Abstract
Dimebon was originally introduced as an antihistamine and subsequently investigated as a possible therapeutic for a variety of disorders, including Alzheimer's disease. One putative mechanism underlying the neuroprotective properties of Dimebon is inhibition of mitochondrial permeability transition, based on the observation that Dimebon inhibited the swelling of rat liver mitochondria induced by calcium and other agents that induce permeability transition. Because liver and brain mitochondria differ substantially in their properties and response to conditions associated with opening of the permeability transition pore, we sought to determine whether Dimebon inhibited permeability transition in brain mitochondria. Dimebon reduced calcium-induced mitochondrial swelling but did not enhance the calcium retention capacity or impair calcium-induced cytochrome C release from non-synaptic mitochondria isolated from rat brain cerebral cortex. These findings indicate that Dimebon does not inhibit mitochondrial permeability transition, induced by excessive calcium uptake, in brain mitochondria.
Keywords: Alzheimer's disease, Apoptosis, Neurodegeneration, Cell death
Introduction
William Markesbery devoted a large portion of his remarkable career to the neuropathology and mechanisms underlying late-onset neurodegenerative disorders including Alzheimer's disease (AD). One of Dr. Markesbery's first papers on AD questioned the aluminum hypothesis, demonstrating no significant difference in the aluminum content of various brain regions in AD when compared to age-matched controls (Markesbery et al. 1981). He subsequently became a major proponent of the oxidative stress theory of AD but cautioned on the difficulty in distinguishing between cause and effect (Markesbery 1997). Dr. Markesbery, together with colleagues including Mark Lovell, Allan Butterfield, and Mark Mattson, investigated the mechanisms and consequences of oxidative damage as well as potential therapeutics (Abdul et al. 2008; Sonnen et al. 2008; Lovell et al. 2009; Jicha and Markesbery 2010; Jo et al. 2010). Oxidative stress, along with an elevation in intracellular calcium, triggers opening of the mitochondrial permeability transition pore (mPTP) which has been implicated in Alzheimer's disease pathology (Bernardi et al. 2006; Du et al. 2009; Reddy 2009). In the present study, we investigated the hypothesis that inhibition of mitochondrial permeability transition may underlie the neuroprotective properties of a putative therapeutic for Alzheimer's disease, Dimebon. Dr. Markesbery was an outstanding investigator, clinician, and colleague, and it is a sincere pleasure to contribute to this special issue in his honor.
Dimebon (Latrepiridine; 2,3,4,5-tetrahydro-2,8-dimethyl-5-(2-(6-methyl-3-pyridyl)ethyl)-1H-pyrido(4,3-b)indole)) was introduced as an antihistamine in Russia in 1983 (Mateeva 1983) and was subsequently investigated as a possible therapeutic for a variety of disorders (Shadurskii et al. 1983; Galenko-Iaroshevskii et al. 1995, 1996). Based on its neuroprotective and cognitive-enhancing properties in the CNS (Shadurskaia et al. 1986; Lermontova et al. 2000; Bachurin et al. 2001; Lermontova et al. 2001), Dimebon was investigated as a possible treatment for Alzheimer's disease (Doody et al. 2008). Results from the initial large-scale clinical trial indicated that Dimebon was safe, improved cognitive performance, and decreased the rate of cognitive decline in patients with mild-to-moderate Alzheimer's disease when compared to placebo controls (Doody et al. 2008). However, a subsequent phase III clinical trial found no significant improvement, and additional clinical trials are ongoing (Miller 2010).
