Abstract
Colorectal cancer is a major health problem in developed countries. Chronic intestinal inflammation predisposes individuals to the development of colorectal cancer. The intracellular NOD-like receptors (NLRs) have emerged as crucial regulators of intestinal inflammation and colorectal tumorigenesis. Activation of several NLRs leads to the formation of a protein complex called the inflammasome, which then triggers the activation of the cysteine protease caspase-1 and the downstream maturation and secretion of the inflammatory cytokines interleukin-1β and -18. Defective inflammasome signaling in the gut contributes to colitis and colorectal tumorigenesis by increasing the permeability of the epithelial barrier, dysregulating the proliferation of epithelial cells, and inducing oncogenic mediators. In this review, we discuss our current knowledge on how the inflammasome protects against colorectal tumorigenesis.
Keywords: Nlrp3, Inflammasome, Colitis, Colorectal tumorigenesis
Introduction
Colorectal cancer is the third most common malignancy in developed countries. Chronic inflammation in the gut in the context of Crohn’s disease and ulcerative colitis represents a major risk factor for the development of colorectal cancer [1, 2]. However, colorectal cancer may also arise in the absence of these inflammatory bowel diseases (IBDs). Although the precise molecular mechanisms contributing to IBD-mediated colorectal cancer largely remain to be defined, recent studies suggest that chronic inflammation in the gut triggers molecular events that result in increased proliferation of mucosal epithelial cells and tumor development.
Chronic inflammation regulates tumorigenesis, at least in part, through the production of proinflammatory cytokines. The interleukin (IL)-1 cytokine subfamily mediates the immune response to infection and injury by activating innate and adaptive immune cells, thereby inducing the production of additional inflammatory cytokines, chemokines, and chemical mediators. Finally, IL-1 cytokines participate in cell proliferation, repair, and the healing process [3]. IL-1 cytokines, particularly IL-1β and IL-18, are synthesized as proforms that require proteolytic maturation to become biologically active. IL-1β and IL-18 are both cleaved by cysteine protease caspase-1, which must first be activated by multiprotein complexes called ‘inflammasomes’ [4, 5]. Given the important functions of IL-1β and IL-18 in inflammation and immunity, the IL-1β/IL-18–activating inflammasome platforms are considered key factors in several inflammatory diseases [3, 6]. In addition to caspase-1, inflammasomes are generally composed of a member of the NLR protein family and the bipartite CARD/PYD