Abstract
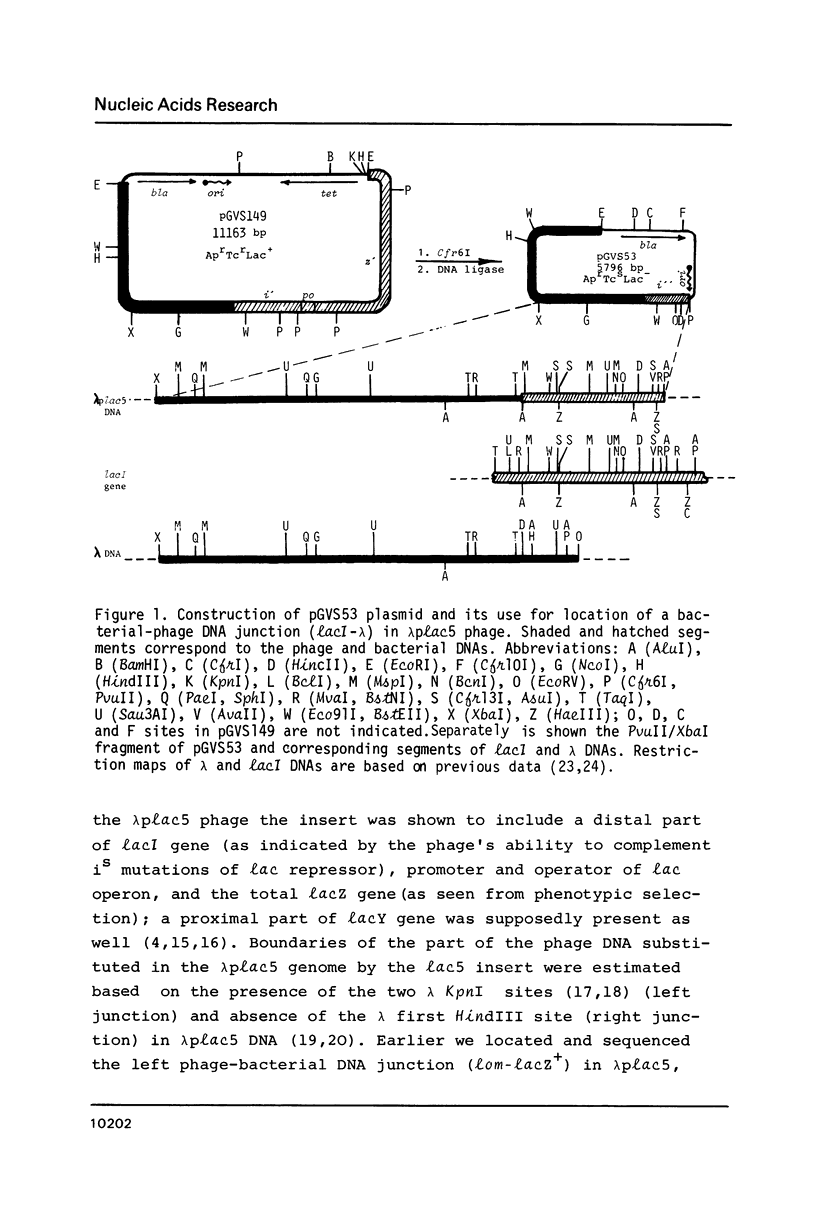
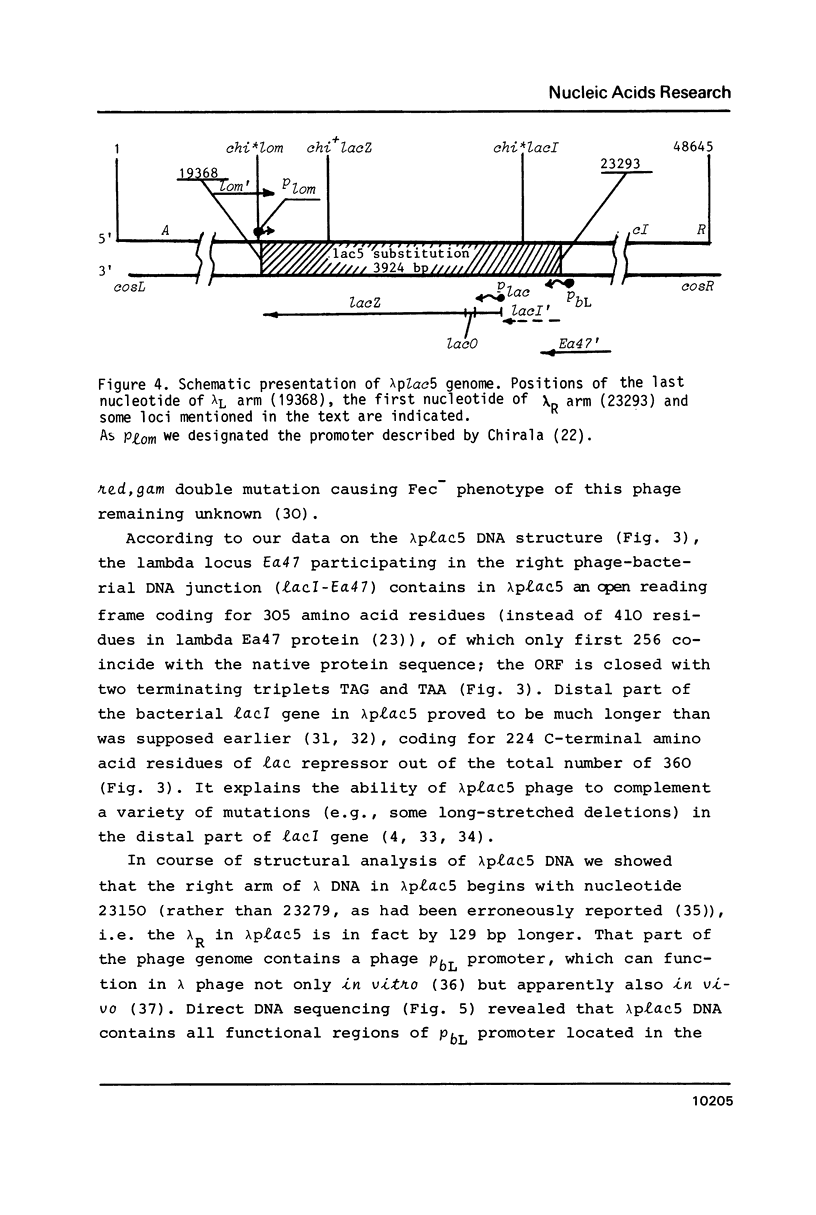
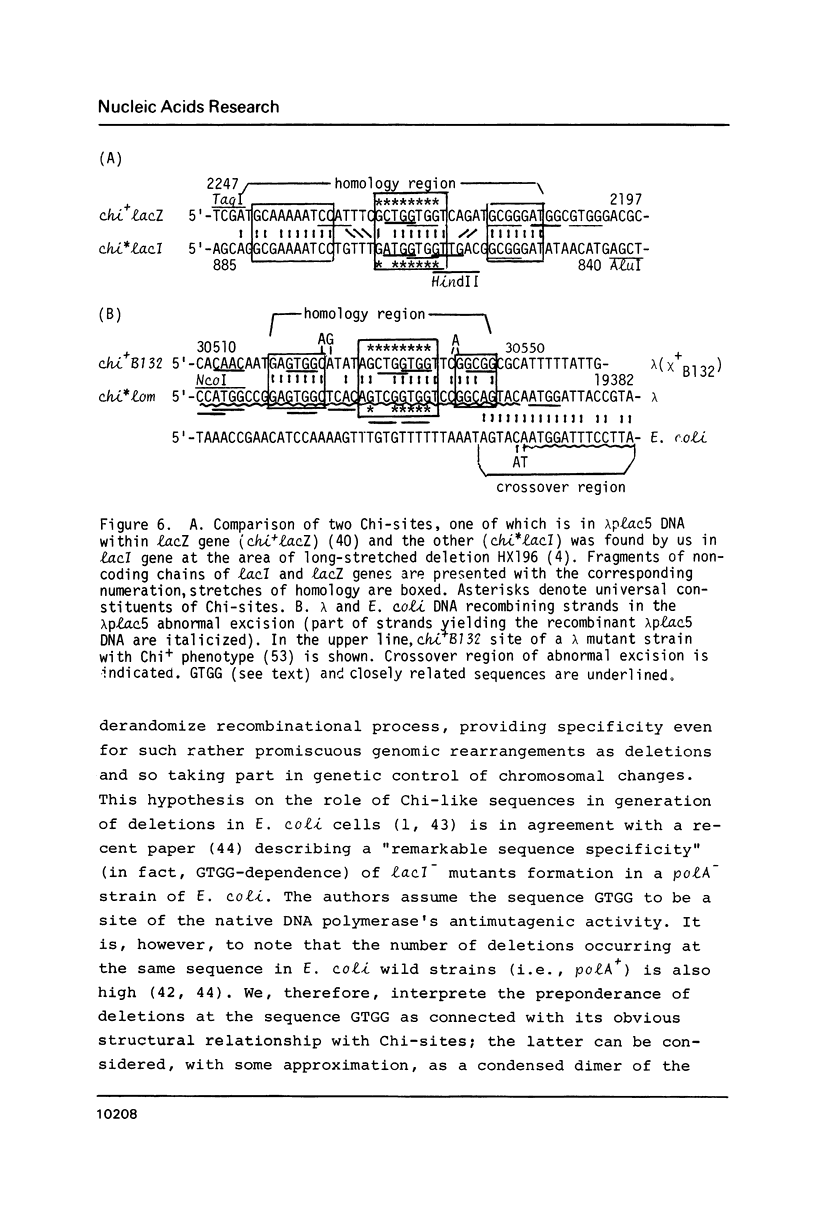
In studying molecular mechanisms of specialised transduction, the lacI (E. coli)-Ea47 (lambda) DNA junction in transducing bacteriophage lambda plac 5 has been structurally elucidated, thus yielding the complete sequence of lambda plac 5 DNA including the lac5 substitution, a well-known segment of lambdoid vectors. The lambda plac5 DNA is shown to consist of 19368 bp (lambda left arm) + 3924 bp (lac5 substitution) + 25353 bp (lambda right arm), totally amounting to 48645 bp. The presence of the phage rho bL promoter near to the right end of the lac5 insert is shown. The lacI gene distal end in lambda plac5 proved to be much longer than it was postulated earlier, coding for 224 C-terminal amino acid residues of lac repressor. Both the recombination studied in this paper and the earlier studied abnormal prophage excision (2, 3) occur near to Chi-like structures (chi*lacI and chi*lom, respectively). On the basis of the data obtained, a key role of the E. coli RecBCD system and Chi-like sequences in the formation of deletions in bacterial cells is suggested.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Amundsen S. K., Taylor A. F., Chaudhury A. M., Smith G. R. recD: the gene for an essential third subunit of exonuclease V. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5558–5562. doi: 10.1073/pnas.83.15.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Smith G. R. Cutting of chi-like sequences by the RecBCD enzyme of Escherichia coli. J Mol Biol. 1987 Apr 20;194(4):747–750. doi: 10.1016/0022-2836(87)90252-x. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Smith G. R. Recombinational hotspot activity of Chi-like sequences. J Mol Biol. 1984 Dec 5;180(2):371–377. doi: 10.1016/s0022-2836(84)80009-1. [DOI] [PubMed] [Google Scholar]