A variety of mechanisms have been proposed to account for the neuroprotective effects of Dimebon. These include inhibition of NMDA receptors (Grigorev et al. 2003), histamine H1 receptors (Wu et al. 2008), voltage-gated calcium channels (Lermontova et al. 2001), or the mPTP (Bachurin et al. 2003; Wu et al. 2008). The mPTP is a non-specific pore in the inner mitochondrial membrane whose opening is triggered by excessive intramitochondrial calcium accumulation and oxidative stress (Haworth and Hunter 1979; Zoratti and Szabo 1995). Opening of the mPTP results in rapid mitochondrial depolarization, mitochondrial swelling, release of calcium from the mitochondrial matrix, and release of pro-apoptotic proteins such as cytochrome C from the intermembrane space as a result of rupture of the outer mitochondrial membrane (Brustovetsky et al. 2002). Bachurin and colleagues found that Dimebon reduced the swelling of rat liver mitochondria induced by Ca2+, phosphate ions, tert-butylhydroxyperoxide, MPP+ (1-methyl-4-phenylpyridinium), and beta-amyloid25–35 (Bachurin et al. 2003). In the present study, we investigated the ability of Dimebon to attenuate Ca2+-induced swelling, cytochrome C release, and Ca2+ release of isolated non-synaptic rat brain mitochondria.
Methods
Mitochondrial Isolation
Non-synaptic mitochondria were isolated from the cortex of male Sprague Dawley rats, approximately 3 months of age, as previously described (Brown et al. 2006; Naga et al. 2007). Briefly, rats were euthanized under CO2 anesthesia, and brains were rapidly removed and dissected. The cortex was homogenized in isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, and 1 mM EGTA, pH adjusted to 7.2 with KOH) using a Dounce homogenizer. The homogenate was mixed with 30% Percoll in isolation buffer and layered onto a discontinuous Percoll density gradient (24%, 40%) and centrifuged at 30,4009×g in a Sorvall SE-12 rotor for 10 min. Mitochondria were obtained from the interface between the 24 and 40% layers. The mitochondrial fraction was resuspended in additional isolation buffer and centrifuged at 16,7009×g for 15 min.
Mitochondrial Swelling
Mitochondria, 100 μg, were suspended in 100 μl of respiration buffer containing 5 mM pyruvate, 2.5 mM malate, and 150uM ADP in a 96-well plate. To the above, 200 μM Dimebon (obtained from Nanosyn, Menlo Park, CA), or 5 μM cyclosporin A was added and the mixture was incubated for 15 min on ice. CaCl2, 250 μM, was added to induce mPTP opening, and mitochondrial swelling was determined by monitoring the absorbance that was recorded for 15 min at 540 nm, 37°C, on a Synergy Biotek Plate reader.
Mitochondrial Calcium Retention
Isolated non-synaptic mitochondria were resuspended in 2 ml of respiration buffer (125 mm KCl, 0.1% BSA, 20 mm HEPES, 2 mm MgCl2, 2.5 mm KH2PO4, pH 7.2, 50 mg protein/ml) and placed in a constantly stirred and thermostatically controlled (37°C) spectrophotometer (Shimadzu RF-5301PC). Ca2+ Green-5 N hexapotassium salt (CaG5N), 100nM, was added and a baseline reading obtained. This was followed by the addition of 5 mM pyruvate and 2.5 mM malate at 1 min, 150uM ADP at 2 min, and 1uM oligomycin at 3 min. Calcium infusion was then initiated at a rate of 160 nmole calcium/mg protein/minute, using a syringe pump (KD Scientific). The calcium infusion was stopped when mitochondria were unable to sequester any more calcium and the CaGN signal increased continuously.
Cytochrome C Release
The protocol was similar to that used for mitochondrial swelling, except that after 10 min of incubation with CaCl2, 37°C, the mitochondrial suspensions were centrifuged (16,2009×g, 4°C) to separate supernatants and pellets. About 25 μl of 4× Laemmili SDS sample buffer was added and the samples were boiled for 10 min. Equal volumes of samples were loaded onto SDS–PAGE gels, followed by western blotting for cytochrome C and the voltage-dependent anion channel as a loading control.
