010;3(5):1694–1710. doi: 10.3390/ph3051694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeyama K, Tamaoki J, Chiyotani A, Tagaya E, Konno K. Effect of macrolide antibiotics on ciliary motility in rabbit airway epithelium in-vitro. Journal of Pharmacy and Pharmacology. 1993;45(8):756–758. doi: 10.1111/j.2042-7158.1993.tb07104.x. [DOI] [PubMed] [Google Scholar]
- 84.Feldman C, Anderson R. Non-antimicrobial activity of macrolides: therapeutic potential in chronic inflammatory airway disorders. South African Journal of Epidemiology Infections. 2009;24(4):21–26. [Google Scholar]
- 85.Shimizu T, Shimizu S, Hattori R, Gabazza EC, Majima Y. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):581–587. doi: 10.1164/rccm.200212-1437OC. [DOI] [PubMed] [Google Scholar]
- 86.Ribeiro CMP, Hurd H, Wu Y, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS ONE. 2009;4(6) doi: 10.1371/journal.pone.0005806. Article ID e5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanabe T, Kanoh S, Tsushima K, et al. Clarithromycin inhibits interleukin-13-induced goblet cell hyperplasia in human airway cells. American Journal of Respiratory Cell and Molecular Biology. 2011;45(5):1075–1083. doi: 10.1165/rcmb.2010-0327OC. [DOI] [PubMed] [Google Scholar]
- 88.Imamura Y, Yanagihara K, Mizuta Y, et al. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrobial Agents and Chemotherapy. 2004;48(9):3457–3461. doi: 10.1128/AAC.48.9.3457-3461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feldman C, Anderson R, Theron AJ, Ramafi G, Cole PJ, Wilson R. Roxithromycin, clarithromycin, and azithromycin attenuate the injurious effects of bioactive phospholipids on human respiratory epithelium in vitro . Inflammation. 1997;21(6):655–665. doi: 10.1023/a:1027342424205. [DOI] [PubMed] [Google Scholar]
- 90.Wu L, Zhang W, Tian L, Bao K, Li P, Lin J. Immunomodulatory effects of erythromycin and its derivatives on human T-lymphocyte in vitro . Immunopharmacology and Immunotoxicology. 2007;29(3-4):587–596. doi: 10.1080/08923970701692841. [DOI] [PubMed] [Google Scholar]
- 91.Ishida Y, Abe Y, Harabuchi Y. Effects of macrolides on antigen presentation and cytokine production by dendritic cells and T lymphocytes. International Journal of Pediatric Otorhinolaryngology. 2007;71(2):297–305. doi: 10.1016/j.ijporl.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 92.Hiwatashi Y, Maeda M, Fukushima H, et al. Azithromycin suppresses proliferation, interleukin production and mitogen-activated protein kinases in human peripheral-blood mononuclear cells stimulated with bacterial superantigen. Journal of Pharmacy and Pharmacology. 2011;63(10):1320–1326. doi: 10.1111/j.2042-7158.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- 93.Noma T, Aoki K, Hayashi M, Yoshizawa I, Kawano Y. Effect of roxithromycin on T lymphocyte proliferation and cytokine production elicited by mite antigen. International Immunopharmacology. 2001;1(2):201–210. doi: 10.1016/s1567-5769(00)00023-0. [DOI] [PubMed] [Google Scholar]
- 94.Pukhalsky AL, Shmarina GV, Kapranov NI, Kokarovtseva SN, Pukhalskaya D, Kashirskaja NJ. Anti-inflammatory and immunomodulating effects of clarithromycin in patients with cystic fibrosis lung disease. Mediators of Inflammation. 2004;13(2):111–117. doi: 10.1080/09629350410001688495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asano K, Kamakazu K, Hisamitsu T, Suzaki H. Modulation of Th2 type cytokine production from human peripheral blood leukocytes by a macrolide antibiotic, roxithromycin, in vitro . International Immunopharmacology. 2001;1(11):1913–1921. doi: 10.1016/s1567-5769(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 96.Williams AC, Galley HF, Watt AM, Webster NR. Differential effects of three antibiotics on T helper cell cytokine expression. Journal of Antimicrobial Chemotherapy. 2005;56(3):502–506. doi: 10.1093/jac/dki251. [DOI] [PubMed] [Google Scholar]
- 97.Park SJ, Lee YC, Rhee YK, Lee HB. The effect of long-term treatment with erythromycin on Th1 and Th2 cytokines in diffuse panbronchiolitis. Biochemical and Biophysical Research Communications. 2004;324(1):114–117. doi: 10.1016/j.bbrc.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 98.Morikawa K, Zhang J, Nonaka M, Morikawa S. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. International Journal of Antimicrobial Agents. 2002;19(1):53–59. doi: 10.1016/s0924-8579(01)00457-5. [DOI] [PubMed] [Google Scholar]
