Abstract
The Pharmaceutical Assets Portal aims to facilitate industry-academic collaborations for discovery of new indications for compounds no longer being developed by pharmaceutical companies, through eliminating barriers to access such compounds. The Portal’s enabling infrastructure includes a national investigator database; a Foci-of-Expertise browser; a material transfer agreement template; and a funding partner. Whereas the goal of creating a shared compound repository remains to be achieved, the Portal has established a mechanism to facilitate future drug repositioning opportunities.
Introduction
Despite remarkable advances, many conditions lack effective therapies. Novel approaches to drug development, such as drug repositioning, or finding new uses for existing compounds, would greatly facilitate discovery of such new therapies. De novo drug development is a lengthy, expensive process whereby compounds can be discontinued at any stage, for reasons including lack of efficacy, insufficient therapeutic index, lack of commercial potential and strategy-driven changes. Discontinued compounds that have been tested in humans represent a significant resource to the international research community searching for therapies for rare and neglected diseases. Clinical attrition rates suggest that thousands of such compounds may exist in industry repositories.
The NIH Chemical Genomics Center (NCGC) has embraced this concept by building a comprehensive collection of small-molecule drugs [1]. This invaluable resource encompasses compounds known through publicly available sources. The Pharma Portal complements the NCGC’s approach by focusing on unpublished compounds discontinued before FDA approval.
The CTSA Pharmaceutical Assets Portal
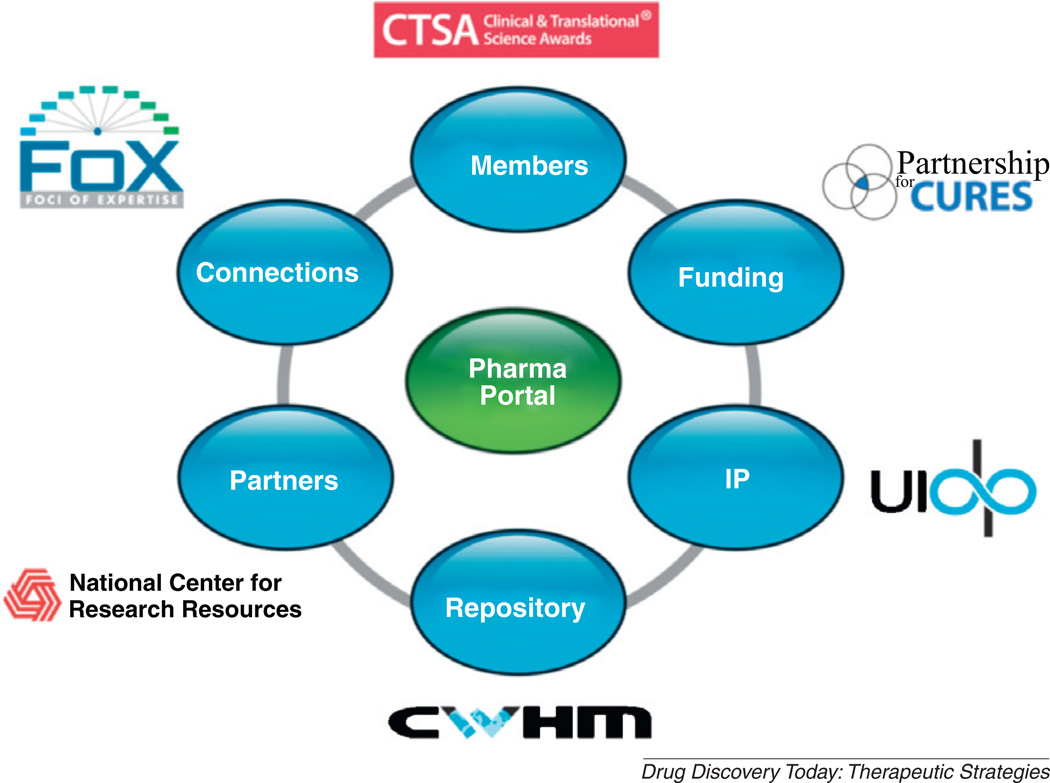
The CTSA Pharmaceutical Assets Portal project was initiated by the consortium of universities linked by the Clinical and Translational Science Award (CTSA) [2]. The ultimate goal of the project was to create a public-private partnership based on collaboration among academic, government, foundation, and industry scientists to facilitate repositioning efforts by effectively leveraging the knowledge/expertise important to drug discovery and development. These matches are envisioned to ultimately result in an increased number of approved drugs for new indications and considerable public benefit. In collaboration with Pfizer’s Indications Discovery Unit and the NCRR (National Center for Research Resources), the Portal has made significant progress in establishing the infrastructure for such partnership. Specifically, key elements comprising the Portal include: membership from 50 universities, a tool for connecting partners and members (Foci-of-Expertise, FoX), a funding arm (Partnership for Cures), an Intellectual Property (IP) arm (the University-Industry Demonstration Partnership, UIDP) and facilities/resources to house, maintain, and distribute the discontinued compounds (the Center for World Health and Medicine, CWHM). Establishing an infrastructure with these elements was considered important to minimizing barriers to collaboration. The resulting infrastructure provides a platform for promoting shelved compounds for further investigation and medical use. This article describes the evolution of the Portal project, the real and perceived barriers and potential opportunities for moving forward (Fig. 1).