Statistical Analysis
Statistical analyses were performed using either an unpaired t test or a one-way ANOVA and Scheffe's post hoc analysis when appropriate. Results were considered significant when P < 0.05. The results are expressed as the group means ± SD from at least three independent experiments. The group size for each experiment is indicated in the figure legends.
Results
Dimebon Attenuates Calcium-Induced Mitochondrial Swelling But Does Not Alter Calcium Uptake Capacity
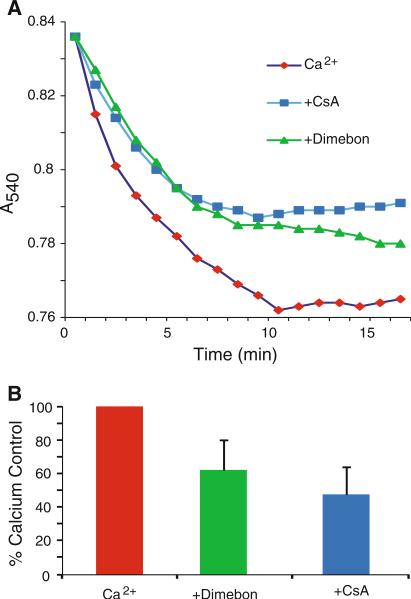
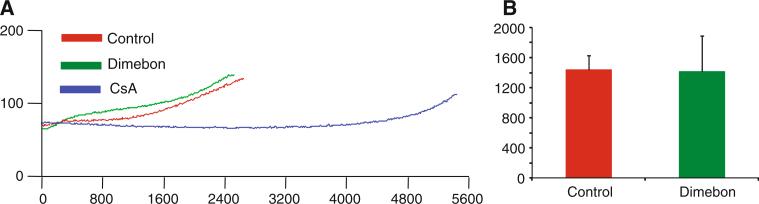
Dimebon, 200 μM, attenuated swelling of non-synaptic rat brain mitochondria induced by 250 μM CaCl2, similar to the reduction in swelling provided by 5 μM cyclosporine A (Fig. 1). However, unlike CsA, Dimebon did not improve the calcium uptake capacity of the rat brain mitochondria (Fig. 2).
Fig. 1.
Dimebon attenuates calcium-induced swelling of rat brain mitochondria. Non-synaptic mitochondria from rat brain were incubated with 250 μM CaCl2 for 15 min in the presence or absence of 200 μM Dimebon or 5 μM cyclosporine A (CsA) for 15 min. Mitochondrial swelling was monitored by a decrease in absorbance at 540 nm. Dimebon attenuated the calcium-induced swelling by 39% (P < 0.01), while CsA reduced swelling by 53% (P < 0.01). The results are expressed as the percentage of swelling induced by incubation with CaCl2 without Dimebon or CsA and represent the mean ± SD, n = 6
Fig. 2.
Dimebon does not alter calcium retention of rat brain mitochondria. Isolated non-synaptic mitochondria were placed in a constantly stirred, temperature-controlled cuvette as described in “Methods”. CaG5N fluorescence was monitored continuously. a Representative traces of CaG5N fluorescence from the onset of CaCl2 administration (160 nmol Ca2+/mg protein/minute). The time and amount of CaCl2 infused until the subsequent rise in CaG5N fluorescence, signifying mitochondrial permeability transition and Ca2+ release, was calculated. CsA (5 μM), included as a positive control, greatly enhanced the calcium retention capacity of non-synaptic rat brain mitochondria as described previously (Naga et al. 2007). b Quantitative results from four animals in each group. The calcium retention capacity of mitochondria treated with Dimebon (200 μM) was similar to that of mitochondria incubated with CaCl2 in the absence of Dimebon. Results are the mean ± SD, n = 4
Dimebon Does Not Alter Calcium-Induced Cytochrome C Release
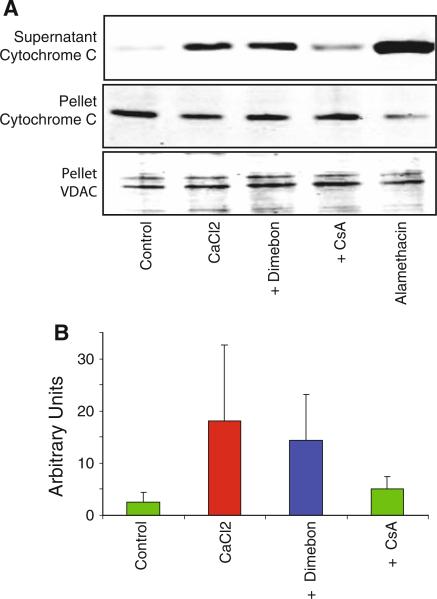
Calcium uptake by mitochondria leads to cytochrome C release (Brustovetsky et al. 2002; Kobayashi et al. 2003). We therefore sought to determine whether Dimebon protected against the Cytochrome C release. Dimebon did not significantly reduce the amount of cytochrome C release following incubation with 250 μM CaCl2 for 30 min, whereas CsA was effective (Fig 3).