adaptor protein ASC. As mentioned above, inflammasomes induce activation of caspase-1, a cysteine protease that activates IL-1β and IL-18 and triggers inflmamatory cell death pathways in myeloid cells [7]. Information about the role of the inflammasomes in colon inflammation and related tumorigenesis has just recently begun to emerge. In this review, we discuss the function of the inflammasome in colon inflammation and colitis-associated colorectal cancer in light of recent findings in this field.
NOD-like receptors initiate the formation of inflammasomes
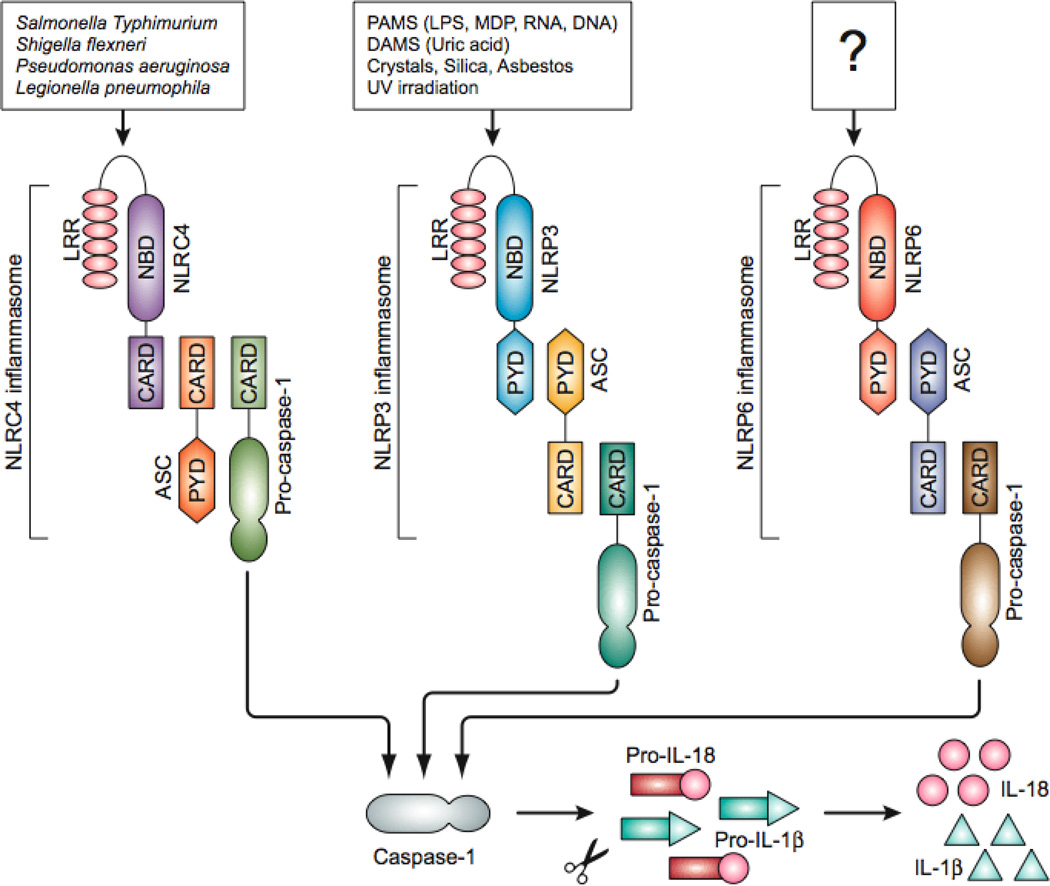
For inflammasomes to form, a group of intracellular pattern recognition receptors called NOD-like receptors (NLRs) must first be activated by microbial or endogenous danger molecules and stress signals. Inflammasomes are named based on their interacting NLRs. Currently, three NLR inflammasomes (the NLRP1, NLRC4 and NLRP3 inflammasomes) and one non-NLR-containing inflammasome (the AIM2 inflammasome) have been characterized in mouse models. Each is activated by a unique range of stimuli. For example, the NLRP1 inflammasome is formed in response to Bacillus anthracis lethal toxin LT [8]. The NLRC4 inflammasome, also known as the IPAF inflammasome, is assembled in response to several bacterial species including Legionella, Pseudomonas, Shigella, and Salmonella [9, 10] (Fig 1). The most thoroughly studied inflammasome is NLRP3 inflammasome, which is activated by a wide range of pathogens, including bacteria, fungi, viruses, and a wide range of microbial stimuli (e.g., LPS, MDP, and bacterial RNA) [4, 6, 11] (Fig. 1).
Figure 1. Inflammasomes involved in intestinal inflammation.
Three different inflammasome complexes consists of NOD-like receptors (NLR) - NLRC4, NLRP3, and NLRP6 - have been known to be involved in colitis and colorectal tumorigenesis. The NLRC4 inflammasome senses the cytosolic presence of several Gram-negative bacteria including S. typhimurium, S. Flexneri, L. pneumophila and P. aeruginosa. The NLRP3 is activated by microbial PAMPs such as LPS, MDP and bacterial RNA, endogenous DAMPs such as ATP, crystals such as monosodium urate, silica and asbestos, or UV irradiation. How the other inflammasome, NLRP6, is activated is still unknown. Once the NLRs are activated they form the inflammasome complex with caspase-1 via adaptor protein ASC. Activated caspase-1 processes the IL-1β and IL-18 precursors into the mature cytokines, which are secreted through an unknown mechanism. CARD, caspase recruitment domain; PYD, pyrin domain; NBD, nucleotide binding and oligomerization domain; LRR, leucine rich repeat; PAMP, pathogen-associated molecular pattern; DAMS, danger-associated molecular patterns.