Figure 1.
The infrastructure of the Pharmaceutical Assets Portal. Shown are the components necessary for achieving the goals of this mission.
The Pharma Portal members
The success of the Portal rests with both industry partners providing access to compounds and Pharma Portal members – those investigators who seek access to shelved pharmaceutical compounds that might have utility for the diseases they study. The Portal used an online, 28-question survey to recruit members. The “Portal” was defined in the survey as a tool that would connect investigators to other researchers with complementary knowledge and skills, as well as to pharmaceutical companies wishing to explore new indications for their ongoing or discontinued drugs. The survey was divided into categories related to demographics, research interests (by MeSH headings), and previous experience with transfer of compounds. The survey team also tracked where investigators obtain information about investigational compounds. In addition, respondents were asked to provide their primary reason for seeking compounds; for example, for use in vitro or in animal models, to conduct clinical trials, or a combination of these reasons. The survey also probed the success rates of obtaining compounds from industry, and attempted to identify barriers to such collaborations (Table 1).
Table 1.
Disease areas most relevant to respondents’ researcha
| Category | All respondents (n=696) |
|
|---|---|---|
| Number | Percentb | |
| Nervous system diseases [CIO] | 236 | 34% |
| Neoplasms [C04] | 181 | 26% |
| Cardiovascular diseases [C14] | 171 | 25% |
| Immune system diseases [C20] | 166 | 24% |
| Nutritional and metabolic diseases [C18] | 127 | 18% |
| Bacterial infections and mycoses [C01] | 112 | 16% |
| Endocrine system diseases [C19] | 110 | 16% |
| Respiratory tract diseases [C08] | 103 | 15% |
| Viral diseases [C02] | 98 | 14% |
| Musculoskeletal diseases [C05] | 98 | 14% |
| Digestive system diseases [C06] | 94 | 14% |
| Hemic and lymphatic diseases [C15] | 72 | 10% |
| Congenital, hereditary, and neonatal diseases and abnormalities [C16] 72 | 10% | |
| Skin and connective tissue diseases [C17] | 71 | 10% |
| Eye diseases [C11] | 48 | 7% |
| Female urogenital diseases and pregnancy complications [C13] 52 | 7% | |
| Male urogenital diseases [C12] | 45 | 6% |
| Parasitic diseases [C03] | 43 | 6% |
| Otorhinolaryngologic diseases [C09] | 23 | 3% |
| Stomatognathic diseases [C07] | 13 | 2% |
Survey respondents were asked to ‘Please check all general disease MeSH Headings that are relevant to your research research’ to categorize their research interests using MeSH headings to facilitate future connections with the Foci Foci-of-Expertise (FoX) Synergy Brower.
The majority (62.8%) of investigators listed multiple interest areas. The total percentages will not add up to 100%.
Between 2008 and 2011, a total of 696 individuals from 47 (85%) CTSA institutions, industry, and the NIH responded to the survey. Most respondents self-identified themselves as institutional leaders or senior faculty, with the majority being physicians. The investigators were primarily focused on clinical applications: 80% said they would conduct clinical trials if the compounds were made available. This response underscores the overwhelming interest in gaining access to clinically tested therapeutics rather than to research reagents. The four most commonly reported diseases of interest included: cancer and non-cancerous diseases of the nervous, cardiovascular, and immune systems (Table 2). Approximately half of those surveyed had access to unique experimental models supporting their clinical research hypotheses, including animal models of disease; high-throughput, cell-based screening assays; or assays for specific molecular targets in the nucleus and cytoplasm. Many investigators expressed an interest in new routes of administration, novel delivery systems and compounds/delivery systems that can cross the blood–brain barrier (Table 3). The survey information may be readily accessed by potential industry collaborators seeking hypothesis-driven drug repositioning.
Table 2.
Highest priority drug delivery routes for respondents’ researcha
| Delivery Mechanism | Number | Percentb |
|---|---|---|
| Oral | 343 | 49% |
| Intravenous | 263 | 38% |
| Transdermal | 152 | 22% |
| Blood-brain barrier | 110 | 16% |
| Inhalation | 94 | 14% |
| Topical Cream | 73 | 11% |
Survey respondents were asked, ‘For your research studies do you have any specific drug delivery requirements?’
The majority (51.3%) of investigators listed multiple interest areas. The total percentages will not add up to 100%.
Table 3.