- 99.Ito T, Ito N, Hashizume H, Takigawa M. Roxithromycin inhibits chemokine-induced chemotaxis of Th1 and Th2 cells but regulatory T cells. Journal of Dermatological Science. 2009;54(3):185–191. doi: 10.1016/j.jdermsci.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 100.Ishimatsu Y, Kadota JI, Iwashita T, et al. Macrolide antibiotics induce apoptosis of human peripheral lymphocytes in vitro . International Journal of Antimicrobial Agents. 2004;24(3):247–253. doi: 10.1016/j.ijantimicag.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 101.Mizunoe S, Kadota JI, Tokimatsu I, Kishi K, Nagai H, Nasu M. Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of Bcl-xL. International Immunopharmacology. 2004;4(9):1201–1207. doi: 10.1016/j.intimp.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 102.Kadota JI, Mizunoe S, Kishi K, Tokimatsu I, Nagai H, Nasu M. Antibiotic-induced apoptosis in human activated peripheral lymphocytes. International Journal of Antimicrobial Agents. 2005;25(3):216–220. doi: 10.1016/j.ijantimicag.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 103.Asano K, Suzuki M, Shimane T, Suzaki H. Suppression of co-stimulatory molecule expressions on splenic B lymphocytes by a macrolide antibiotic, roxithromycin in vitro . International Immunopharmacology. 2001;1(7):1385–1392. doi: 10.1016/s1567-5769(01)00070-4. [DOI] [PubMed] [Google Scholar]
- 104.Aoki Y, Kao PN. Erythromycin inhibits transcriptional activation of NF-κB, but not NFAT, through calcineurin-independent signaling in T cells. Antimicrobial Agents and Chemotherapy. 1999;43(11):2678–2684. doi: 10.1128/aac.43.11.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clinical Microbiology Reviews. 2010;23(3):590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fecik RA, Nguyen PL, Venkatraman L. Approaches to the synthesis of immunolides: selective immunomodulatory macrolides for cystic fibrosis. Current Opinion in Drug Discovery and Development. 2005;8(6):741–747. [PubMed] [Google Scholar]
- 107.Mereu A, Moriggi E, Napoletano M, et al. Design, synthesis and in vivo activity of 9-(S)-dihydroerythromycin derivatives as potent anti-inflammatory agents. Bioorganic and Medicinal Chemistry Letters. 2006;16(22):5801–5804. doi: 10.1016/j.bmcl.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 108.Sugawara A, Sueki A, Hirose T, et al. Novel 12-membered non-antibiotic macrolides from erythromycin A; EM900 series as novel leads for anti-inflammatory and/or immunomodulatory agents. Bioorganic & Medicinal Chemistry Letters. 2011;21(11):3373–3376. doi: 10.1016/j.bmcl.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Gotfried MH. Macrolides for the treatment of chronic sinusitis, asthma, and COPD. Chest. 2004;125(2, supplement):52S–61S. doi: 10.1378/chest.125.2_suppl.52s. [DOI] [PubMed] [Google Scholar]
- 110.Crosbie PAJ, Woodhead MA. Long-term macrolide therapy in chronic inflammatory airway diseases. European Respiratory Journal. 2009;33(1):171–181. doi: 10.1183/09031936.00042208. [DOI] [PubMed] [Google Scholar]
- 111.Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010;138(5):1202–1212. doi: 10.1378/chest.10-0196. [DOI] [PubMed] [Google Scholar]
- 112.Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. European Journal of Clinical Pharmacology. 2012;68(5):479–503. doi: 10.1007/s00228-011-1161-x. [DOI] [PubMed] [Google Scholar]
- 113.Tateda K, Ishii Y, Kimura S, Horikawa M, Miyairi S, Yamaguchi K. Suppression of Pseudomonas aeruginosa quorum-sensing systems by macrolides: a promising strategy or an oriental mystery? Journal of Infection and Chemotherapy. 2007;13(6):357–367. doi: 10.1007/s10156-007-0555-2. [DOI] [PubMed] [Google Scholar]
- 114.Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. International Journal of Antimicrobial Agents. 2008;31(1):12–20. doi: 10.1016/j.ijantimicag.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 115.Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics—part 2: advantages and disadvantages of long-term, low-dose macrolide therapy. Respiration. 2010;81(1):75–87. doi: 10.1159/000320320. [DOI] [PubMed] [Google Scholar]
- 116.Guillot L, Tabary O, Nathan N, Corvol H, Clement A. Macrolides: new therapeutic perspectives in lung diseases. International Journal of Biochemistry and Cell Biology. 2011;43(9):1241–1246. doi: 10.1016/j.biocel.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Nagai H, Shishido H, Yoneda R, Yamaguchi E, Tamura A, Kurashima A. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58(3-4):145–149. doi: 10.1159/000195915. [DOI] [PubMed] [Google Scholar]
- 118.Kobayashi H, Ohgaki N, Takeda H. Therapeutic possibilities for diffuse panbronchiolitis. International Journal of Antimicrobial Agents. 1993;3(1):S81–S86. doi: 10.1016/0924-8579(93)90039-8. [DOI] [PubMed] [Google Scholar]