The NLRP3 inflammasome can detect signs of metabolic stress (monosodium urate crystals, extracellular glucose), environmental pollutants (silica, asbestos), UV irradiation, and skin irritants such as trinitrophenyl chloride and trinitrobenzene [7, 12]. The non-NLR inflammasome sensor AIM2 activates caspase-1 in response to double-stranded viral DNA and the etiological agent of tularemia, Francisella tularensis [13, 14]. Recently, NLRP6 was proposed to exert inflammasome activity against pathogenic microbiota in the gut [15, 16]. However, the precise composition of the NLRP6 inflammasome and its molecular triggers require further analysis.
Inflammasomes are crucial regulators of intestinal inflammation and tumorigenesis
Since its discovery in 2002, the inflammasome has emerged as a key regulator of inflammatory responses in a variety of diseases that include periodic fever syndromes, type I and II diabetes, atherosclerosis, obesity and colitis [3, 17–19]. Hence, inhibiting caspase-1 activity and neutralizing IL-1β appear effective strategies for treating several inflammatory diseases. Initial studies aimed toward understanding the role of caspase-1, IL-1β and IL-18 in colitis made use of chemical inhibitors of these molecules, and the results emphasized their detrimental activity in disease pathogenesis [20–23].
Our understanding of the inflammasome and its contribution to colitis and colorectal tumorigenesis has markedly advanced in recent years thanks to recent landmark studies from multiple laboratories. We and others reported that mice lacking NLRP3 inflammasome components (namely ASC, caspase-1 and NLRP3) are susceptible to dextran sodium sulfate (DSS)-induced colitis with signs of increased inflammation and colonic damage [24–27]. These reports provided mechanistic data explaining the previously reported observation that missense mutations in Nlrp3 associated with increased susceptibility to Crohn’s disease [28]. In agreement with a protective role for the NLRP3 inflammasome against colorectal inflammation, the inflammasome substrates IL-1β and IL-18 were previously suggested to have beneficial role in IBDs [29–31]. The increased chronic inflammation in mice lacking NLRP3 or caspase-1 resulted in a markedly increased susceptibility to colorectal tumorigenesis in the azoxymethane plus DSS (AOM+DSS) model [24, 25, 32].
Unlike the four studies cited above, two additional reports failed to confirm the critical role of NLRP3 in protection against DSS-induced colitis reported [33, 34]. One study confirmed caspase-1–deficient mice to be hypersusceptible to colitis-associated adenomatous polyp formation in the gut, but could not find association of these events to NLRP3 [34]. This apparent discrepancy may be attributed to the presence of different gut microflora in animals housed in different facilities, and differences in experimental protocols. In agreement, both the studies that failed to detect a protective role for NLRP3 in DSS-induced colitis used a lower dose of DSS (2%) [33, 34], whereas the other studies that support protective role of NLRP3 made use of ≥3% DSS to induce gut inflammation [24–27]. Thus, the beneficial effect of the NLRP3 inflammasome and the downstream cytokines IL-1β and IL-18 may be apparent only when severe colonic damage occurs, whereas low levels of DSS may not induce colitis that is severe enough that the protective functions of IL-1β and IL-18 become apparent.
Nevertheless, NLRP3 was consistently found to be a weaker regulator of tumor development relative to caspase-1 and ASC as both Asc-knockout (KO) mice and caspase-1–KO mice suffered from increased colorectal tumorigenesis [24, 32]. This suggests that aside from NLRP3, additional NLRs may regulate inflammasome activation during colitis. In agreement, Hu et al. demonstrated a role for NLRC4 in protection against colorectal tumorigenesis as well [34]. Moreover, 3 recent reports described a protective role for NLRP6 in colitis and colorectal tumorigenesis, which was correlated with the detection of reduced IL-18 levels in circulation and in colon tissue of Nlrp6-KO mice[15, 16]. Although these observations provide support for the notion that NLRP6 assembles an inflammasome, further analysis is required to identify the composition of this inflammasome and the molecules inducing its assembly in the gut.
