Abstract
Oxidative stress is a key factor linked renal function decline with age. However, there is still no large cohort study exploring the potential role of oxidative stress in mild insufficiency of kidney function (MIKF) and chronic kidney disease (CKD) after adjusting for confounding factors. This study tested the hypothesis that oxidative stress, indicated by plasma malondialdehyde (MDA), is associated with the prevalence of MIKF and CKD after controlling the effects of confounding factors.
Plasma levels of MDA and serum levels of fasting glucose, cholesterol, triglycerides, creatinine, alanine aminotransferase, and aspartate aminotransferase were analyzed from 2,169 Chinese Han adults. A questionnaire and physical examination were performed to identify and suspect risk factors of renal function decline with age. Kidney function, as indicated by estimated glomerular filtration rate, showed a significant decline with age in both male and female. Although the association between age and plasma MDA levels was nonlinear, MDA was negatively related to kidney function. The multivariate-adjusted odds ratios showed that plasma MDA had a significantly graded relation to the prevalence of MIKF and CKD with or without adjustment for covariates. By comparison with the lowest quartile, individuals with the highest quartile of MDA level had a 99% and 223% increased risk of developing MIKF and CKD, respectively. Further results from multiinteraction analysis demonstrated that plasma MDA may be the mediator linking different covariates with renal function decline. The most striking finding of this study was that oxidative stress, as indicated by plasma MDA levels, is associated with the prevalence of MIKF and/or CKD. Although imposing an increasing burden on the kidney and/or promoting a cyclical process of oxidative stress in the body, high levels of MDA in plasma may link the decline of kidney function with age.
Introduction
Aging is a naturally developing biological process associated with gradual structural changes and functional loss of most systems, and is often characterized by declining adaptive capacity and increasing morbidity or mortality of an organism.1 As one of the fastest aging organs, the kidney shows an age-related reduction in some structures and functions. The annual decrease of renal parenchyma is about 1%,2 and the decline of creatinine clearance or glomerular filtration rate (GFR) is approximately 1.0 mL/min per 1.73 m2 per year in elderly subjects.3,4 This may explain why the prevalence of chronic kidney disease (CKD) and end-stage renal disease is especially common in elderly persons.5,6 Furthermore, outcomes of age-related changes of kidney function include not only growing incidence and prevalence of CKD, but also increasing risk of development and progression of cardiovascular diseases.7–9
The glomerular filtration barrier comprises five structural components—the glomerular endothelial fenestrae, glomerular basement membrane, podocyte interfoot process/slit diaphragms, endothelial surface layer, and subpodocyte space. Any change to these components may affect glomercular permeability.10 Intriguingly, oxidative stress and inflammation are involved in almost all alterations of these components11–13 and also are increased in CKD.14,15 However, it is unclear if oxidative stress functions as an initiating or progressing cause or is just the consequence of renal function decline with age.
Based on the oxidative stress theory of aging, on inflammation or stress conditions, the increased generation of reactive oxygen species (ROS) and the corresponding response to oxidative stress are key factors linked to aging and the development of age-related diseases,16,17 Accumulating evidence supports the potential links between oxidative stress and renal function decline with age. Increased oxidative stress has been widely used to evaluate the progression of kidney function decline and the effectiveness of treatment for CKD.18–21 Furthermore, oxidative stress, through increasing age-related renal cell apoptosis22,23 or mitochondrial dysfunction,24 may account for alternations of renal function prior to the development of age-related pathology and loss of renal function.18 Given that mild insufficiency of kidney function (MIKF) also occurs early before the appearance of CKD,25,26 oxidative stress may cause specific damage to renal structures and may also directly contribute to MIKF and further promote the development and progression of CKD. However, there is still no large cohort study to explore the potential correlation of oxidative stress with MIKF and CKD after adjustment for confounding factors.
For these reasons, we tested the hypothesis that oxidative stress, indicated by plasma malondialdehyde (MDA), is associated with the prevalence of MIKF and CKD under controlling the effects of covariates, including age, gender, lifestyle factors, blood glucose, blood lipids, and serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Therefore, plasma levels of MDA and serum levels of above-mentioned factors were deterrmined from the study participants, and a questionnaire and physical examination were performed to identify and suspect risk factors for renal function decline.
Materials and Methods
Chemicals
1,1,3,3-Tetramethoxypropane and thiobarbituric acid were purchased from Sigma-Aldrich (St. Louis, MO). Other chemicals (analytical grade) were from Sangon Biotech Co. Ltd., (Shanghai, China). Water was produced by a Milli-Q Plus purification system (Millipore, Bedford, MA).
Participants and questionnaire
All study volunteers signed informed consents, and the Institutional Review Board of Hunan Normal University approved the study protocol. A total of 2,169 Chinese Han adults of both sexes living in the urban areas of Changsha City and its surrounding cities in the central region of China were included in the study. The basic characteristics of the participants are shown in Table 1.
Table 1.
