Abstract
Transgenic zebrafish have been utilized for in vivo analysis of cell behaviors using advanced imaging techniques, for analyzing spatiotemporal gene regulation, and for targeted mis-expression of transgenes. The Tg(fli1a:EGFP)y1 vascular reporter has been particularly useful for examining the development of blood and lymphatic vessels, but it has been suggested that whole-mount in situ hybridization may result high background staining in this line, potentially limiting its usefulness. Here, we show that off-target hybridization of plasmid vector-derived probes to tissues expressing transgenes occurs in a number of different commonly used transgenic lines as a result of multiple cloning site sequences present in the cloning vectors, suggesting this may be a more general problem. However, we also show that this problem is easily avoided by performing in situ hybridization using probes synthesized from PCR templates lacking vector sequences.
Introduction
Transgenic zebrafish lines have become crucial tools for in vivo analysis of developmental- and tissue-specific gene regulation, and for targeted expression of transgenes of interest.1 The high transparency of zebrafish embryos, together with advanced imaging techniques such as confocal or two-photon microscopy, permits visualization of cell behaviors at the single cell level in the living transgenic zebrafish expressing fluorescent reporters such as green fluorescent protein (GFP) in specific cells or tissues.2,3 The Tg(fli1a:EGFP)y1 line,4 a vascular-specific transgenic zebrafish with fluorescently “tagged” blood and lymphatic vessels, has become very widely used for studying the development of these vessels via in vivo time-lapse imaging, for genetic and chemical screening, and for observing the vascular consequences of other experimental manipulations.3
It is often useful to perform whole-mount in situ hybridization (WISH) to analyze gene expression changes in the same transgenic zebrafish embryos that exhibit phenotypic alterations induced by experimental manipulation. However, a report in ZFIN (ZFIN ID: ZDB-ALT-011017-8) has suggested that WISH with probes generated from linearized plasmid templates give rise to “high background staining” in Tg(fli1a:EGFP)y1 embryos and larvae. Since this issue has not been clarified, we analyzed this in more detail.
Materials and Methods
Zebrafish
Zebrafish (Danio rerio) embryos were obtained from natural spawning of laboratory lines. Embryos were raised and fish maintained as previously described.5,6 Zebrafish lines used in the study are wild-type EK, Tg(fli1a:EGFP)y1,4 Tg(flk:EGFP)la116, Tg(gata1:DsRed)sd2,7,8 and Tg(HuC:GFP).9 Embryos were treated with 1-phenyl-2-thiourea (PTU) to inhibit pigment formation6 to facilitate imaging and staining for whole-mount in situ hybridization (WISH).
Plasmid constructs and a PCR-based template for in situ RNA probes
A 552 bp PCR product for nadl1.2 was generated with Nadl1.2F (AAGGACTGCCCATCATCAAG) and Nadl1.2R (GACTGGCTGTACGCTTCTCC) primers. A 565 bp PCR product for tnnt2 was generated with tnnt2F (GAGAAAGAGTCGATTTTGATGACA) and tnnt2R (GTTTTCTGATGGTCACTGACTCTG) primers. Stage mixed cDNA synthesized using ThermoScript RT-PCR system (Invitrogen) according to the manufacturer's instruction was used as a template for PCR reaction. PCR conditions were as follows: PCR activation step 95°C, 5 min; denaturation 95°C, 20 sec; annealing 55°C, 30 sec; extension 72°C, 30 sec; cycles from denaturation to extension were repeated 32 times; final extension 72°C, 7 min. The PCR product was cloned into pGEM-T vector (Promega) and pCRII-TOPO vector (Invitrogen). For PCR-based in situ template, SP6_Nadl1.2R primer (GCCAAGCTATTTAGGTGACACTATAGAAGACTGGCTGTACGCTTCTCC; SP6 promoter sequence is underlined) and SP6_tnnt2R (GCCAAGCTATTTAGGTGACACTATAGAAGTTTTCTGATGGTCACTGACTCTG; SP6 promoter sequence is underlined) were used with Nadl1.2F and tnnt2F primer respectively.