Fig. 3.
Dimebon does not alter calcium-induced cytochrome c release from rat brain mitochondria. Non-synaptic mitochondria from rat brain were incubated in 250 μM CaCl2 for 30 min, then centrifuged and cytochrome C evaluated in the supernatant and pellet by western blotting. Dimebon (200 μM) or cyclosporin A (CsA, 5 μM) was added to the incubation media. The voltage-dependent anion channel (VDAC), a mitochondrial protein, was monitored as a loading control. Alamethacin was used to induce maximal cytochrome C release for comparison with CaCl2. a Representative western blots of the pellet and supernatant fractions. b Quantitative results from four animals in each group (mean ± SD). The results demonstrated calcium-induced cytochrome C release from rat brain mitochondria but that Dimebon did not influence the extent of cytochrome C release, in contrast to CsA
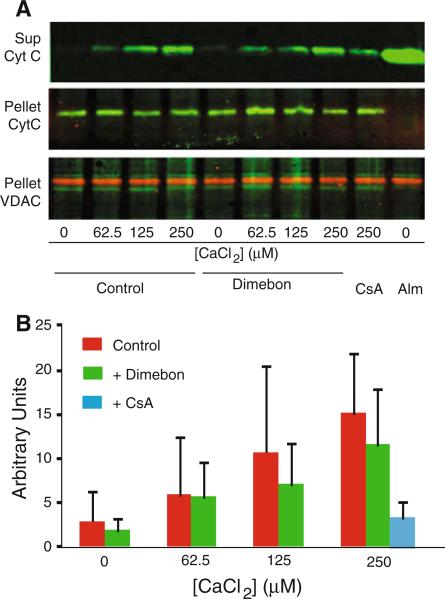
To determine whether Dimebon might be more effective against milder or shorter insults, we evaluated the ability of Dimebon to reduce cytochrome C release following incubation of non-synaptic rat brain mitochondria with 62.5-250 μM CaCl2 for 10 min. The results demonstrate that Dimebon was ineffective at each of the CaCl2 concentrations (Fig 4).
Fig. 4.
Dimebon does not alter calcium-induced cytochrome c release from rat brain mitochondria following 10-min incubation. To determine whether Dimebon could attenuate cytochrome C release following milder or shorter insults, we incubated non-synaptic rat brain mitochondria with 62.5–250 μM CaCl2 for 10 min. Dimebon was ineffective at each of the CaCl2 concentrations. Quantitative results are the mean ± SD, n = 3
Discussion
The previously demonstrated ability of Dimebon to attenuate swelling of rat liver mitochondria induced by Ca2+ and other insults suggested that inhibition of the mitochondrial permeability transition pore may be one mechanism by which Dimebon protects brain mitochondria from neurodegeneration associated with AD (Bachurin et al. 2003). However, swelling of brain mitochondria following mPTP opening is less extensive than in liver mitochondria (Berman et al. 2000; Kristian et al. 2000; Hansson et al. 2003; Kobayashi et al. 2003). In the present study, we therefore investigated the ability of Dimebon to protect against calcium-induced permeability transition in rat brain mitochondria using additional indicators of permeability transition including mitochondrial calcium uptake capacity and cytochrome C release.