- Chirala S. S. The nucleotide sequence of the lac operon and phage junction in lambda gt11. Nucleic Acids Res. 1986 Jul 25;14(14):5935–5935. doi: 10.1093/nar/14.14.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Johnson P. Nucleotide sequence changes produced by mutations in the lac promoter of Escherichia coli. J Mol Biol. 1977 Mar 25;111(1):65–75. doi: 10.1016/s0022-2836(77)80132-0. [DOI] [PubMed] [Google Scholar]
- Edlind T. D., Cooley T. E., Richards S. H., Ihler G. M. Long range base-pairing in the leftward transcription unit of bacteriophage lambda. Characterization by electron microscopy and computer-aided sequence analysis. J Mol Biol. 1984 Nov 5;179(3):351–365. doi: 10.1016/0022-2836(84)90070-6. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J. Sequence of the lacI gene. Nature. 1978 Aug 24;274(5673):765–769. doi: 10.1038/274765a0. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Storey A., Brown K., Hickson I. D., Emmerson P. T. Complete nucleotide sequence of recD, the structural gene for the alpha subunit of Exonuclease V of Escherichia coli. Nucleic Acids Res. 1986 Nov 11;14(21):8583–8594. doi: 10.1093/nar/14.21.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix D. F., Burns P. A., Glickman B. W. DNA sequence analysis of spontaneous mutation in a PolA1 strain of Escherichia coli indicates sequence-specific effects. Mol Gen Genet. 1987 May;207(2-3):267–272. doi: 10.1007/BF00331588. [DOI] [PubMed] [Google Scholar]
- Gronenborn B. Overproduction of phage lambda repressor under control of the lac promotor of Escherichia coli. Mol Gen Genet. 1976 Nov 17;148(3):243–250. doi: 10.1007/BF00332898. [DOI] [PubMed] [Google Scholar]
- Ippen K., Shapiro J. A., Beckwith J. R. Transposition of the lac region to the gal region of the Escherichia coli chromosome: isolation of lambda-lac transducing bacteriophages. J Bacteriol. 1971 Oct;108(1):5–9. doi: 10.1128/jb.108.1.5-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko V. V., Vasilenko S. K., Grachev M. A. A rightward promoter to the left of the att site of lambda phage DNA: possible participant in site-specific recombination. Gene. 1979 Nov;7(3-4):181–195. doi: 10.1016/0378-1119(79)90045-3. [DOI] [PubMed] [Google Scholar]