Basic Characteristics of Participants
| Factor | All (n=2169) | NKF (n=1115) | MIKF (n=588) | CKD (n=72) |
|---|---|---|---|---|
| Age, mean (SD), years | 44.49 (13.84) | 39.50 (11.08) | 51.93 (13.35)a | 61.06 (16.17)a |
| Gender, male, n (%) | 1373 (63.30) | 689 (61.79) | 418 (71.09)a | 44 (61.11) |
| BMI, mean (SD), kg/m2 | 23.87 (3.26) | 23.65 (3.29) | 24.23 (3.12)a | 24.40 (3.48) |
| Blood pressure, mean (SD), mmHg | ||||
| Systolic | 123.98 (15.66) | 121.67 (14.79) | 127.45 (16.46)a | 131.28 (14.76)a |
| Diastolic | 75.78 (11.52) | 74.85 (11.34) | 77.21 (11.64)a | 78.44 (11.75)b |
| BGL, mean(SD), mmol/L | 4.98 (1.17) | 4.87 (1.05) | 5.12 (1.32)a | 5.47 (1.33)a |
| Total cholesterol, mean(SD), mmol/L | 4.49 (0.85) | 4.45 (0.88) | 4.54 (0.80) | 4.66 (0.79) |
| Triacylglycerol, mean(SD), mmol/L | 1.63 (1.26) | 1.54 (1.25) | 1.79 (1.27)a | 1.64 (1.08) |
| ALT, mean(SD), U/L | 28.90 (23.26) | 29.69 (22.22) | 28.13 (25.55) | 22.94 (17.88)b |
| AST, mean(SD), U/L | 23.37 (10.57) | 23.15 (9.85) | 24.03 (11.96) | 21.33 (8.50) |
| eGFR, mean(SD), ml/min/1.73m2 | 95.54 (18.52) | 107.04 (10.88) | 78.88 (7.74)a | 53.40 (6.02)a |
| MDA, mean (SD), μmol/L | 3.58 (1.13) | 3.44 (0.89) | 3.82 (1.39)a | 3.95 (1.64)b |
| Tobacco consumption, n (%) | 887 (40.89) | 477 (42.78) | 268 (45.58) | 15 (20.83)a |
| Alcohol intake, n (%) | 1057 (48.73) | 541 (48.52) | 316 (53.74) | 28 (38.89) |
| Sleep quality, well, n (%) | 1343 (61.92) | 660 (59.19) | 420 (71.43)b | 42 (58.33) |
| Physical inactivity, n (%) | 1159 (53.43) | 665 (59.64) | 276 (46.94)a | 32 (44.44)b |
p<0.01 compared to subjects with NKF.
p<0.05 compared to subjects with NKF.
NKF, Normal kidney function; MIKF, mild insufficiency of kidney function; CKD, chronic kidney disease; SD, standard deviation; n, number; BMI, body mass index; BGL, blood glucose level; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; MDA, reactive carbonyl species.
Information about demographics (gender and age), lifestyle habits (sleep, drinking history, smoking history, and physical activity), and detailed medical history was assessed by a self-administered questionnaire. The questionnaire for sleep quality contained five aspects—well, insomnia, dreaminess, restless sleep, and other problems. Subjects who answered “well” were not classified into the sleep with some problems categories.
Blood sampling
Between 2009 and 2011, blood samples (5 mL) of all participants from the median cubital vein on the inside of the elbow were collected into vacutainer tubes containing ethylenediaminetetraacetic acid, according to standard blood collection procedures,27,28 and stored at 0–4°C. All detections were performed within 8 hr of sampling.
Physical examination
Stature, body weight, and body mass index (BMI) were determined using an ultrasonic body scale SK-CK (Sonka Electronic Technologies Co. Ltd., Shenzhen, China). The BMI cutoff point for overweight was 25 kg/m2, as advocated by the World Health Organization (WHO).29
After resting for at least 30 min, each participant's blood pressure was measured three times in the sitting position, with the right arm relaxed and well supported by a table at an angle of 45° from the trunk, using an automatic electronic sphygmomanometer (Ken2-BPMSP-1; Pengcheng Healthcare Products Co. Ltd., Shenzhen, China).
According to our previous study,30 blood lipids (serum total cholesterol and triglycerides), fasting glucose, kidney function (serum creatinine), and liver function (AST and ALT) were measured using a chromatographic enzymatic method in a MINDRAY automatic analyzer BS-40 (MINDRAY Co. Ltd., Shenzhen, China). Serum hepatitis B viral antigens and antibodies were detected by MR-96 microplate reader (MINDRAY Co. Ltd., Shenzhen, China).
The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.6 Based on the eGFR and the National Kidney Foundation Practice Guidelines,25,26 kidney function was classified into three categories: Normal kidney function (eGFR >90 mL/min per 1.73 m2 and no proteinuria), MIKF (60 mL/min per 1.73 m2 ≤ eGFR <90 mL/min per 1.73 m2 and no proteinuria), and CKD (eGFR <60 mL/min per 1.73 m2 or overt proteinuria).
MDA assay
According to our previous studies,28,31 a modified TBA method was used to determine the concentration of MDA in plasma. The MDA was expressed as thiobarbituric acid–reactive substances and detected by a LS-50B spectrofluorometer (Perkin-Elmer Corp., Norwalk, CT).