Inflammasomes protect the integrity of the epithelial barrier and maintain homeostasis
Considerable efforts have been aimed at addressing how inflammasome formation leads to protection from colitis and colon tumorigenesis. Although findings from different laboratories varied in details, a common theme that emerged from multiple studies is that defective inflammasome activation results in increased colonic epithelial injury during DSS-induced colitis [16, 24, 25, 27] (Table 1). Using dextran-FITC, multiple groups showed that the gut epithelial barrier of Nlrp3-, Nlrp6-, and caspase-1–KO mice was significantly more permeable to this reporter molecule than that of DSS-fed wild-type animals [16, 25, 27]. Increased permeability of the epithelial layer in the gut of Nlrp3−/− and caspase-1−/− mice during acute colitis was also apparent from the systemic dispersion of gut microbiota in animals of these genotypes [16, 25, 27]. Thus, activation of inflammasomes after cytotoxic assault on the intestinal epithelium may trigger repair responses characterized by increased division of stem cells at the base of the crypt to replace the damaged enterocytes [35]. Notably, IL-1β and IL-18 were previously implicated in repairing the ulcerated epithelium [36]. In agreement, epithelial cell proliferation in Nlrp3-deficient and Nlrp6-deficient mice was remarkably arrested during acute colitis [16, 27].
Table 1.
Summary of studies on the role of inflammasomes in colitis and colorectal tumorigenesis.
| Study | Mouse | Study model | Results | Conclusion |
|---|---|---|---|---|
| Zaki et al, Immunity, 2010 | Nlrp3−/−, caspase-1−/−, ASC−/− | DSS colitis: 3% DSS for 5 days | Nlrp3−/−, caspase-1−/− and ASC−/− mice are susceptible to colitis | NLRP3 inflammasome protects from colitis |
| Dupaul-Chicoinie et al, Immunity, 2010 | ASC−/−, caspase-1−/− | DSS colitis: 3% DSS for 5 days | ASC−/− and caspase-1−/− mice are susceptible to colitis | Inflammasomes protects from colitis |
| Allen et al, J Exp Med, 2010 | Nlrp3−/−, ASC−/−, caspase-1−/− | AOM+DSS colitis: 3 cycles of 2.5% DSS (5 day each cycle) | Nlrp3−/−, ASC−/−, and caspase-1−/− mice are susceptible to colorectal tumorigenesis | NLRP3 inflammasome protects colon tumroigenesis |
| Hu et al, PNAS, 2010 | Nlrp3−/−, Nlrc4−/−, caspase-1−/− | DSS colitis: 2% DSS for 7 days; AOM+DSS colitis: 3 cycles of 2.5% DSS (5 day each cycle) | Caspase-1−/− and Nlrc4−/−, but not Nlrp3−/−, mice are susceptible to colorectal tumorigenesis. | NLRC4 inflammasome protects from colon tumorigenesis |
| Bauer et al, Gut, 2010 | Nlrp3−/− | DSS colitis: 2% DSS for 9 days | Nlrp3−/− mice are resistant to colitis | NLRP3 inflammasome contributes to colitis |
| Hirota et al. Inflam Bowel Dis, 2011 | Nlrp3−/− | DSS colitis: 2.5% DSS for 7 days; TNBS colitis: TNBS (30mg/ml) in 20% ethanol | Nlrp3−/− mice are susceptible to colitis | NLRP3 inflammasome protects from colitis |
| Zaki et al, J Immunol, 2010 | Nlrp3−/−, ASC−/−, caspase-1−/−, IL-18−/− | AOM+DSS: 3cycles of 3% DSS (5 day each cycle) | Nlrp3−/−, ASC−/−, caspase-1−/−, and IL-18−/− mice are susceptible to colorectal tumorigenesis | IL-18 downstream of NLRP3 inflammasome protects from colon tumorigenesis |
| Chen et al, J Immunol, 2011 | Nlrp6−/− | DSS colitis: 3.5% DSS for 7 days; AOM+DSS colitis: 3 cycles of 2% DSS (5 day each cycle) | Nlrp6−/− mice are susceptible to colorectal tumorigenesis | NLRP6-inflammasome protects from colitis and colon tumroigenesis |
| Elinav et al, Cell, 2011 | ASC−/−, Nlrp6−/− | DSS colitis: 2% DSS for 7 days | ASC−/− and Nlrp6−/− mice are susceptible to colitis | NLRP6 inflammasome protects from colitis |
| Normand et al, PNAS, 2011 | Nlrp6−/− | DSS colitis: 3% DSS for 6–8 days; AOM+DSS colitis: 4 cycles of 2% DSS (5 day each cycle) | Nlrp6−/− mice are susceptible to colitis and colorectal tumorigenesis | NLRP6 protects from colitis and colon tumorigenesis |