- 119.Koyama H, Geddes DM. Erythromycin and diffuse panbronchiolitis. Thorax. 1997;52(10):915–918. doi: 10.1136/thx.52.10.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kudoh S. Erythromycin treatment in diffuse panbronchiolitis. Current Opinion in Pulmonary Medicine. 1998;4(2):116–121. doi: 10.1097/00063198-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. American Journal of Respiratory and Critical Care Medicine. 1998;157(6):1829–1832. doi: 10.1164/ajrccm.157.6.9710075. [DOI] [PubMed] [Google Scholar]
- 122.Yanagihara K, Kadoto J, Kohno S. Diffuse panbronchiolitis—pathophysiology and treatment mechanisms. International Journal of Antimicrobial Agents. 2001;18(1):S83–S87. doi: 10.1016/s0924-8579(01)00403-4. [DOI] [PubMed] [Google Scholar]
- 123.Keicho N, Kudoh S. Diffuse panbronchiolitis: role of macrolides in therapy. American Journal of Respiratory Medicine. 2002;1(2):119–131. doi: 10.1007/BF03256601. [DOI] [PubMed] [Google Scholar]
- 124.Kadota J, Mukae H, Ishii H, et al. Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respiratory Medicine. 2003;97(7):844–850. doi: 10.1016/s0954-6111(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 125.Kudoh S. Applying lessons learned in the treatment of diffuse panbronchiolitis to other chronic inflammatory diseases. The American Journal of Medicine. 2004;117(supplement 9):12S–19S. doi: 10.1016/j.amjmed.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 126.Poletti V, Chilosi M, Casoni G, Colby TV. Diffuse panbronchiolitis. Sarcoidosis Vasculitis and Diffuse Lung Diseases. 2004;21(2):94–104. [PubMed] [Google Scholar]
- 127.Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and crystic fibrosis. Journal of Antimicrobial Chemotherapy. 2004;54(1):21–28. doi: 10.1093/jac/dkh309. [DOI] [PubMed] [Google Scholar]
- 128.Azuma A, Kudoh S. Diffuse panbronchiolitis in East Asia. Respirology. 2006;11(3):249–261. doi: 10.1111/j.1440-1843.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- 129.Poletti V, Casoni G, Chilosi M, Zompatori M. Diffuse panbronchiolitis. European Respiratory Journal. 2006;28(4):862–871. doi: 10.1183/09031936.06.00131805. [DOI] [PubMed] [Google Scholar]
- 130.Yang M, Dong BR, Lu J, Lin X, Wu HM. Macrolides for diffuse panbronchiolitis. Cochrane Database of Systematic Reviews (Online) 2010;12 doi: 10.1002/14651858.CD007716.pub2. Article ID CD007716. [DOI] [PubMed] [Google Scholar]
- 131.Jaffè A. The anti-inflammatory effects of macrolides in cystic fibrosis. Japanese Journal of Antibiotics. 2001;54(supplement):77–82. [PubMed] [Google Scholar]
- 132.Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. The Lancet. 2002;360(9338):978–984. doi: 10.1016/s0140-6736(02)11081-6. [DOI] [PubMed] [Google Scholar]
- 133.Nguyen T, Louie SG, Beringer PM, Gill MA. Potential role of macrolide antibiotics in the management of cystic fibrosis lung disease. Current Opinion in Pulmonary Medicine. 2002;8(6):521–528. doi: 10.1097/00063198-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 134.Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax. 2002;57(3):212–216. doi: 10.1136/thorax.57.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolter JM, Seeney SL, McCormack JG. Macrolides in cystic fibrosis: is there a role? American Journal of Respiratory Medicine. 2002;1(4):235–241. doi: 10.1007/BF03256614. [DOI] [PubMed] [Google Scholar]
- 136.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patient with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA: Journal of the American Medical Association. 2003;290(13):1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 137.Schöni MH. Macrolide antibiotic therapy in patients with cystic fibrosis. Swiss Medical Weekly. 2003;133(21-22):297–301. doi: 10.4414/smw.2003.10018. [DOI] [PubMed] [Google Scholar]
- 138.Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2, supplement):70S–78S. doi: 10.1378/chest.125.2_suppl.70s. [DOI] [PubMed] [Google Scholar]
- 139.Saiman L. The use of macrolide antibiotics in patients with cystic fibrosis. Current Opinion in Pulmonary Medicine. 2004;10(6):515–523. doi: 10.1097/01.mcp.0000142101.53084.f0. [DOI] [PubMed] [Google Scholar]
- 140.Bell SC, Senini SL, McCormack JG. Macrolides in cystic fibrosis. Chronic Respiratory Disease. 2005;2(2):85–98. doi: 10.1191/1479972305cd066rs. [DOI] [PubMed] [Google Scholar]
- 141.Dinwiddie R. Anti-inflammatory therapy in cystic fibrosis. Journal of Cystic Fibrosis. 2005;4(2, supplement):45–48. doi: 10.1016/j.jcf.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 142.Hansen CR, Pressler T, Koch C, Høiby N. Long-term azitromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study. Journal of Cystic Fibrosis. 2005;4(1):35–40. doi: 10.1016/j.jcf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 143.Prescott WA, Jr., Johnson GE. Antiinflammatory therapies for cystic fibrosis: past, present, and future. Pharmacotherapy. 2005;25(4):555–573. doi: 10.1592/phco.25.4.555.61025. [DOI] [PubMed] [Google Scholar]