The results demonstrate that in isolated mitochondria from rat brain, Dimebon attenuates Ca2+-induced swelling but does not alter cytochrome C release or Ca2+-uptake capacity. The finding that Dimebon reduces mitochondrial swelling but not other consequences of permeability transition is puzzling but not unprecedented. In screening putative inhibitors of mPTP for brain disorders, Morota and colleagues found that Propofol reduced brain mitochondrial swelling but did not improve calcium retention capacity, whereas Topiramate improved calcium retention but did not reduce swelling (Morota et al. 2009). This supports previous observations that mitochondrial swelling can occur independently of mitochondrial permeability transition, depending upon experimental conditions (Morota et al. 2009). It is therefore important to demonstrate that pharmacologic inhibitors protect against more than one symptom of permeability transition such as swelling, depolarization, and release of calcium and proapoptotic proteins. The inability of Dimebon to improve the calcium retention capacity or impair cytochrome C release following incubation of rat brain mitochondria with CaCl2 argues against inhibition of the mPTP as a neuroprotective mechanism for Dimebon.
Acknowledgments
This research was supported by NIH grants PO1NS058484, PO1AG010836, R01NS062993, and P30NS051220, as well as funding from the Kentucky Spinal Cord and Head Injury Research Trust.
References
- Abdul HM, Sultana R, St Clair DK, Markesbery WR, Butterfield DA. Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radical Biology and Medicine. 2008;45:1420–1425. doi: 10.1016/j.freeradbiomed.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, et al. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Annals of the New York Academy of Sciences. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Annals of the New York Academy of Sciences. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. discussion 345–339. [DOI] [PubMed] [Google Scholar]
- Berman SB, Watkins SC, Hastings TG. Quantitative biochemical and ultrastructural comparison of mitochondrial permeability transition in isolated brain and liver mitochondria: Evidence for reduced sensitivity of brain mitochondria. Experimental Neurology. 2000;164:415–425. doi: 10.1006/exnr.2000.7438. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. The FEBS Journal. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2 + overload than nonsynaptic mitochondria. The Journal of Biological Chemistry. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. Journal of Neurochemistry. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: A randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.003. doi:10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galenko-Iaroshevskii PA, Chekanova OA, Skibitskii VV, Bartashevich VV, Khankoeva AI, Poliashova TI. Antiarrhythmic properties of dimebone. Biulleten’ eksperimental’noǐ biologii i meditsiny. 1995;119:375–377. [PubMed] [Google Scholar]
- Galenko-Iaroshevskii PA, Sheikh-Zade Iu R, Chekanova OA, Melkumova ER, Bartashevich VV, Khankoeva AI. Effect of dimebone on coronary blood flow and myocardial contractibility. Biulleten’ eksperimental’noǐ biologii i meditsiny. 1996;121:506–508. [PubMed] [Google Scholar]
- Grigorev VV, Dranyi OA, Bachurin SO. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bulletin of Experimental Biology and Medicine. 2003;136:474–477. doi: 10.1023/b:bebm.0000017097.75818.14. [DOI] [PubMed] [Google Scholar]
- Hansson MJ, Persson T, Friberg H, Keep MF, Rees A, Wieloch T, et al. Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Research. 2003;960:99–111. doi: 10.1016/s0006-8993(02)03798-8. [DOI] [PubMed] [Google Scholar]
- Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Archives of Biochemistry and Biophysics. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Markesbery WR. Omega-3 fatty acids: Potential role in the management of early Alzheimer's disease. Clinical Interventions in Aging. 2010;5:45–61. doi: 10.2147/cia.s5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo DG, Arumugam TV, Woo HN, Park JS, Tang SC, Mughal M, et al. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer's disease. Neurobiology of Aging. 2010;31:917–925. doi: 10.1016/j.neurobiolaging.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kuroda S, Tada M, Houkin K, Iwasaki Y, Abe H. Calcium-induced mitochondrial swelling and cytochrome c release in the brain: Its biochemical characteristics and implication in ischemic neuronal injury. Brain Research. 2003;960:62–70. doi: 10.1016/s0006-8993(02)03767-8. [DOI] [PubMed] [Google Scholar]
- Kristian T, Gertsch J, Bates TE, Siesjo BK. Characteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: Effect of cyclosporin A and ubiquinone O. Journal of Neurochemistry. 2000;74:1999–2009. doi: 10.1046/j.1471-4159.2000.0741999.x. [DOI] [PubMed] [Google Scholar]
- Lermontova NN, Lukoyanov NV, Serkova TP, Lukoyanova EA, Bachurin SO. Dimebon improves learning in animals with experimental Alzheimer's disease. Bulletin of Experimental Biology and Medicine. 2000;129:544–546. doi: 10.1007/BF02434871. [DOI] [PubMed] [Google Scholar]
- Lermontova NN, Redkozubov AE, Shevtsova EF, Serkova TP, Kireeva EG, Bachurin SO. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bulletin of Experimental Biology and Medicine. 2001;132:1079–1083. doi: 10.1023/a:1017972709652. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xiong S, Lyubartseva G, Markesbery WR. Organoselenium (Sel-Plex diet) decreases amyloid burden and RNA and DNA oxidative damage in APP/PS1 mice. Free Radical Biology and Medicine. 2009;46:1527–1533. doi: 10.1016/j.freeradbiomed.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radical Biology and Medicine. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Ehmann WD, Hossain TI, Alauddin M, Goodin DT. Instrumental neutron activation analysis of brain aluminum in Alzheimer disease and aging. Annals of Neurology. 1981;10:511–516. doi: 10.1002/ana.410100604. [DOI] [PubMed] [Google Scholar]
- Mateeva IA. Action of Dimebon on histamine receptors. Farmakologiia i toksikologiia (Russ.) 1983;46:27–29. [PubMed] [Google Scholar]
- Miller G. Pharmacology. The puzzling rise and fall of a dark-horse Alzheimer's drug. Science. 2010;327:1309. doi: 10.1126/science.327.5971.1309. [DOI] [PubMed] [Google Scholar]
- Morota S, Mansson R, Hansson MJ, Kasuya K, Shimazu M, Hasegawa E, et al. Evaluation of putative inhibitors of mitochondrial permeability transition for brain disorders–spec-ificity vs. toxicity. Experimental Neurology. 2009;218:353–362. doi: 10.1016/j.expneurol.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. Journal of Neuroscience. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Experimental Neurology. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadurskaia SK, Khomenko AI, Pereverzev VA, Balaklevskii AI. Neuromediator mechanisms of the effect of the antihistamine agent dimebone on the brain. Biulleten’ eksperimental’noǐ biologii i meditsiny. 1986;101:700–702. [PubMed] [Google Scholar]
- Shadurskii KS, Matveeva IA, Il'iuchenok T. Therapeutic and protective properties of dimebon in burns. Farmakologiia i toksikologiia. 1983;46:90–92. [PubMed] [Google Scholar]
- Sonnen JA, Breitner JC, Lovell MA, Markesbery WR, Quinn JF, Montine TJ. Free radical-mediated damage to brain in Alzheimer's disease and its transgenic mouse models. Free Radical Biology and Medicine. 2008;45:219–230. doi: 10.1016/j.freeradbiomed.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li Q, Bezprozvanny I. Evaluation of Dimebon in cellular model of Huntington's disease. Molecular Neurodegeneration. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochimica et Biophysica Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]