The importance of inflammasomes in epithelial barrier integrity and protection against colitis and colorectal tumorigenesis was further supported by bone marrow chimera studies. Three different studies showed that inflammasome activation in the non-hematopoietic compartment is critical for attenuation of acute colitis. On the other hand, inflammasome activity in the myeloid compartment may contribute to suppression of polyp formation during chronic inflammation in the AOM+DSS-tumorigenesis model [16, 24]. This suggests that inflammasome activation in different compartments and tissues may help limit inflammation, tissue damage and tumorigenesis in a concerted manner at different stages of disease. Cells of the gut epithelium may support inflammasome activation during the acute stage of colon inflammation to restore the epithelial barrier, whereas inflammasome activation in myeloid cells may play a more important role in preventing the production of tumorigenic factors that contribute to the formation of a tumor-supporting microenvironment during chronic stages of inflammation. Thus, depending on the spatiotemporal parameters, inflammasome activation in the epithelial layer and lamina propria might contribute to the homeostasis of the gut epithelium and protection from colitis-associated colorectal tumorigenesis.
In addition to increased transmural permeability, defective antimicrobial defense appears to be a trait of Nlrp3−/− colonic epithelial cells. Hirota et al showed that Nlrp3−/− mice express low levels of defensin, a peptide that blocks microbial colonization and invasion of the gut [26]. This notion was further supported by the presence of altered gut microflora in Nlrp3−/−, Nlrp6−/−, and ASC−/− mice [15, 26]. Caspase-1 or a downstream cytokine may mediate the production of antimicrobial peptides in paneth cells, which protect the gut from colitogenic commensal bacteria. Future studies should focus on the role of inflammasomes in the production of antimicrobial peptides by the epithelial cell population.
Tumorigenesis is associated with dysregulated cytokine response in inflammasome-deficient mice
The development of colorectal tumors is governed by complex cellular and molecular processes. These include the mutagenic transformation of epithelial cells, the rapid proliferation of neoplastic cells, and the simultaneous induction of angiogenesis. These processes are regulated by cytokines, chemokines, reactive-oxygen species, and oncogenic factors [37, 38]. The increased tumor burden seen in Nlrp3-deficient mice was associated with deregulated cytokine and chemokine production and markedly reduced IL-18 production in circulation and in the colorectal tract [24, 27, 32]. Similarly, the IL-18 levels were significantly reduced at the initial stages of tumor development in DSS-treat Nlrp6-deficient mice [16]. From these studies, it can be concluded that IL-18 may play central regulatory role in anti-tumor immunity exhibited by the inflammasome. Such a role for IL-18 is supported by previous reports indicating that both IL-18−/− and IL-18R−/− mice are hyper-susceptible to colitis and colorectal tumorigenesis [31]. Furthermore, mice lacking Myd88, an adaptor that is required for both the production of IL-1β and IL-18 as well as for signaling downstream of their cognate receptors, showed increased intestinal damage, hyperproliferation of gut epithelial cells and colorectal tumorigenesis [39]. In contrast, the gut of IL-1R−/− mice and wild-type littermates contained similar numbers of adenomatous polyps, a finding that further highlighted the unique and essential role of IL-18 in regulating colon tumorigenesis [39]. Finally, these conclusions were further supported by the observation that administration of exogenous IL-18 reduces the severity of colitis and colorectal tumorigenesis in Nlrp3- and caspase-1-deficient mice [25, 27, 38].