Statistics
Continuous results were presented as means and standard deviations (SDs). Statistical analysis of data was done using predictive analytics software (PASW) statistics 18.0 (SPSS Inc., Chicago, IL). Because data showed a normal distribution, parametric statistical methods were used. In comparison to the groups (different physical activity levels), one-way analysis of variance (ANOVA) was performed. To assess significant F-ratios obtained by analysis of variance, the least significant difference (LSD) or the Tamhan's T2 post hoc test was used. Quartiles of MDA were created based on the distribution of plasma MDA levels. The associations with MDA and eGFR were analyzed by Pearson partial correlation with or without adjusting for age and other confounding factors. The odds ratio (OR) and 95% confidence interval (CI) of MIKF or CKD associated with different MDA levels were examined using multinomial logistic regression32 and adjusted for the potential confounding effects of age, gender, alcohol intake, tobacco consumption, sleep quality, physical activity, BMI, systolic blood pressure, diastolic blood pressure, blood glucose, and serum levels of cholesterol, triglycerides, ALT, and AST. Because there were only 72 persons with CKD and the cases were not enough for further multinomial logistic regression, further risk analysis associated with MDA levels stratified by different covariates was just performed in MIKF. A p value less than 0.05 was considered statistically significant.
Results
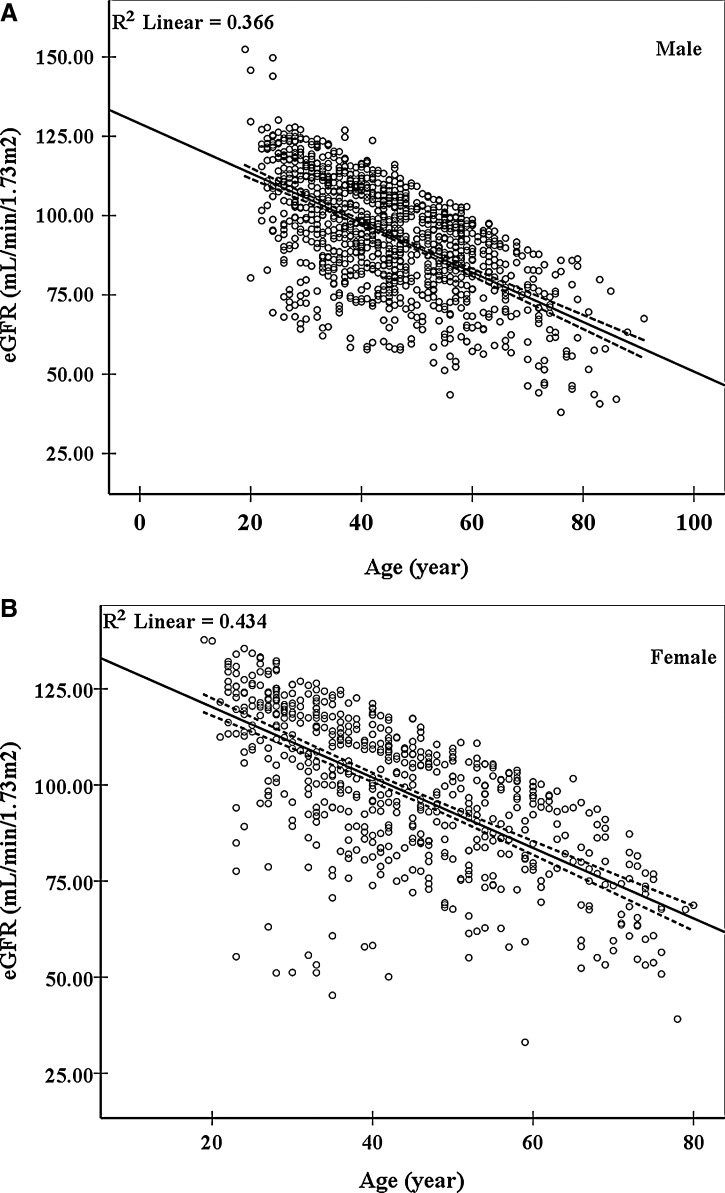
Among the 2,169 participants included in the cross-sectional analysis, kidney function was significantly associated with age, male, BMI, blood pressure, blood glucose, and plasma MDA (p<0.05) (Table 1). In contrast, there was no significant correlation of kidney function with blood lipids, liver function, and most lifestyle factors (Table 1). As indicated by eGFR, kidney function showed a significant decline with age in both male (R2 linear=0.366, p<0.01) (Fig. 1A) and female (R2 linear=0.434, p<0.01) (Fig. 1B).
FIG. 1.
The association of estimated glomerular filtration rate (eGFR) with age. The eGFR showed a significantly linear decline with increasing age in both male (A) (R2 linear=0.366, p=0.000) and female (B) (R2 linear=0.434, p=0.000). Dotted lines show the 95% confidence interval (CI). The eGFR was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.6 Data are from 1,150 male and 623 female subjects.