- Kröger M., Hobom G. A chain of interlinked genes in the ninR region of bacteriophage lambda. Gene. 1982 Nov;20(1):25–38. doi: 10.1016/0378-1119(82)90084-1. [DOI] [PubMed] [Google Scholar]
- Luk K. C., Mark K. K. The separation of phage promoter from bacterial lac promoter for beta-galactosidase expression in transducing phage lambda plac5. Biochem Biophys Res Commun. 1979 Jul 12;89(1):64–70. doi: 10.1016/0006-291x(79)90943-4. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Polisky B., Bishop R. J., Gelfand D. H. A plasmid cloning vehicle allowing regulated expression of eukaryotic DNA in bacteria. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3900–3904. doi: 10.1073/pnas.73.11.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli A. S., Schultz D. W., Taylor A. F., Smith G. R. Chi-dependent DNA strand cleavage by RecBC enzyme. Cell. 1985 May;41(1):145–151. doi: 10.1016/0092-8674(85)90069-8. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Breitmeyer J. B., Tabachnik N. F., Myers P. A. A second specific endonuclease from Haemophilus aegyptius. J Mol Biol. 1975 Jan 5;91(1):121–123. doi: 10.1016/0022-2836(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Robinson L. H., Landy A. HindII, HindIII, and HpaI restriction fragment maps of the left arm of bacteriophage lambda DNA. Gene. 1977 Sep;2(1):33–54. doi: 10.1016/0378-1119(77)90020-8. [DOI] [PubMed] [Google Scholar]
- Rosenvold E. C., Calva E., Burgess R. R., Szybalski W. In vitro transcription from the b2 region of bacteriophage lambda. Virology. 1980 Dec;107(2):476–487. doi: 10.1016/0042-6822(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Danforth B. N., Glickman B. W. Mechanisms of spontaneous mutagenesis: an analysis of the spectrum of spontaneous mutation in the Escherichia coli lacI gene. J Mol Biol. 1986 May 20;189(2):273–284. doi: 10.1016/0022-2836(86)90509-7. [DOI] [PubMed] [Google Scholar]