- 144.Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais JP. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax. 2006;61(10):895–902. doi: 10.1136/thx.2005.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McArdle JR, Talwalkar JS. Macrolides in cystic fibrosis. Clinics in Chest Medicine. 2007;28(2):347–360. doi: 10.1016/j.ccm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 146.McCormack J, Bell S, Senini S, et al. Daily versus weekly azithromycin in cystic fibrosis patients. European Respiratory Journal. 2007;30(3):487–495. doi: 10.1183/09031936.00163306. [DOI] [PubMed] [Google Scholar]
- 147.Skindersoe ME, Alhede M, Phipps R, et al. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa . Antimicrobial Agents and Chemotherapy. 2008;52(10):3648–3663. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Steinkamp G, Schmitt-Grohe S, Döring G, et al. Once-weekly azithromycin in cystic fibrosis with chronic Pseudomonas aeruginosa infection. Respiratory Medicine. 2008;102(11):1643–1653. doi: 10.1016/j.rmed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 149.Florescu DF, Murphy PJ, Kalil AC. Effects of prolonged use of azithromycin in patients with cystic fibrosis: a meta-analysis. Pulmonology, Pharmacology and Therapy. 2009;22(6):467–472. doi: 10.1016/j.pupt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 150.Winstanley C, Fothergill JL. The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections. FEMS Microbiology Letters. 2009;290(1):1–9. doi: 10.1111/j.1574-6968.2008.01394.x. [DOI] [PubMed] [Google Scholar]
- 151.Kabra SK, Pawaiya R, Lodha R, et al. Long-term daily high and low doses of azithromycin in children with cystic fibrosis: a randomized controlled trial. Journal of Cystic Fibrosis. 2010;9(1):17–23. doi: 10.1016/j.jcf.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 152.Yousef AA, Jaffe A. The role of azithromycin in patients with cystic fibrosis. Paediatric Respiratory Reviews. 2010;11(2):108–114. doi: 10.1016/j.prrv.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 153.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Systematic Reviews. 2011;12 doi: 10.1002/14651858.CD002203.pub3. Article ID CD002203. [DOI] [PubMed] [Google Scholar]
- 154.Mikami M. Clinical and pathophysiological significance of neutrophil elastase in sputum and the effect of erythromycin in chronic respiratory diseases. Nihon Kyobu Shikkan Gakkai Zasshi. 1991;29(1):72–83. [PubMed] [Google Scholar]
- 155.Ohgaki N. Bacterial biofilm in chronic airway infection. Kansenshogaku zasshi. 1994;68(1):138–151. doi: 10.11150/kansenshogakuzasshi1970.68.138. [DOI] [PubMed] [Google Scholar]
- 156.Tsang KWT, Roberts P, Read RC, Kees F, Wilson R, Cole PJ. The concentrations of clarithromycin and its 14-hydroxy metabolite in sputum of patients with bronchiectasis following single dose oral administration. Journal of Antimicrobial Chemotherapy. 1994;33(2):289–297. doi: 10.1093/jac/33.2.289. [DOI] [PubMed] [Google Scholar]
- 157.Cole PJ. Bronchiectasis. In: Brewis RAL, Corrin B, Geddes DM, Gibson GJ, editors. Respiratory Medicine. 2nd edition. London, UK: W. B. Saunders; 1995. pp. 1286–1317. [Google Scholar]
- 158.Koh YY, Lee MH, Sun YH, Sung KW, Chae JH. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double-blind, placebo-controlled study. European Respiratory Journal. 1997;10(5):994–999. doi: 10.1183/09031936.97.10050994. [DOI] [PubMed] [Google Scholar]
- 159.Nakamura H, Fujishima S, Inoue T, et al. Clinical and immunoregulatory effects of roxithromycin therapy for chronic respiratory tract infection. European Respiratory Journal. 1999;13(6):1371–1379. doi: 10.1183/09031936.99.13613809. [DOI] [PubMed] [Google Scholar]
- 160.Tsang KWT, Ho PI, Chan KN, et al. A pilot study of low-dose erythromycin in bronchiectasis. European Respiratory Journal. 1999;13(2):361–364. doi: 10.1183/09031936.99.13236199. [DOI] [PubMed] [Google Scholar]
- 161.Gorrini M, Lupi A, Viglio S, et al. Inhibition of human neutrophil elastase by erythromycin and flurythromycin, two macrolide antibiotics. American Journal of Respiratory Cell and Molecular Biology. 2001;25(4):492–499. doi: 10.1165/ajrcmb.25.4.4552. [DOI] [PubMed] [Google Scholar]
- 162.Jaff A, Bush A. Anti-inflammatory effects of macrolides in lung disease. Pediatric Pulmonology. 2001;31(6):464–473. doi: 10.1002/ppul.1076. [DOI] [PubMed] [Google Scholar]