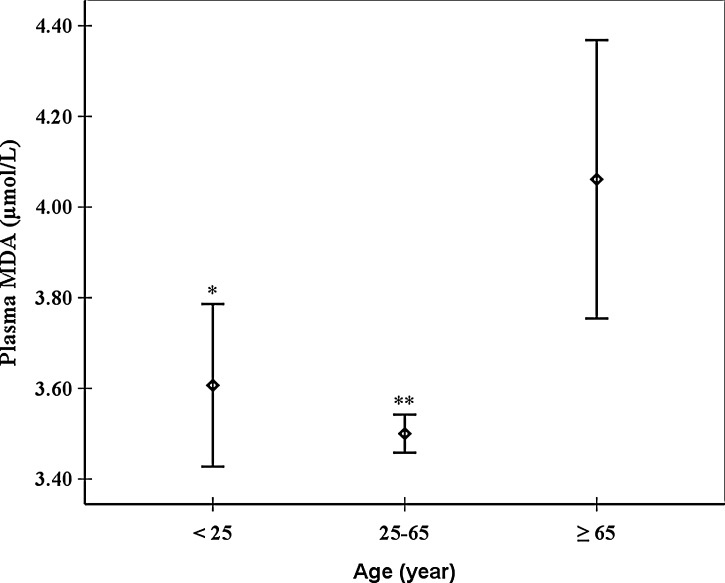
Intriguingly, the association between age and plasma MDA levels was nonlinear. Both the adult group (25–65 years) and the young one (<25 years) had a significantly lower levels of plasma MDA compared to the old group (≥65 years) (p<0.05) (Fig. 2). The adult group had the lowest levels of plasma MDA but did not show significant difference from the young group (Fig. 2).
FIG. 2.
Means (◊) and 95% confidence interval (CI) (⊤) of plasma malondialdehyde (MDA) in different age groups. (*) p<0.05 compared to the group of age ≥65 years. (**) p<0.01 compared to the group of age ≥65 years.
The correlation of eGFR with MDA illustrated that MDA was negatively related to kidney function (p<0.01) (Table 2). Although there were some differences in the Pearson correlation coefficients between with and without adjusted for age, gender, and/or other confounding factors (Table 2), as displayed by the p values, all of the associations were statistically significant (p<0.01) (Table 2).
Table 2.
Pearson Correlation Coefficient between Malondialdehyde and Estimated Glomerular Filtration Rate
| MDA | MDAa | MDAb | |
|---|---|---|---|
| eGFR | |||
| r | −0.170 | −0.152 | −0.142 |
| p value | 0.000 | 0.000 | 0.000 |
| Degrees of freedom | 1771 | 1767 | 1631 |
Adjusted for age and gender.
Adjusted for age, gender, alcohol intake, tobacco consumption, sleep quality, physical activity, BMI, systolic blood pressure, diastolic blood pressure, blood glucose level, and serum levels of cholesterol, triglyceride, ALT and AST.
MDA, Reactive carbonyl species; eGFR, estimated glomerular filtration rate; r, Pearson correlation coefficient.
As demonstrated by multinomial logistic regression, plasma MDA showed a significantly graded relation to the prevalence of MIKF and CKD with or without adjustment for age, gender, alcohol intake, tobacco consumption, sleep quality, physical activity, BMI, systolic blood pressure, diastolic blood pressure, blood glucose level, and serum levels of cholesterol, triglyceride, ALT, and AST (p for trend <0.05) (Table 3). The multiadjusted ORs of MIKF for plasma MDA by quartiles of plasma MDA were 1.33 (95% CI 0.94–1.88) for the second quartile, 1.46 (95% CI 1.02–2.07) for the third quartile, and 1.99 (95% CI 1.41–2.81) for the fourth quartile (p for trend <0.001) compared to the lowest quartile of plasma MDA (Table 3). The multiadjusted ORs of CKD for plasma MDA by quartiles of plasma MDA were 2.01 (95% CI 0.86–4.67) for the second quartile, 3.11 (95% CI 1.39–6.93) for the third quartile, and 3.23 (95% CI 1.45–7.19) for the fourth quartile (p for trend=0.004) compared with the lowest quartile of plasma MDA (Table 3).
Table 3.
The Odds Ratio and Adjusted Odds Ratio of Mild Insufficiency of Kidney Function and Chronic Kidney Disease Associated with Different Malondialdehyde Levels
| MDA (μmol/L) | OR (95% CI) | ORa (95% CI) | ORb (95% CI) | |
|---|---|---|---|---|
| MIKF | <2.90 | 1.00 | 1.00 | 1.00 |
| 2.90–3.43 | 1.12 (0.83–1.51) | 1.34 (0.96–1.86) | 1.33 (0.94–1.88) | |
| 3.43–4.01 | 1.23 (0.91–1.65) | 1.49 (1.07–2.08) | 1.46 (1.02–2.07) | |
| ≥4.01 | 2.23 (1.68–2.97) | 2.19 (1.59–3.03) | 1.99 (1.41–2.81) | |
| p=0.000 | p=0.000 | p=0.000 | ||
| CKD | <2.90 | 1.00 | 1.00 | 1.00 |
| 2.90–3.43 | 1.17 (0.55–2.49) | 2.00 (0.89–4.53) | 2.01 (0.86–4.67) | |
| 3.43–4.01 | 1.63 (0.79–3.33) | 2.57 (1.18–5.56) | 3.11 (1.39–6.93) | |
| ≥4.01 | 2.41 (1.20–4.83) | 2.70 (1.29–5.70) | 3.23 (1.45–7.19) | |
| p=0.013 | p=0.009 | p=0.004 | ||
| Nc | 1111/588/72 | 1111/588/72 | 1017/560/70 |
Adjusted for age and gender.