- Shapiro J., Machattie L., Eron L., Ihler G., Ippen K., Beckwith J. Isolation of pure lac operon DNA. Nature. 1969 Nov 22;224(5221):768–774. doi: 10.1038/224768a0. [DOI] [PubMed] [Google Scholar]
- Shpakovski G. V., Berlin Y. A. Site-specificity of abnormal excision: the mechanism of formation of a specialized transducing bacteriophage lambda plac5. Nucleic Acids Res. 1984 Sep 11;12(17):6779–6795. doi: 10.1093/nar/12.17.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpakovskii G. V., Akhrem A. A., Berlin Iu A. Polnaia pervichnaia struktura DNK transdutsiruiushchego bakteriofaga lambda plac5. Bioorg Khim. 1986 May;12(5):695–698. [PubMed] [Google Scholar]
- Shpakovskii G. V., Berlin Iu A. Struktura uchastka rekombinatsii v DNK transdutsiruiushchego bakteriofaga lambda plac5. Bioorg Khim. 1983 May;9(5):711–713. [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Schultz D. W., Crasemann J. M. Generalized recombination: nucleotide sequence homology between Chi recombinational hotspots. Cell. 1980 Mar;19(3):785–793. doi: 10.1016/s0092-8674(80)80054-7. [DOI] [PubMed] [Google Scholar]
- Sokolov N. N., Fitsner A. B., Anikeitcheva N. V., Choroshoutina YuB, Samko O. T., Kolosha V. O., Fodor I., Votrin I. I. A site-specific endonuclease from Pseudomonas aeruginosa. Mol Biol Rep. 1985 Apr;10(3):159–161. doi: 10.1007/BF00778522. [DOI] [PubMed] [Google Scholar]
- Triman K. L., Chattoraj D. K., Smith G. R. Identity of a Chi site of Escherichia coli and Chi recombinational hotspots of bacteriophage lambda. J Mol Biol. 1982 Jan 15;154(2):393–399. doi: 10.1016/0022-2836(82)90072-9. [DOI] [PubMed] [Google Scholar]
- Williams B. G., Blattner F. R. Construction and characterization of the hybrid bacteriophage lambda Charon vectors for DNA cloning. J Virol. 1979 Feb;29(2):555–575. doi: 10.1128/jvi.29.2.555-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]