- 163.Shibuya Y, Wills PJ, Cole PJ. The effect of erythromycin on mucociliary transportability and rheology of cystic fibrosis and bronchiectasis sputum. Respiration. 2001;68(6):615–619. doi: 10.1159/000050582. [DOI] [PubMed] [Google Scholar]
- 164.Tagaya E, Tamaoki J, Kondo M, Nagai A. Effect of a short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretion. Chest. 2002;122(1):213–218. doi: 10.1378/chest.122.1.213. [DOI] [PubMed] [Google Scholar]
- 165.Bush A, Rubin BK. Macrolides as biological response modifiers in cystic fibrosis and bronchiectasis. Seminars in Respiratory and Critical Care Medicine. 2003;24(6):737–747. doi: 10.1055/s-2004-815669. [DOI] [PubMed] [Google Scholar]
- 166.Davies G, Wilson R. Prophylactic antibiotic treatment of bronchiectasis with azithromycin. Thorax. 2004;59(6):540–541. [PMC free article] [PubMed] [Google Scholar]
- 167.Cymbala AA, Edmonds LC, Bauer MA, et al. The disease-modifying effects of twice-weekly oral azithromycin in patients with bronchiectasis. Treatments in Respiratory Medicine. 2005;4(2):117–122. doi: 10.2165/00151829-200504020-00005. [DOI] [PubMed] [Google Scholar]
- 168.Máiz Carro L. Long-term treatment with azithromycin in a patient with idiopathic bronchiectasis. Archivos de Bronconeumologia. 2005;41(5):p. 295. doi: 10.1016/s1579-2129(06)60226-7. [DOI] [PubMed] [Google Scholar]
- 169.Verleden GM, Dupont LJ, Vanhaecke J, Daenen W, Van Raemdonck DEM. Effect of azithromycin on bronchiectasis and pulmonary function in a heart-lung transplant patient with severe chronic allograft dysfunction: a case report. Journal of Heart and Lung Transplantation. 2005;24(8):1155–1158. doi: 10.1016/j.healun.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 170.Yalcin E, Kiper N, Ozcelik U. Effects of clarithromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. Journal of Clinical Pharmacology and Therapeutics. 2006;31(1):49–55. doi: 10.1111/j.1365-2710.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 171.Vila-Justribo M, Dorca-Sargatal J, Bello-Dronda S. Bronchiectasis and macrolides. Archivos de Bronconeumologia. 2006;42(4):p. 206. doi: 10.1016/s1579-2129(06)60446-1. [DOI] [PubMed] [Google Scholar]
- 172.King P. Is there a role for inhaled corticosteroids and macrolide therapy in bronchiectasis? Drugs. 2007;67(7):965–974. doi: 10.2165/00003495-200767070-00002. [DOI] [PubMed] [Google Scholar]
- 173.Anwar GA, Bourke SC, Afolabi G, Middleton P, Ward C, Rutherford RM. Effects of long-term low-dose azithromycin in patients with non-CF bronchiectasis. Respiratory Medicine. 2008;102(10):1494–1496. doi: 10.1016/j.rmed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 174.Tsang KW, Bilton D. Clinical challenges in managing bronchiectasis series. Respirology. 2009;14(5):637–650. doi: 10.1111/j.1440-1843.2009.01569.x. [DOI] [PubMed] [Google Scholar]
- 175.Metersky ML. New treatment options for bronchiectasis. Therapeutic Advances in Respiratory Disease. 2010;4(2):93–99. doi: 10.1177/1753465810366858. [DOI] [PubMed] [Google Scholar]
- 176.Bochet M, Garin N, Janssens JP, Gerstel E. Is there a role for prophylactic antibiotic treatment with macrolides in bronchiectasis? Revue Medicale Suisse. 2011;7(280):308–312. [PubMed] [Google Scholar]
- 177.Serisier DJ, Martin ML. Long-term, low-dose erythromycin in bronchiectasis subjects with frequent infective exacerbations. Respiratory Medicine. 2011;105(6):946–949. doi: 10.1016/j.rmed.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 178.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. American Journal of Respiratory and Critical Care Medicine. 2003;168(1):121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- 179.Verleden GM, Dupont LJ. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;77(9):1465–1467. doi: 10.1097/01.tp.0000122412.80864.43. [DOI] [PubMed] [Google Scholar]
- 180.Crowley S, Egan JJ. Macrolide antibiotics and bronchiolitis obliterans following lung transplantation. Expert Review of Anti-Infective Therapy. 2005;3(6):923–930. doi: 10.1586/14787210.3.6.923. [DOI] [PubMed] [Google Scholar]
- 181.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. American Journal of Respiratory and Critical Care Medicine. 2005;172(6):772–775. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]
- 182.Fietta AM, Meloni F. Lung transplantation: the role of azithromycin in the management of patients with bronchiolitis obliterans syndrome. Current Medicinal Chemistry. 2008;15(7):716–723. doi: 10.2174/092986708783885228. [DOI] [PubMed] [Google Scholar]
- 183.Gottlieb J, Szangolies J, Koehnlein T, Golpon H, Simon A, Welte T. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85(1):36–41. doi: 10.1097/01.tp.0000295981.84633.bc. [DOI] [PubMed] [Google Scholar]