Adjusted for age, gender, alcohol intake, tobacco consumption, sleep quality, physical activity, BMI, systolic blood pressure, diastolic blood pressure, blood glucose level, and serum levels of cholesterol, triglyceride, ALT and AST.
Nc=normal kidney function/MIKF/CKD.
MIKF, Mild insufficiency of kidney function; CKD, chronic kidney disease; MDA, reactive carbonyl species; OR, odds ratio; CI, confidence interval.
Given that different confounding factors may have potential effects on MIKF risk, the MIKF risk related to quartiles of plasma MDA was stratified by subgroups (Table 4). All values were adjusted for age and other covariates. The ratios (N$) of MIKF/healthy showed an obvious risk increase in the subgroups of male, elder age, higher BMI, current alcohol intake, current tobacco consumption, sleep “well,” and active physical activity compared with the corresponding subgroup, respectively (Table 4). Although the cases of the group of age <25 years were not enough for precise multinomial logistic regression, the overall trend of multiadjusted ORs in each subgroup were similar to the trend of multiadjusted ORs in Table 3. As indicated by the p value for trend, most subgroups of the confounding factors showed a significant risk increase along with the increment of the quartiles of plasma MDA (p<0.05), except for the subgroups of age ≥65 years, no tobacco consumption, sleep not too well, and active physical activity (p>0.05) (Table 4).
Table 4.
Mild Insufficiency of Kidney Function Risk Associated with Malondialdehyde Levels Stratified by Gender, Age, Body Mass Index, Alcohol Intake, Tobacco Consumption, Sleep Quality, and Physical Activity
| |
|
|
ORa (95%CI) |
||||
|---|---|---|---|---|---|---|---|
| MIKF | Nb | <2.90 | 2.90–3.43 | 3.43–4.01 | ≥4.01 | p value | |
| Gender | male | 400/612 | 1.00 | 1.32 (0.86–2.03) | 1.41 (0.91–2.18) | 1.85 (1.21–2.82) | 0.004 |
| female | 160/405 | 1.00 | 1.15 (0.61–2.15) | 1.33 (0.71–2.49) | 1.92 (1.01–3.63) | 0.046 | |
| Age(years) | <25 | 6/62 | 1.00 | N/A | N/A | N/A | N/A |
| 25–65 | 443/943 | 1.00 | 1.30 (0.90–1.86) | 1.36 (0.94–1.98) | 1.92 (1.34–2.75) | 0.000 | |
| ≥65 | 111/12 | 1.00 | 3.53 (0.18–71.21) | 1.94 (0.17–22.15) | 4.80 (0.26–87.67) | 0.289 | |
| BMI (kg/m2) | <25 | 343/693 | 1.00 | 1.24 (0.81–1.90) | 1.40 (0.91–2.16) | 1.96 (1.27–3.04) | 0.002 |
| ≥25 | 217/324 | 1.00 | 1.58 (0.85–2.91) | 1.58 (0.85–2.96) | 2.03 (1.13–3.65) | 0.017 | |
| Alcohol intake | no | 168/314 | 1.00 | 0.95 (0.49–1.87) | 1.38 (0.72–2.65) | 2.05 (1.08–3.90) | 0.028 |
| yes | 302/491 | 1.00 | 1.61 (1.00–2.59) | 1.47 (0.91–2.39) | 1.99 (1.24–3.20) | 0.005 | |
| Tobacco consumption | no | 226/390 | 1.00 | 1.06 (0.61–1.86) | 1.04 (0.58–1.84) | 1.51 (0.88–2.58) | 0.131 |
| yes | 255/432 | 1.00 | 1.69 (1.00–2.87) | 1.81 (1.07–3.08) | 2.10 (1.23–3.58) | 0.007 | |
| Sleep quality | well | 402/606 | 1.00 | 1.52 (0.99–2.33) | 1.44 (0.93–2.22) | 2.18 (1.43–3.30) | 0.000 |
| not too well | 106/221 | 1.00 | 1.47 (0.66–3.27) | 1.69 (0.72–4.00) | 1.52 (0.65–3.55) | 0.329 | |
| Physical activity | sedentary | 259/610 | 1.00 | 1.22 (0.74–2.02) | 1.81 (1.10–2.99) | 2.10 (1.29–3.41) | 0.003 |
| active | 240/205 | 1.00 | 1.42 (0.77–2.60) | 0.90 (0.48–1.68) | 1.84 (1.00–3.40) | 0.051 | |
Adjusted for age, gender, alcohol intake, tobacco consumption, sleep quality, physical activity, BMI, systolic blood pressure, diastolic blood pressure, blood glucose level, and serum levels of cholesterol, triglyceride, ALT and AST.
Nb=MIKF/normal kidney function.
MIKF, Mild insufficiency of kidney function; BMI, body mass index; OR, odds ratio; CI, confidence interval; N/A, not available (cases not enough for multinomial logistic regression).
To evaluate the contributions of different confounding factors to plasma MDA, means and SDs of plasma MDA were compared between subgroups (Table 5). Intriguingly, the effects of different covariates on levels of plasma MDA were similar to the effects on MIKF. The subgroups of male, older age, and overweight had significantly higher levels of plasma MDA in comparison to correspondent subgroup, respectively (p<0.05) (Table 5). The subjects with alcohol intake, tobacco consumption, sleep well, or active physical activity also contributed to a relatively increase of MDA (Table 5).