Whole-mount in situ hybridization
RNA probes were labeled with DIG using a DIG RNA labeling kit (Roche). Before the labeling reaction, all pGEM-T and pCRII-TOPO vectors with or without inserts were digested with SacII and EcoRV restriction enzymes, respectively, for linearization. In vitro transcription from plasmid vector- and PCR-based templates were carried out using SP6 RNA polymerase. WISH was carried out as described previously with some modifications.10 After proteinase K treatment, embryos were pre-hybridized for 5 h at 70°C. Hybridization was carried out in HB4 buffer (50% formamide, 5X SSC, 50 μg/mL heparin, 0.1% Tween-20, 5 mg/mL torula RNA) for 16 h at 70°C. For the consistency of the in situ condition, same concentration of in situ probes (120 ng/100 μL HB4 buffer) was used for hybridization. Washing was carried out as following: 1 round of washing with HB4 buffer for 10 min at 70°C; 2 rounds of washing with washing solution 1 (50% formamide, 2X SSC, 0.1% Tween-20) for 50 min at 70°C; 1 round of washing with washing solution 2 (2X SSC, 0.1% Tween-20) for 30 min at 70°C; 2 rounds of washing with washing solution 3 (0.2X SSC, 0.1% Tween-20) for 50 min at 70°C. After washing, embryos were blocked with 1X blocking reagent solution (Roche) for 1 h at room temperature and incubated with anti-DIG-AP antibody (1/3000 dilution) in 1X blocking reagent solution for 2 h at room temperature. After overnight washing, DIG-labeled probes were color stained with BM purple (Roche). After fixation, stained embryos were mounted with 9% methyl cellulose and imaged with a ProgRes C14 camera mounted on a Leica MZ12 stereomicroscope for single time point imaging.
Results and Discussion
To examine the whole mount in situ hybridization (WISH) “background” problem in Tg(fli1a:EGFP)y1 embryos and larvae, we performed WISH using probes generated from different template sources to determine whether this affected the specificity of hybridization. We used neural adhesion molecule L1.2 (nadl1.2)11 as a test probe, since the expression of this gene is known to be restricted to the brain and central nervous system.12 In wild-type EK strain embryos, nadl1.2 probes generated from pCRII-TOPO vector (Figs. 1A and B) or pGEM-T vectors (Figs. 1G and H), or from a gene-only template amplified from nadl1.2 cDNA with an SP6 promoter-containing primer by PCR (Figs. 1M and N), all consistently and specifically detected nadl1.2 expression only in the brain and central nervous system. In contrast, in Tg(fli1a:EGFP)y1 embryos probes generated from the two plasmid templates resulted in in situ staining of blood vessels such as the dorsal aorta, cardinal vein, and growing intersegmental vessels consistent with EGFP expression domains in Tg(fli1a:EGFP)y1 embryos (Supplementary Figs. S1C and S1D; supplementary material is available online at www.liebertonline.com/zeb) in addition to the endogenous nadl1.2 expression domains in the brain and central nervous system (Figs. 1C, D, I, and J). In addition, a probe generated from pGEM-T vector showed nonspecific staining in the head (Fig. 1I), which is consistent with the staining with a probe generated from multi-cloning site (MCS) sequence of pGEM-T vector only without insert (Fig. 3K). However, a probe generated from a PCR-based template still showed in situ staining only in the endogenous nadl1.2 expression domains, not in the vasculature (Figs. 1O and P). These results suggest that in situ hybridization probes generated from cloning vectors are detecting both their target nadl1.2 transcripts and transcripts expressed from the Tg(fli1a:EGFP)y1 transgene.
FIG. 1.