- 184.Porhownik NR, Batobara W, Kepron W, Unruh HW, Bshouty Z. Effect of maintenance azithromycin on established bronchiolits obliterans syndrome in lung transplant patients. Canadian Respiratory Journal. 2008;15(4):199–202. doi: 10.1155/2008/158681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vanaudenaerde BM, Meyts I, Vos R, et al. A dichotomy in bronchiolitis obliterans syndrome after lung transplantation revealed by azithromycin therapy. European Respiratory Journal. 2008;32(4):832–843. doi: 10.1183/09031936.00134307. [DOI] [PubMed] [Google Scholar]
- 186.Benden C, Boehler A. Long-term clarithromycin therapy in the management of lung transplant recipients. Transplantation. 2009;87(10):1538–1540. doi: 10.1097/TP.0b013e3181a492b2. [DOI] [PubMed] [Google Scholar]
- 187.Maimon N, Lipton JH, Chan CKN, Marras TK. Macrolides in the treatment of bronchiolitis obliterans in allograft recipients. Bone Marrow Transplantation. 2009;44(2):69–73. doi: 10.1038/bmt.2009.106. [DOI] [PubMed] [Google Scholar]
- 188.Jain R, Hachem RR, Morrell MR, et al. Azithromycin is associated with increased survival in lung transplant recipients with bronchiolitis obliterans syndrome. Journal of Heart and Lung Transplantation. 2010;29(5):531–537. doi: 10.1016/j.healun.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vos R, Vanaudenaerde BM, Ottevaere A, et al. Long-term azithromycin therapy for bronchiolitis obliterans syndrome: divide and conquer? Journal of Heart and Lung Transplantation. 2010;29(12):1358–1368. doi: 10.1016/j.healun.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 190.Fisher AJ. Azithromycin and bronchiolitis obliterans syndrome after lung transplantation: is prevention better than cure? European Respiratory Journal. 2011;37(1):10–12. doi: 10.1183/09031936.00137110. [DOI] [PubMed] [Google Scholar]
- 191.Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. European Respiratory Journal. 2011;37(1):164–172. doi: 10.1183/09031936.00068310. [DOI] [PubMed] [Google Scholar]
- 192.Tamaoki J, Takeyama K, Tagaya E, Konno K. Effect of clarithromycin on sputum production and its rheological properties in chronic respiratory tract infections. Antimicrobial Agents and Chemotherapy. 1995;39(8):1688–1690. doi: 10.1128/aac.39.8.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Swords WE, Rubin BK. Macrolide antibiotics, bacterial populations and inflammatory airway disease. The Netherlands Journal of Medicine. 2003;61(7):242–248. [PubMed] [Google Scholar]
- 194.Banerjee D, Honeybourne D, Khair OA. The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomized controlled trial. Treatments in Respiratory Medicine. 2004;3(1):59–65. doi: 10.2165/00151829-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 195.Basyigit I, Yildiz F, Ozkara SK, Yildirim E, Boyaci H, Ilgazli A. The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data. Annals of Pharmacotherapy. 2004;38(9):1400–1405. doi: 10.1345/aph.1D634. [DOI] [PubMed] [Google Scholar]
- 196.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacological Reviews. 2004;56(4):515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 197.Banerjee D, Khair OA, Honeybourne D. The effect of oral clarithromycin on health status and sputum bacteriology in stable COPD. Respiratory Medicine. 2005;99(2):208–215. doi: 10.1016/j.rmed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 198.Bishai WR. Macrolide immunomodulatory effects and symptom resolution in acute exacerbation of chronic bronchitis and acute maxillary sinusitis: a focus on clarithromycin. Expert Review of Anti-Infective Therapy. 2006;4(3):405–416. doi: 10.1586/14787210.4.3.405. [DOI] [PubMed] [Google Scholar]
- 199.Murphy DM, Forrest IA, Curran D, Ward C. Macrolide antibiotics and the airway: antibiotic or non-antibiotic effects? Expert Opinion on Investigational Drugs. 2010;19(3):401–414. doi: 10.1517/13543781003636480. [DOI] [PubMed] [Google Scholar]
- 200.Amayasu H, Yoshida S, Ebana S, et al. Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Annals of Allergy, Asthma and Immunology. 2000;84(6):594–598. doi: 10.1016/S1081-1206(10)62409-X. [DOI] [PubMed] [Google Scholar]
- 201.Cazzola M, Salzillo A, Diamare F. Potential role of macrolides in the treatment of asthma. Monaldi Archives for Chest Disease. 2000;55(3):231–236. [PubMed] [Google Scholar]
- 202.Ekici A, Ekici M, Kemal Erdemoğlu A. Effect of azithromycin on the severity of bronchial hyperresponsiveness in patients with mild asthma. Journal of Asthma. 2002;39(2):181–185. doi: 10.1081/jas-120002199. [DOI] [PubMed] [Google Scholar]
- 203.Beuther DA, Martin RJ. Antibiotics in asthma. Current Allergy and Asthma Reports. 2004;4(2):132–138. doi: 10.1007/s11882-004-0058-5. [DOI] [PubMed] [Google Scholar]