Table 5.
Comparison of Mean±Standard Deviation of Plasma Malondialdehyde Levels within Strata of Gender, Age, Body Mass Index, Alcohol Intake, Tobacco Consumption, Sleep Quality, and Physical Activity
| Risk factor | n | MDA | p valuea | |
|---|---|---|---|---|
| Gender | male | 1371 | 3.63±1.06 | |
| female | 758 | 3.41±1.15 | 0.000 | |
| Age (year) | <25 | 132 | 3.61±1.04 | |
| 25-65 | 1786 | 3.50±0.91 | 0.585 | |
| ≥65 | 191 | 4.06±2.15 | 0.036 | |
| BMI (kg/m2) | <25 | 1267 | 3.51±1.12 | |
| ≥25 | 642 | 3.73±1.13 | 0.000 | |
| Alcohol intake | no | 642 | 3.56±1.28 | |
| yes | 1056 | 3.58±1.00 | 0.713 | |
| Tobacco consumption | no | 846 | 3.54±1.16 | |
| yes | 884 | 3.62±1.05 | 0.124 | |
| Sleep quality | well | 1342 | 3.62±1.15 | |
| not too well | 425 | 3.50±1.07 | 0.064 | |
| Physical activity | sedentary | 1157 | 3.57±1.03 | |
| active | 586 | 3.61±1.26 | 0.416 |
Comparing differences between mean values by category.
MDA, Reactive carbonyl species; BMI, body mass index; n, number.
Discussion
Debates continue regarding the decrease of renal function with age. The National Health and Nutrition Examination Survey cross-sectional study supports kidney function decline with age in normal-aged individuals,33 whereas some subjects have no absolute reduction in renal function.34,35 Nonetheless, this population-based study confirmed that renal function, as indicated by eGFR, showed a significantly progressive decrease with aging.
Although the rate of kidney function decline was slow, this process might have negative effects on multiple organ systems and decrease overall health and physical function in elderly individuals. Our results showed that the subjects with reduced kidney function had significantly higher levels of blood pressure and fasting glucose than those with normal renal function. These data are consistent with the observations that impaired kidney function, especially end-stage renal disease, is a major risk factor for cardiovascular diseases and premature death.9,36 A rational explanation for the importance of kidney function in overall health and other physical functions may be that the presence of adequate kidney function is critical to effectively keeping the body load of stressors at nontoxic levels. Insufficient kidney function, by contrast, causes stress load in the body and subsequently promotes an increase in inflammation and/or oxidative stress, leading to further structural and functional damage to organ systems.
Intriguingly, both MIKF and CKD participants of this large cohort study had significantly higher levels of plasma MDA as compared with normal renal function participants. The values of eGFR also showed a negative correlation with plasma MDA. Because MDA is a widely used biomarker of oxidative stress,37,38 this result seems to support the notion that insufficient kidney function, with decreased capacity to excrete oxidative stressors, will further enhance oxidative stress. However, is the increased level of MDA just a consequence of insufficient renal function?
The major finding of this study is that the higher plasma MDA level was a direct risk factor for developing of MIKF or CKD. As indicated by the multivariate-adjusted ORs, individuals with the highest quartile of MDA level had a 99% and 223% increased risk of developing MIKF and CKD, respectively, as compared to the lowest quartile. Intriguingly, the effects of different lifestyles or undesirable physical conditions on both the risk of MIKF and the increase of MDA were similar. As a corollary, the increased risk of MIKF by these factors may be, at least in part, through increasing MDA levels. These findings seem logical and are consistent with most existing evidence that supports the potential role of oxidative stress in the progression of kidney function decline.18,21 However, to the best of our knowledge, the present study is the first report to provide conceivable support that plasma MDA is one of the pathogenic factors and an important index for MIKF and CKD in a large population-based study.
Given that MDA are molecules with high nucleophilic reactivity, they can readily attack most macromolecules, especially the sulfhydryl and/or amino groups of protein.39 As a result, we can postulate that the increased levels of MDA in the body cause specific damage to macromolecules, leading to structural and functional changes to cell components, further promoting oxidative stress and inflammation and accelerating the generation of MDA, a cyclical process that underlies the progressive decline in physiologic function and the development of diseases. In fact, some studies have illustrated a direct risk role of MDA in a series of pathophysiologic processes,28,30,40,41 and some potential antioxidant agents, including pyridoxamine and melatonin, perform their roles in the body by acting as MDA scavengers.42,43 However, whether MDA is merely an index of oxidative stress or also a direct risk factor for developing MIKF and/or CKD is still an open question at present. Further investigation is needed to determine the underlying mechanisms that contribute directly to the pathogenesis of MIKF and/or CKD from molecular, animal, and other model systems.
This study had several limitations. First, although MDA is the most widely used biomarker of oxidative stress, other in vivo biomarkers exist, such as F2-isoprostanes.44 Second, the method for evaluating the demographics by a self-administered questionnaire should not be absolute. Finally, the sample is limited to Chinese Han adults from urban areas in the central region of China, and the applicability of the results to rural residents and other nationalities and ethnic groups is unknown.