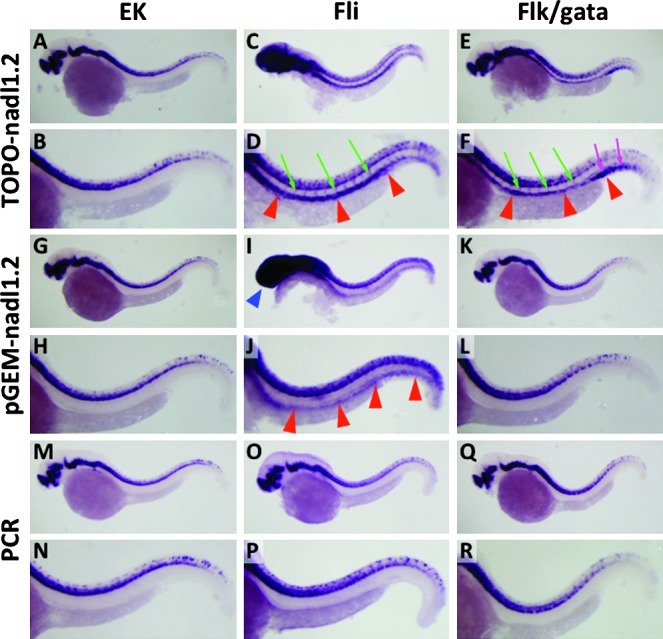
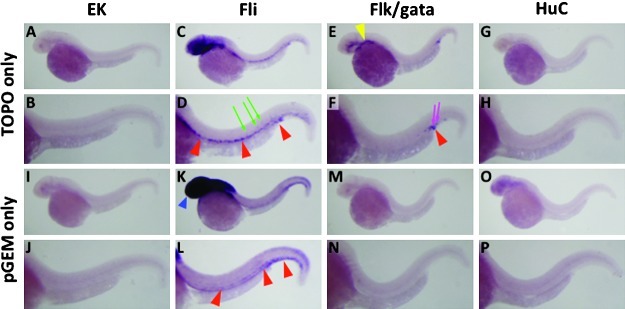
Comparative whole-mount in situ hybridization (WISH) staining of 32 hpf wild-type EK strain (A, B, G, H, M, N), Tg(fli1a:EGFP)y1 (C, D, I, J, O, P), and Tg(flk:EGFP)la116;Tg(gata1:DsRed)sd2 (E, F, K, L, Q, R) embryos using Nadl1.2 probes generated from pCRII-TOPO vector (A–F), pGEM-T vector (G–L), or PCR-based template (M–R). Panels B, D, F, H, J, L, N, P, and R are magnified views of the trunk regions of the embryos in panels A, C, E, G, I, K, M, O, and Q, respectively. Red arrowheads indicate off-target staining in axial vessels such as dorsal aorta and posterior cardinal vein. Green arrows show off-target staining in intersegmental vessels. Blue arrowhead indicates nonspecific staining by pGEM-T vector in the head of Tg(fli1a:EGFP)y1 embryo (see Fig. 3K). Magenta arrows note off-target staining in the posterior blood island.
FIG. 3.
Comparative whole-mount in situ hybridization (WISH) staining of 32 hpf wild type EK strain (A, B, I, J), Tg(fli1a:EGFP)y1 (C, D, K, L), Tg(flk:EGFP)la116;Tg(gata1:DsRed)sd2 (E, F, M, N), and Tg(Huc:EGFP) (G, H, O, P) embryos using multi-cloning site (MCS) sequence probes generated from pCRII-TOPO vector (A–H), pGEM-T vector (I–P). Panels B, D, F, H, J, L, N, and P are magnified views of the trunk regions of the embryos in panels A, C, E, G, I, K, M, and O, respectively. Red arrowheads indicate off-target staining in axial vessels such as dorsal aorta and posterior cardinal vein. Green arrows show off-target staining in intersegmental vessels. Blue arrowhead indicates nonspecific staining by pGEM-T vector in the head of Tg(fli1a:EGFP)y1 embryo. Magenta arrows note off-target staining in the posterior blood island. Yellow arrowhead shows off-target staining in head blood vessels.