- 204.Hatipoğlu U, Rubinstein I. Low-dose, long-term macrolide therapy in asthma: an overview. Clinical and Molecular Allergy. 2004;2(1, article 4) doi: 10.1186/1476-7961-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kostadima E, Tsiodras S, Alexopoulos EI, et al. Clarithromycin reduces the severity of bronchial hyperresponsiveness in patients with asthma. European Respiratory Journal. 2004;23(5):714–717. doi: 10.1183/09031936.04.00118404. [DOI] [PubMed] [Google Scholar]
- 206.Ferrara G, Losi M, Franco F, Corbetta L, Fabbri LM, Richeldi L. Macrolides in the treatment of asthma and cystic fibrosis. Respiratory Medicine. 2005;99(1):1–10. doi: 10.1016/j.rmed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 207.Richeldi L, Ferrara G, Fabbri LM, Lasserson TJ, Gibson PG. Macrolides for chronic asthma. Cochrane Database of Systematic Reviews (Online) 2005;4(3) doi: 10.1002/14651858.CD002997.pub3. Article ID CD002997. [DOI] [PubMed] [Google Scholar]
- 208.Richeldi L, Ferrara G, Fabbri LM, Lasserson TJ, Gibson PG. Macrolides for chronic asthma. Cochrane Database of Systematic Reviews (Online) 2005;3 doi: 10.1002/14651858.CD002997.pub2. Article ID CD002997. [DOI] [PubMed] [Google Scholar]
- 209.Black PN. Antibiotics for the treatment of asthma. Current Opinion in Pharmacology. 2007;7(3):266–271. doi: 10.1016/j.coph.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 210.Sharma S, Jaffe A, Dixon G. Immunomodulatory effects of macrolide antibiotics in respiratory disease: therapeutic implications for asthma and cystic fibrosis. Pediatric Drugs. 2007;9(2):107–118. doi: 10.2165/00148581-200709020-00004. [DOI] [PubMed] [Google Scholar]
- 211.Hernando-Sastre V. Macrolide antibiotics in the treatment of asthma. An update. Allergologia et Immunopathologia. 2010;38(2):92–98. doi: 10.1016/j.aller.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 212.Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Current Allergy and Asthma Reports. 2010;10(1):67–73. doi: 10.1007/s11882-009-0086-2. [DOI] [PubMed] [Google Scholar]
- 213.Oliveinstein R, Jahdali HA, Alkhamis N, Halwani R, Al-Muhsen S, Hamid Q. Challenges in the management of severe asthma: role of current and future therapies. Current Pharmaceutical Design. 2011;17(7):703–711. doi: 10.2174/138161211795428993. [DOI] [PubMed] [Google Scholar]
- 214.Good JT, Jr., Rollins DR, Martin RJ. Macrolides in the treatment of asthma. Current Opinion in Pulmonary Medicine. 2012;18(1):76–84. doi: 10.1097/MCP.0b013e32834daff8. [DOI] [PubMed] [Google Scholar]
- 215.Gleason PP, Meehan TP, Fine JM, Galusha DH, Fine MJ. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Archives of Internal Medicine. 1999;159(21):2562–2572. doi: 10.1001/archinte.159.21.2562. [DOI] [PubMed] [Google Scholar]
- 216.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Archives of Internal Medicine. 2001;161(15):1837–1842. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 217.Brown RB, Iannini P, Gross P, Kunkel M. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: analysis of a hospital claims-made database. Chest. 2003;123(5):1503–1511. doi: 10.1378/chest.123.5.1503. [DOI] [PubMed] [Google Scholar]
- 218.Martinez JA, Horcajada JP, Almela M, et al. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clinical Infectious Diseases. 2003;36(4):389–395. doi: 10.1086/367541. [DOI] [PubMed] [Google Scholar]
- 219.Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. American Journal of Respiratory and Critical Care Medicine. 2004;170(4):440–444. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 220.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The impact of empiric antimicrobial therapy with a β-lactam and fluoroquinolone on mortality for patients hospitalized with severe pneumonia. Critical Care. 2005;10(1, article R8) doi: 10.1186/cc3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131(2):466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 222.Rodriguez A, Mendia A, Sirvent JM, et al. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Critical Care Medicine. 2007;35(6):1493–1498. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 223.Feldman C, Anderson R. Therapy for pneumococcal bacteremia: monotherapy or combination therapy? Current Opinion in Infectious Diseases. 2009;22(2):137–142. doi: 10.1097/QCO.0b013e3283232a30. [DOI] [PubMed] [Google Scholar]
- 224.Restrepo MI, Mortensen EM, Waterer GW, Wunderink RG, Coalson JJ, Anzueto A. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. European Respiratory Journal. 2009;33(1):153–159. doi: 10.1183/09031936.00054108. [DOI] [PubMed] [Google Scholar]