In summary, our findings provide credible evidence that oxidative stress, as indicated by plasma MDA levels, is associated with the prevalence of MIKF and/or CKD. On the basis of the characteristics of MDA, high levels of MDA in plasma impose an increasing burden on the kidney and/or promote a cyclical process of oxidative stress in human body, which contributes to kidney function decline with age. The potential role of MDA in MIKF and/or CKD may be a practical target in pharmacology and clinical settings.
Acknowledgments
We are very grateful to Ms. Fang Lei for her critical reading of this paper. This work was supported by National Natural Science Funds of China (30800207), National 863 Grants of China (2008AA02Z411), National Basic Research Program of China (2010CB530500, 2010CB530503), and Scientific Research Funds of Hunan Provincial Education Department.
Author Disclosure Statement
The authors declare that they have no conflicts of interest relevant to this study.
References
- 1.Yin DZ. Chen KJ. The essential mechanisms of aging: Irreparable damage accumulation of biochemical side-reactions. Exp Gerontol. 2005;40:455–465. doi: 10.1016/j.exger.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Gourtsoyiannis N. Prassopoulos P. Cavouras D. Pantelidis N. The thickness of the renal parenchyma decreases with age: A CT study of 360 patients. AJR. Am J Roentgenol. 1990;155:541–544. doi: 10.2214/ajr.155.3.2117353. [DOI] [PubMed] [Google Scholar]
- 3.Rowe JW. Andres R. Tobin JD. Norris AH. Shock NW. The effect of age on creatinine clearance in men: A cross-sectional and longitudinal study. J Gerontol. 1976;31:155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 4.Fehrman-Ekholm I. Skeppholm L. Renal function in the elderly (>70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand J Urol Nephro. 2004;38:73–77. doi: 10.1080/00365590310015750. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J. Selvin E. Stevens LA. Manzi J. Kusek JW. Eggers P. Van Lente F. Levery AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS. Stevens LA. Schmid CH. Zhang Y. Castro AF., III Feldman HI. Kusek JW. Eggers P. Van Lente F. Greene T. Coresh J for the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Zee S. Baber U. Elmariah S. Winston J. Fuster V. Cardiovascular risk factors in patients with chronic kidney disease. Nat Rev Cardiol. 2009;6:580–589. doi: 10.1038/nrcardio.2009.121. [DOI] [PubMed] [Google Scholar]
- 8.Schiffrin EL. Lipman ML. Mann JF. Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 9.Go AS. Chertow GM. Fan D. McCulloch CE. Hsu C-y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10.Salmon AH. Neal CR. Harper SJ. New aspects of glomerular filtration barrier structure and function: Five layers (at least) not three. Curr Opin Nephrol Hypertens. 2009;18:197–205. doi: 10.1097/MNH.0b013e328329f837. [DOI] [PubMed] [Google Scholar]
- 11.Kim J. Sohn E. Kim CS. Kim JS. Renal podocyte apoptosis in Zucker diabetic fatty rats: Involvement of methylglyoxal-induced oxidative DNA damage. J Comp Pathol. 2011;144:41–47. doi: 10.1016/j.jcpa.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Tojo A. Asaba K. Onozato ML. Suppressing renal NADPH oxidase to treat diabetic nephropathy. Expert Opin Ther Targets. 2007;11:1011–1018. doi: 10.1517/14728222.11.8.1011. [DOI] [PubMed] [Google Scholar]
- 13.Nagasu H. Satoh M. Yorimitsu D. Tomita N. Sasaki T. Kashihara N. Comparison of combination therapy of olmesartan plus azelnidipine or hydrochlorothiazide on renal and vascular damage in SHR/NDmcr-cp rats. Kidney Blood Press Res. 2011;34:87–96. doi: 10.1159/000323535. [DOI] [PubMed] [Google Scholar]
- 14.Forbes JM. Coughlan MT. Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 15.Oberg BP. McMenamin E. Lucas FL. McMonagle E. Morrow J. Alp Ikizler T. Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 17.Baynes JW. Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Silva E. Pinto V. Simao S. Serrao MP. Afonso J. Amaral J. Pinho MJ. Gomes P. Soares-da-Silva P. Renal aging in WKY rats: changes in Na+,K+-ATPase function and oxidative stress. Exp Gerontol. 2010;45:977–983. doi: 10.1016/j.exger.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Uribarri J. Cai W. Peppa M. Goodman S. Ferrucci L. Striker G. Vlassara H, et al. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaminathan S. Shah SV. Novel approaches targeted toward oxidative stress for the treatment of chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:143–148. doi: 10.1097/MNH.0b013e3282f4e539. [DOI] [PubMed] [Google Scholar]