We then sought to determine whether off-target in situ hybridization is specific to Tg(fli1a:EGFP)y1 transgenic embryos or a more generalized problem in embryos containing transgenes. To examine this question, we hybridized the same nadl1.2 probes to Tg(flk:EGFP);Tg(gata1:DsRed) double transgenic embryos.7,8 All three probes tested showed robust expression in the brain and central nervous system, as expected for endogenous nadl1.2 (Figs. 1E, F, K, L, Q, and R). In addition, the pCRII-TOPO probe strongly stained both the posterior blood island as well as blood vessels including the dorsal aorta, posterior cardinal vein, and primary angiogenic sprouts (Figs. 1E and F) that are consistent with EGFP and DsRed expression domains in Tg(flk:EGFP);Tg(gata1:DsRed) double transgenic embryos (Supplementary Figs. S1E and S1F), while the pGEM-T probe did not give rise to any off-target staining (Figs. 1K and L). However, off-target staining in the posterior blood island was detected with the pGEM-T probe at lower hybridization temperature (65°C; data not shown). As in Tg(fli1a:EGFP)y1 embryos, off-target hybridization was not detected in Tg(flk:EGFP);Tg(gata1:DsRed) double transgenic embryos when probes generated from PCR-based templates were used (Figs. 1Q and R). These results suggest that off-target hybridization to transgene transcripts could be a more general problem when performing in situ hybridization on embryos and larvae from transgenic lines.
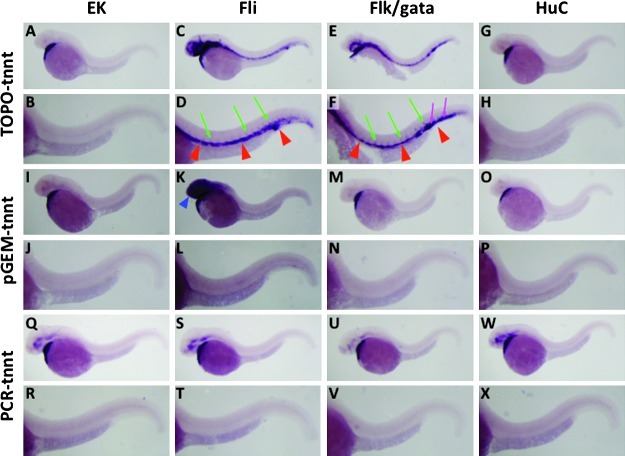
Next, we further analyzed whether off-target hybridization is found when using probes generated from plasmid templates for different genes in transgenic embryos. We selected cardiac troponin T (tnnt2) as a test probe since its expression is known to be highly specific to heart.13 In wild-type EK strain embryos, tnnt2 probes generated from plasmids and PCR product (Figs. 2A, B, I, J, Q, and R), all consistently and specifically detected tnnt2 expression only in the heart. In contrast, in Tg(fli1a:EGFP)y1 embryos a probe generated from the pCRII-TOPO vector template resulted in in situ staining of blood vessels such as the dorsal aorta, cardinal vein, and growing intersegmental vessels, in addition to the endogenous tnnt2 expression in the heart (Figs. 2C and D). In contrast, a probe generated from pGEM-T vector did not show strong off-target staining in the trunk blood vessel but instead had nonspecific staining in the head (Figs. 2K and L) that was also seen when using probes made only from vector sequences in the pGEM-T vector plasmid (Fig. 3K). Like the nadl1.2 probe, a tnnt2 probe synthesized from a PCR-based template still showed in situ staining only in its endogenous expression domains, not in the vasculature or head (Figs. 2S and T). In Tg(flk:EGFP);Tg(gata1:DsRed) double transgenic embryos, all three probes tested showed robust expression in the heart, as expected for endogenous tnnt2 (Figs. 2E, M, and U). In addition, the pCRII-TOPO probe strongly stained both the posterior blood island as well as blood vessels including the dorsal aorta, posterior cardinal vein, and primary angiogenic sprouts (Figs. 2E and F), while the pGEM-T probe did not give rise to any off-target staining with a 70°C hybridization (Figs. 2M and N). As in Tg(fli1a:EGFP)y1 embryos, off-target hybridization was not detected in Tg(flk:EGFP);Tg(gata1:DsRed) double transgenic embryos when probes generated from PCR-based templates were used (Figs. 2U and V). We also performed in situ hybridization with tnnt2 probe in the Tg(HuC:GFP) transgenic line in which GFP is specifically expressed in neurons of the brain and spinal cord (Supplementary Figs. 1G and 2H).9 In contrast to the other two transgenic lines, tnnt2 probes did not show off-target hybridization in the Tg(HuC:GFP) embryos with either plasmid vector- or PCR-template-derived probes (Figs. 2G, H, O, P, W, and X). These results suggest that off-target staining is dependent both on the cloning plasmids used for making probes and on the specific transgenic line used for in situ hybridization.