Although conclusive evidence describing the precise role of IL-1β during colitis and colorectal tumorigenesis is currently lacking, excessive IL-1β signaling might support adenomatous polyp development as mice lacking SIGIRR, negative regulator of IL-1 signaling, develop more severe colorectal tumrigenesis during AOM/DSS treatment [40]. It thus appears that balancing the level of the inflammasome-dependent cytokines IL-1β and IL-18 is essential for maintaining intestinal homeostasis and for protection against inflammation and cancer in the gut.
Interestingly, reduced IL-18 production by Nlrp3- and caspase-1-deficient mice during colitis and colorectal tumorigenesis was accompanied by an increased production of tumor-associated cytokines such as IL-6 and TNF-α, and chemokines such as KC, eotaxin, MIP2, and MIP1α. These proinflammatory cytokines and chemokines are typically produced as an initial response to injury of the mucosal epithelia and are known to contribute to healing and the host’s antimicrobial defense responses [16, 27, 38]. However, these molecules may become detrimental when produced in excess; IL-6 and TNF-α induce cell proliferation and the induction of signaling pathways that promote tumorigenesis. Similarly, chemokines shape the microenvironment of the tumor by recruiting macrophages and neutrophils, which produce reactive-oxygen and nitrogen species, enzymes such as MMPs, growth factors, and other oncogenic molecules [37].
IL-18–mediated regulation of tumorigenesis
As discussed above, IL-18 helps maintain homeostatic balance in cytokine and chemokine production in the colon. IL-18 was previously noted as having antitumor function in various experimental tumor models [41–43]. It was reported to inhibit tumor growth and angiogenesis [36, 44] and to be associated with repair and restitution of ulcerated epithelium [35]. However, the exact mechanism of IL-18–mediated protection of colon tumorigenesis is not clear. A potential mechanism that may contribute to IL-18–mediated attenuation of colon tumorigenesis is the regulation of the of intestinal epithelial cell proliferation. We observed increased proliferation of colonic epithelial cells and a higher incidence of hyperplasia in Nlrp3-inflammasome–deficient mice during the resting stage of AOM+DSS-induced colitis [32]. Notably, during the acute colitis stage, intestinal proliferation in Nlrp3-deficient mice was defective [27]. This result suggests a biphasic role of IL-18 in colitis—induction of proliferation during the early stage and suppression at the later resting stage.
Although it is intriguing that IL-18 regulates intestinal epithelial proliferation in a context-dependent manner, this phenomenon can be explained, at least in part, by IL-18–dependent production of other factors. IL-18 was originally identified as the IFN-γ–inducing factor, and IFN-γ has been described as a pleiotropic cytokine with potent antitumor activity [45, 46]. It may thus not be surprising that diminished production of IL-18 in Nlrp3- and caspase-1–deficient mice was accompanied by significantly reduced expression of IFN-γ [32]. Moreover, IFN-γ production was markedly reduced in IL-18–KO mice following AOM+DSS treatment [39]. In agreement with a biphasic role for Nlrp3-mediated IL-18 production in colitis-associated tumorigenesis, a biphasic role for IFN-γ during DSS-induced colitis, with promotion of intestinal epithelial cell proliferation at early stages and induction of antiproliferative responses at later stages, was recently reported [47].
IFN-γ mediates its effects through the IFN-γ receptor (IFN-γR), which is expressed on normal and malignant cells. The biological effects of IFN-γR are mediated by a number of intracellular signaling pathways, the best characterized of which is the JAK-STAT pathway. Once IFN-γR is activated, it phosphorylates JAK1 and JAK2, which further induces phosphorylation and nuclear translocation of STAT1. Phosphorylated STAT1 levels were found to be markedly reduced in colon tissue of AOM+DSS–treated caspase-1−/− mice, but were restored upon stimulation with either IFN-γ or IL-18 [32]. These results suggest that STAT1 signaling may be affected in the absence of a functional Nlrp3 inflammasome. Once in the nucleus, STAT1 binds with gamma-activated sequences in IFN-γ–responsive genes to induce the transcription of genes involved in cell proliferation, differentiation, and cell death [48]. Thus, IFN-γ–mediated STAT1 activation downstream of IL-18 may help maintain gut homeostasis and inhibit tumor development during colitis (Fig. 2).