- 225.Tessmer A, Welte T, Martus P, Schnoor M, Marre R, Suttorp N. Impact of intravenous β-lactam/macrolide versus β-lactam monotherapy on mortality in hospitalized patients with community-acquired pneumonia. Journal of Antimicrobial Chemotherapy. 2009;63(5):1025–1033. doi: 10.1093/jac/dkp088. [DOI] [PubMed] [Google Scholar]
- 226.Martin-Loeches I, Lisboa T, Rodriguez A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Medicine. 2010;36(4):612–620. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 227.Bratzler DW, Ma A, Nsa W. Initial antibiotic selection and patient outcomes: observations from the National Pneumonia Project. Clinical Infectious Diseases. 2008;47:S193–201. doi: 10.1086/591404. [DOI] [PubMed] [Google Scholar]
- 228.Torres A, Garau J, Arvis P, et al. Moxifloxacin monotherapy is effective in hospitalized patients with community-acquired pneumonia: the MOTIV study—a randomized clinical trial. Clinical Infectious Diseases. 2008;46(10):1499–1509. doi: 10.1086/587519. [DOI] [PubMed] [Google Scholar]
- 229.Ewig S, Hecker H, Suttorp N, Marre R, Welte T. Moxifloxacin monotherapy versus β-lactam mono- or combination therapy in hospitalized patients with community-acquired pneumonia. Journal of Infection. 2011;62(3):218–225. doi: 10.1016/j.jinf.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 230.Epstein BJ, Gums JG. Optimal pharmacological therapy for community-acquired pneumonia the role of dual antibacterial therapy. Drugs. 2005;65(14):1949–1971. doi: 10.2165/00003495-200565140-00004. [DOI] [PubMed] [Google Scholar]
- 231.Giamarellos-Bourboulis EJ, Pechère JC, Routsi C, et al. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clinical Infectious Diseases. 2008;46(8):1157–1164. doi: 10.1086/529439. [DOI] [PubMed] [Google Scholar]
- 232.Kovaleva A, Remmelts HHF, Rijkers GT, et al. Immunomodulatory effects of macrolides during community-acquired pneumonia: a literature review. Journal of Antimicrobial Chemotherapy. 2012;67(3):5305–540. doi: 10.1093/jac/dkr520. [DOI] [PubMed] [Google Scholar]
- 233.Walkey AJ, Wiener RS. Macrolide antibiotics and survival in patients with acute lung injury. Chest. 2012;141(5):1153–1159. doi: 10.1378/chest.11-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cervin A. The anti-inflammatory effect of erythromycin and its derivatives, with special reference to nasal polyposis and chronic sinusitis. Acta Oto-Laryngologica. 2001;121(1):83–92. doi: 10.1080/000164801300006326. [DOI] [PubMed] [Google Scholar]
- 235.Suzuki H, Ikeda K. Mode of action of long-term low-dose macrolide therapy for chronic sinusitis in the light of neutrophil recruitment. Current Drug Targets—Inflammation & Allergy. 2002;1(1):117–126. doi: 10.2174/1568010023344832. [DOI] [PubMed] [Google Scholar]
- 236.Garey KW, Alwani A, Danziger LH, Rubinstein I. Tissue reparative effects of macrolide antibiotics in chronic inflammatory sinopulmonary diseases. Chest. 2003;123(1):261–265. doi: 10.1378/chest.123.1.261. [DOI] [PubMed] [Google Scholar]
- 237.Majima Y. Clinical implications of the immunomodulatory effects of macrolides on sinusitis. The American Journal of Medicine. 2004;117(supplement 9):20S–25S. doi: 10.1016/j.amjmed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 238.Cervin A, Wallwork B. Anti-inflammatory effects of macrolide antibiotics in the treatment of chronic rhinosinusitis. Otolaryngologic Clinics of North America. 2005;38(6):1339–1350. doi: 10.1016/j.otc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 239.Hatipoglu U, Rubinstein I. Treatment of chronic rhinosinusitis with low-dose, long-term macrolide antibiotics: an evolving paradigm. Current Allergy and Asthma Reports. 2005;5(6):491–494. doi: 10.1007/s11882-005-0031-y. [DOI] [PubMed] [Google Scholar]
- 240.Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006;116(2):189–193. doi: 10.1097/01.mlg.0000191560.53555.08. [DOI] [PubMed] [Google Scholar]
- 241.Cervin A, Wallwork B. Macrolide therapy of chronic rhinosinusitis. Rhinology. 2007;45(4):259–267. [PubMed] [Google Scholar]
- 242.Harvey RJ, Wallwork BD, Lund VJ. Anti-inflammatory effects of macrolides: applications in chronic rhinosinusitis. Immunology and Allergy Clinics of North America. 2009;29(4):689–703. doi: 10.1016/j.iac.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 243.Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clinic Proceedings. 2011;86(5):427–443. doi: 10.4065/mcp.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Piromchai P, Thanaviratananich S, Laopaiboon M. Systemic antibiotics for chronic rhinosinusitis without nasal polyps in adults. Cochrane Database of Systematic Reviews (Online) 2011;5 doi: 10.1002/14651858.CD008233.pub2. Article ID CD008233. [DOI] [PubMed] [Google Scholar]