- 21.Locatelli F. Canaud B. Eckardt KU. Stenvinkel P. Wanner C. Zoccali C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH. Jung KJ. Kim JW. Kim HJ. Yu BP. Chung HY. Suppression of apoptosis by calorie restriction in aged kidney. Exp Gerontol. 2004;39:1361–1368. doi: 10.1016/j.exger.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Qiao X. Chen X. Wu D. Ding R. Wang J. Hong Q. Shi S. Li J. Xie Y. Lu Y. Wang Z. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci. 2005;60:830–839. doi: 10.1093/gerona/60.7.830. [DOI] [PubMed] [Google Scholar]
- 24.Choksi KB. Nuss JE. Boylston WH. Rabek JP. Papaconstantinou J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes. Free Radic Biol Med. 2007;43:1423–1438. doi: 10.1016/j.freeradbiomed.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS. Coresh J. Balk E. Kausz AT. Levin A. Steffes MW. Hogg RJ. Perrone RD. Lau J. Eknoyan G National Kideny Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 26.National-Kidney-Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 27.Baskurt OK. Boynard M. Cokelet GC. Connes P. Cooke BM. Forconi S. Liao F. Hardeman MR. Jung F. Meiselman HJ. Nash G. Nemeth N. Neu B. Sandhagen B. Shin S. Thurston G. Wautier JL International Expert Panel for Standardization of Hemorheological Methods. New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc. 2009;42:75–97. doi: 10.3233/CH-2009-1202. [DOI] [PubMed] [Google Scholar]
- 28.Chen K. Xie F. Liu S. Li G. Chen Y. Shi W. Hu H. Liu L. Yin D. Plasma reactive carbonyl species: Potential risk factor for hypertension. Free Radic Res. 2011;45:568–574. doi: 10.3109/10715762.2011.557723. [DOI] [PubMed] [Google Scholar]
- 29.WHO-Expert-Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 30.Liu S. Shi W. Li G. Jin B. Chen Y. Hu H. Liu L. Xie F. Chen K. Yin D. Plasma reactive carbonyl species levels and risk of nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26:1010–1015. doi: 10.1111/j.1440-1746.2011.06672.x. [DOI] [PubMed] [Google Scholar]
- 31.Li G. He H. Yan H. Zhao Q. Yin D. Does carbonyl stress cause increased blood viscosity during storage? Clin Hemorheol Microcirc. 2010;44:145–154. doi: 10.3233/CH-2010-1263. [DOI] [PubMed] [Google Scholar]
- 32.Hosmer DW. Lemeshow S. Applied Logistic Regression. 2nd. Wiley; New York: 2000. [Google Scholar]
- 33.Coresh J. Astor BC. Greene T. Eknoyan G. Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 34.Lindeman RD. Tobin J. Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 35.Dols LF. Weimar W. Ijzermans JN. Long-term consequences of kidney donation. N Engl J Med. 2009;360:2371–2372. author reply 2372. [PubMed] [Google Scholar]
- 36.Pizzarelli F. Lauretani F. Bandinelli S. Windham GB. Corsi AM. Giannelli SV. Ferrucci L. Guralnik JM. Predictivity of survival according to different equations for estimating renal function in community-dwelling elderly subjects. Nephrol Dial Transplant. 2009;24:1197–1205. doi: 10.1093/ndt/gfn594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadiiska MB. Gladen BC. Baird DD. Germolec D. Graham LB. Parker CE. Nyska A. Wachsman JT. Ames BN. Basu S. Brot N. Fitzgerald GA. Floyd RA. George M. Heinecke JW. Hatch GE. Hensley K. Lawson JA. Marnett LJ. Morrow JD. Murray DM. Plastaras J. Roberts LJ., 2nd Rokach J. Shigenaga MK. Sohal RS. Sun J. Tice RR. Van Thiel DH. Wellner D. Walter PB. Tomer KB. Mason RP. Barrett JC. Biomarkers of oxidative stress study II. Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Gil L. Siems W. Mazurek B. Gross J. Schroeder P. Voss P. Grune T. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res. 2006;40:495–505. doi: 10.1080/10715760600592962. [DOI] [PubMed] [Google Scholar]
- 39.Aldini G. Dalle-Donne I. Colombo R. Maffei Facino R. Molzani A. Carini M. Lipoxidation-derived reactive carbonyl species as potential drug targets in preventing protein carbonylation and related cellular dysfunction. ChemMedChem. 2006;1:1045–1058. doi: 10.1002/cmdc.200600075. [DOI] [PubMed] [Google Scholar]
- 40.Levine RL. Stadtman ER. Carbonylated proteins and their implication in physiology and pathology. In: Desiderio DM, editor; Nibbering NM, editor. Redox Proteomics: From Protein Modifications to Cellular Dysfunction and Diseases. vol 1. John Wiley & Sons, Inc.; New Jersey: 2006. pp. 123–168. [Google Scholar]
- 41.Weismann D. Hartvigsen K. Lauer N. Bennett KL. Scholl HP. Charbel Issa P. Cano M. Brandstatter H. Tsimikas S. Skerka C. Superti-Furga G. Handa JT. Zipfel PF. Witztum JL. Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang Z. Li H. Li G. Yin D. Reaction of pyridoxamine with malondialdehyde: Mechanism of inhibition of formation of advanced lipoxidation end-products. Amino Acids. 2006;30:55–61. doi: 10.1007/s00726-005-0209-6. [DOI] [PubMed] [Google Scholar]
- 43.Li G. Li L. Yin D. A novel observation: Melatonin's interaction with malondiadehyde. Neuroendocrinol Lett. 2005;26:61–66. [PubMed] [Google Scholar]
- 44.Roberts LJ. Morrow JD. Measurement of F-2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]