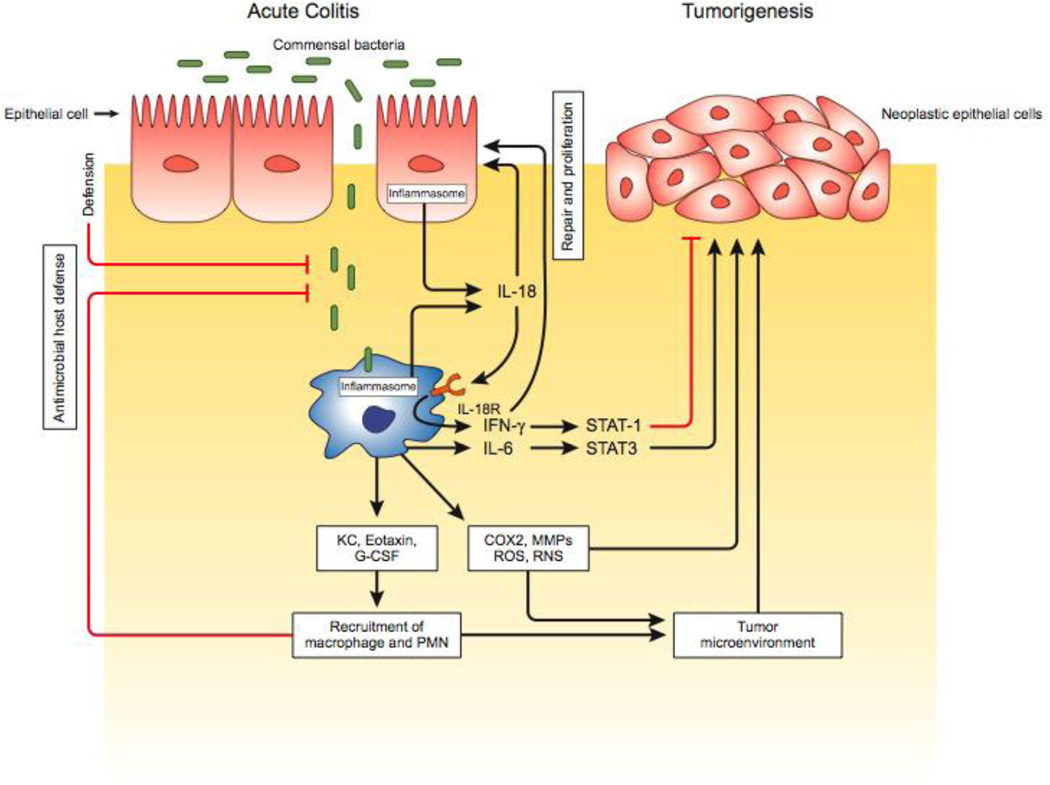
Figure 2. Inflammasome-mediated regulation of colitis and colorectal tumorigenesis.
Damage of the gut epithelial layer leads to invasion of commensal bacteria into submucosa and lamina propria, and rapid activation of inflammasome complex in the epithelial cells and macrophages. Inflammasomes activate proinflammatory cytokines IL-1β and IL-18 as an early response of injury and inflammation. These two cytokines, particularly IL-18, play regulating roles in colitis and colorectal tumorigenesis depending on their concentration and context of the disease. In general, IL-18 protects colitis by inducing proliferation of epithelial cells to repair the damaged epithelia during acute colitis and inhibits tumorigeneis by activating STAT1 antitumor signaling pathway. Defects in IL-18 production leads to increased epithelial barrier permeability and hyperproduction of other proinflammatory cytokines including IL-6, TNF-α, chemokines KC, MIP2, eotaxin, and tumor promoting factors COX2, reactive oxygen species (ROS), reactive nitrogen species (RNS) and matrix metaloprotineases (MMPs). Although cytokines, chemkines, ROS and RNS exerts antimicrobial and healing functions, they also contribute to chronic inflammation and neoplastic transformation of epithelial cells.