FIG. 2.
Comparative whole-mount in situ hybridization (WISH) staining of 32 hpf wild-type EK strain (A, B, I, J, Q, R), Tg(fli1a:EGFP)y1 (C, D, K, L, S, T), Tg(flk:EGFP)la116;Tg(gata1:DsRed)sd2 (E, F, M, N, U, V) and Tg(Huc:EGFP) (G, H, O, P, W, X) embryos using tnnt2 probes generated from pCRII-TOPO vector (A–H), pGEM-T vector (I–P), or PCR-based template (Q–X). Panels B, D, F, H, J, L, N, P, R, T, V, and X are magnified views of the trunk regions of the embryos in panels A, C, E, G, I, K, M, O, Q, S, U, and W, respectively. Red arrowheads indicate off-target staining in axial vessels such as dorsal aorta and posterior cardinal vein. Green arrows show off-target staining in intersegmental vessels. Blue arrowhead indicates nonspecific staining by pGEM-T vector in the head of Tg(fli1a:EGFP)y1 embryo (see Fig. 3K). Magenta arrows note off-target staining in the posterior blood island.
Our results show that off-target in situ hybridization staining by cloning vector-derived nadl1.2 and tnnt2 probes in transgenic lines matches the transgene expression patterns, such as axial vessels and primary angiogenic sprouts and the posterior blood island in Tg(fli1a:EGFP)y1 and Tg(flk:EGFP);Tg(gata1:DsRed) embryos. This suggests that the off-target staining may be the result of residual cloning vector sequences in the transgene. We tested this possibility using probes containing only vector multiple cloning site (MCS) sequences, generated from “vector only” plasmids. A pCRII-TOPO MCS probe showed no hybridization in wild-type EK embryos (Figs. 3A and B), but detected transgene expression domains such as blood vessels in Tg(fli1a:EGFP)y1 embryos (Figs. 3C and D) and blood vessels and posterior blood island in Tg(flk:EGFP);Tg(gata1:DsRed) embryos (Figs. 3E and F). A pGEM-T MCS probe also demonstrated off-target staining of axial vessels in Tg(fli1a:EGFP)y1 embryos (Figs. 3K and L) but not in Tg(flk:EGFP);Tg(gata1:DsRed) embryos (Figs. 3M and N) or in wild-type EK embryos (Figs. 3I and J). This probe also showed nonspecific head staining in the Tg(fli1a:EGFP)y1 embryos (blue arrowhead in Fig. 3K). None of the probes showed off-target in situ staining in Tg(HuC:GFP) embryos (Figs. 3G, H, O, and P).
Most transgene constructs are generated using cloning procedures that involve the MCS of cloning vectors, with portions of the MCS sequences retained within the resulting transgene transcripts. These same MCS sequences would also be transcribed together with specific target sequences during the synthesis of in situ probes from plasmid templates, resulting in off-target cross-hybridization. This interpretation is supported by our findings that probes synthesized from PCR-derived templates, which contained only an SP6 initiation site in addition to the endogenous nadl1.2 and tnnt2 sequences, and did not contain any vector sequences, specifically detected only the endogenous nadl1.2 and tnnt2 expression domains both in Tg(fli1a:EGFP)y1 embryos (Figs. 1O and P; Fig. 2S and T) and in Tg(flk:EGFP);Tg(gata1:DsRed) double transgenic embryos (Figs. 1Q and R; Fig. 2U and V). Conversely, probes that contain only plasmid vector MCS sequences recapitulated the off-target in situ staining in the transgene expression domains in some transgenic embryos such as Tg(fli1a:EGFP)y1 embryos (Figs. 3C, D, K, and L; Supplementary Figs. S1C and S1D) and Tg(flk:EGFP);Tg(gata1:DsRed) embryos (Figs. 3E and F; Supplementary Figs. S1E and S1F), matching the off-target in situ staining with plasmid vector-derived nadl1.2 and tnnt2 probes in Tg(fli1a:EGFP)y1 embryos (Figs. 1C, D, I, and J; Figs. 2C and D) and Tg(flk:EGFP);Tg(gata1:DsRed) embryos (Figs. 1E and F; Figs. 2E and F). Indeed, sequence comparison clearly showed that 60 consecutive nucleotides in linker 1 located upstream of the EGFP coding sequence in the fli1a transgene exactly match MCS sequence in the pCRII-TOPO plasmid (Supplementary Fig. S2), confirming the idea that off-target hybridization in Tg(fli1a:EGFP)y1 embryos using plasmid-derived in situ probes is the result of cross-hybridization between MCS sequences in the fli1a transgene and cloning vector.