Another mechanism of IL-18–mediated protection against colorectal tumorigenesis is the increased production of proinflammatory cytokines. The absence of IL-18 leads to mucosal translocation of gut microflora into colon tissue, where they may trigger resident myeloid cells epithelial cells to produce proinflammatory cytokines. Several proinflammatory cytokines are strong inducers of tumorigenic pathways. For example, IL-6 activates STAT3, which regulates a number of genes involved in proliferation, apoptosis, and angiogenesis [37]. Given that STAT3 phosphorylation is increased in MyD88-deficient and IL-18–deficient mouse colonic epithelial cells during AOM+DSS–induced colitis [39], it appears likely that inflammasomes may regulate tumorigenesis by limiting STAT3 phosphorylation (Fig. 2).
Concluding remarks
A growing body of recent studies indicates a central role for inflammasomes in driving protection against intestinal inflammation and tumorigenesis. Inflammasomes may exert their effects through a variety of mechanisms that include the production of IL-18. IL-18 regulates intestinal epithelial cell proliferation and tissue repair through IFN-γ–mediated STAT1 activation and the production of inflammatory cytokines and chemokines. Nevertheless, additional work is required to define the precise molecular mechanisms by which inflammasomes and IL-18 regulate these processes. Such studies are likely to suggest new approaches for the development of more effective therapies against intestinal inflammation and to counter colorectal cancer.
ACKNOWLEDGEMENTS
We acknowledge researchers who have contributed to this field, whose work was not cited or was cited through review articles because of space limitations. This work was supported by National Institute of Health Grants (R01AR056296 and AI088177), and the American Lebanese Syrian Associated Charities (ALSAC) to T-D.K. M.H.Z is supported by Gephardt fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950–954. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaden JA, et al. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 4.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, et al. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 6.Kanneganti TD, et al. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 12.Martinon F, et al. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elinav E, et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen GY, et al. A functional role for nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masters SL, et al. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001902. 81ps17. [DOI] [PubMed] [Google Scholar]
- 18.Shaw PJ, et al. Inflammasomes and autoimmunity. Trends Mol Med. 2011;17:57–64. doi: 10.1016/j.molmed.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer C, et al. The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNF-alpha mRNA expression. Dig Dis Sci. 2007;52:1642–1652. doi: 10.1007/s10620-007-9802-8. [DOI] [PubMed] [Google Scholar]
- 21.Sivakumar PV, et al. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegmund B. Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol. 2002;64:1–8. doi: 10.1016/s0006-2952(02)01064-x. [DOI] [PubMed] [Google Scholar]
- 23.Siegmund B, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–R1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 24.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Hirota SA, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villani AC, et al. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, et al. Regulation of IL-8 and IL-1beta expression in Crohn's disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 30.Tamura K, et al. IL18 polymorphism is associated with an increased risk of Crohn's disease. J Gastroenterol. 2002;37(Suppl 14):111–116. doi: 10.1007/BF03326428. [DOI] [PubMed] [Google Scholar]
- 31.Takagi H, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 32.Zaki MH, et al. IL-18 Production Downstream of the Nlrp3 Inflammasome Confers Protection against Colorectal Tumor Formation. J Immunol. 2010 doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 34.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 36.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34:2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 37.Grivennikov SI, et al. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaki MH, et al. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salcedo R, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garlanda C, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micallef MJ, et al. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57:4557–4563. [PubMed] [Google Scholar]
- 42.Micallef MJ, et al. In vivo antitumor effects of murine interferon-gamma-inducing factor/interleukin-18 in mice bearing syngeneic Meth A sarcoma malignant ascites. Cancer Immunol Immunother. 1997;43:361–367. doi: 10.1007/s002620050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osaki T, et al. Potent antitumor effects mediated by local expression of the mature form of the interferon-gamma inducing factor, interleukin-18 (IL-18) Gene Ther. 1999;6:808–815. doi: 10.1038/sj.gt.3300908. [DOI] [PubMed] [Google Scholar]
- 44.Hegardt P, et al. Nitric oxide synthase inhibitor and IL-18 enhance the anti-tumor immune response of rats carrying an intrahepatic colon carcinoma. Cancer Immunol Immunother. 2001;50:491–501. doi: 10.1007/s002620100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dighe AS, et al. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 46.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 47.Nava P, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darnell JE, Jr., et al. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]