Our data clearly demonstrate that the previously reported WISH “high background” in Tg(fli1a:EGFP)y1 embryos is not in fact background hybridization but rather off-target in situ staining in the transgene expression domain by MCS sequences in the plasmid vector-derived in situ probes. The same type of off-target in situ staining was generated in Tg(flk:EGFP);Tg(gata1:DsRed) embryos with pCRII-TOPO-derived probes but not pGEM-T-derived probes. In contrast, the off-target in situ hybridization was not detected in Tg(HuC:GFP) transgenic embryos. Although off-target in situ staining was not always present in all the transgenic animals that we tested, our results suggest that caution should be exercised in interpreting WISH data from transgenic animals with plasmid vector-derived in situ probes because of the common procedures used for making transgenic constructs. We would suggest that WISH using probes generated from PCR-based templates will facilitate the analysis of gene expression in experimentally manipulated transgenic animals without resulting in potentially misleading results from off-target hybridization to transgenes.
Supplementary Material
Acknowledgments
We thank members of the Weinstein lab for technical help and suggestions. This research was supported by the intramural program of the NICHD, NIH.
Disclosure Statement
No competing financial interests exist.
References
- 1.Udvadia AJ. Linney E. Windows into development: Historic, current, and future perspectives on transgenic zebrafish. Dev Biol. 2003;256:1–17. doi: 10.1016/s0012-1606(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 2.Beis D. Stainier DY. In vivo cell biology: Following the zebrafish trend. Trends Cell Biol. 2006;16:105–112. doi: 10.1016/j.tcb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Cha YR. Weinstein BM. Visualization and experimental analysis of blood vessel formation using transgenic zebrafish. Birth Defects Res C Embryo Today. 2007;81:286–296. doi: 10.1002/bdrc.20103. [DOI] [PubMed] [Google Scholar]
- 4.Lawson ND. Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 5.Kimmel CB. Ballard WW. Kimmel SR. Ullmann B. Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 6.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4th. Eugene, OR: Univ. of Oregon Press; 2000. [Google Scholar]
- 7.Choi J. Dong L. Ahn J. Dao D. Hammerschmidt M. Chen JN. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev Biol. 2007;304:735–744. doi: 10.1016/j.ydbio.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traver D. Paw BH. Poss KD. Penberthy WT. Lin S. Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 9.Park HC. Kim CH. Bae YK. Yeo SY. Kim SH. Hong SK, et al. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000;227:279–293. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- 10.Hauptmann G. Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- 11.Holm J. Hillenbrand R. Steuber V. Bartsch U. Moos M. Lubbert H, et al. Structural features of a close homologue of L1 (CHL1) in the mouse: A new member of the L1 family of neural recognition molecules. Eur J Neurosci. 1996;8:1613–1629. doi: 10.1111/j.1460-9568.1996.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 12.Rauch GJ. Lyons DA. Middendorf I. Friedlander B. Arana N. Reyes T, et al. Submission and curation of gene expression data. http://zfinorg. 2003. http://zfinorg ZFIN Direct Data Submission. ZFIN ID: ZDB-PUB-031103–24.
- 13.Hsiao CD. Tsai WY. Horng LS. Tsai HJ. Molecular structure and developmental expression of three muscle-type troponin T genes in zebrafish. Dev Dyn. 2003;227:266–279. doi: 10.1002/dvdy.10305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.