Prior experience with obtaining investigational compounds a
| Experience | Number | Percent w/experience |
Percent successful |
|---|---|---|---|
| Yes b | 397 | 58% | |
| Yes, successful | 227 | 57% | |
| Yes, sometimes successful | 146 | 37% | |
| Yes, but not successful | 17 | 4% | |
| Yes, not answered (2) | 7 | 2% | |
| No | 293 | 42% |
Survey respondents were asked, ‘Do you have prior experience in obtaining or seeking to obtain pharmaceutical compounds for your research?’
Survey respondents with experience seeking compounds were asked, ‘Were your requests approved?’
Most respondents (58%) had prior experience requesting compounds from the pharmaceutical industry. Approximately 60% had been “successful”, whereas another 37% reported being “sometimes successful”. The relatively small fraction (4%) of those who had prior experience requesting compounds but were “unsuccessful”, often reported contractual issues as limiting their success. Importantly, of those who sought compounds, whether ultimately successful or not, there was a desire to make the process more efficient by streamlining the negotiation process. Whereas the survey did not seek specific details of the contractual limitations, our experience suggests that intellectual property and publication language are typically the most contentious. Additionally, lack of sufficient quantities of compound or a compelling business case were frequently cited. Most investigators identified compounds of interest through public sources, such as the scientific literature, professional conferences, web searches, or by word of mouth. Fewer than 40% of investigators reported using commercial drug information databases; subscription fees may have discouraged their use. Our own informal research revealed that commercial databases capture only a portion of the potentially useful shelved compounds, perhaps 50%, underscoring the importance of projects like the Pharma Portal, which aims to make available compounds not described in publicly accessible literature. As shown by the fact that the majority of respondents (82% of 570) became Pharma Portal members, the goals of the Portal project appear to be in alignment with the needs of this community. The Portal members are specifically highlighted in the Foci-of-Expertise, a tool for facilitating the industry-academia collaborations.
Identifying potential collaborators using the Foci-of-Expertise (FoX) tool
One barrier to forming productive public-private partnerships is the difficulty identifying companies and academic investigators with complementary interests. The Foci-of-Expertise (FoX) Synergy Browser was specifically developed to address this challenge. FoX links three search categories (target gene/protein, authors, disease) to enable users to readily identify potential collaborators, as well as to explore new collaborative relationships that may not be immediately obvious. The links generated by FoX rely on information derived from MEDLINE (publications), NIH RePORTER (funded grant abstracts) and Reactome (gene-gene interactions). Examples of the many potential uses for the FoX system include facilitating organizing a symposium for collaborative research on a given disease at a specific institution; identifying potential members of a translational research team to investigate a new pharmacological agent; identifying key opinion leaders in certain disease areas; identifying researchers with expertise in a specific area of interest. The tool was developed in an iterative fashion with researchers at Pfizer, together refining the usage model, user interface and capabilities for querying, highlighting, and filtering.
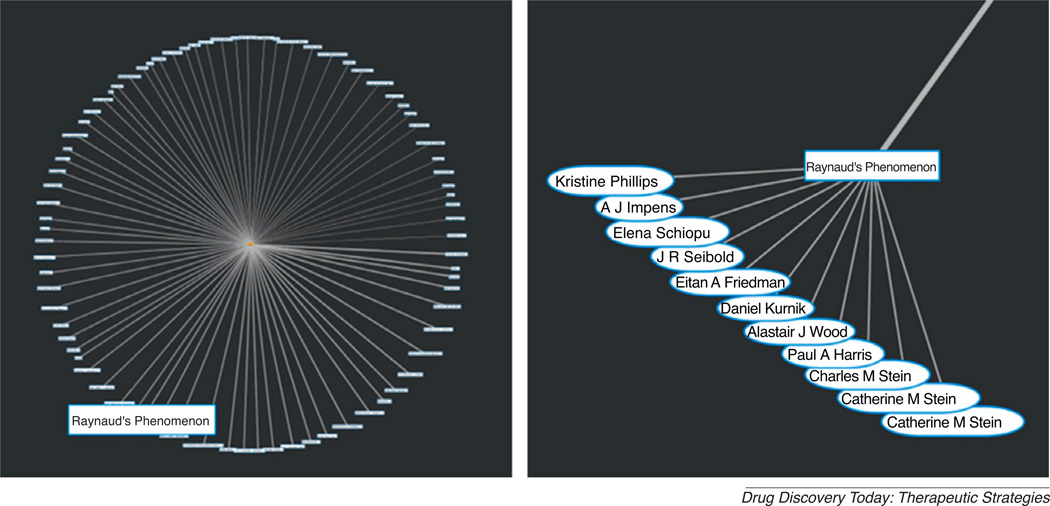
Whereas FoX can be used broadly to develop new collaborations, for the purposes of the Pharma Portal we used the tool in a more focused manner – to find complementary overlap between drug characteristics (including the biological target and its clinical history) and existing academic expertise, including those of the Portal members. For instance, if a drug’s primary mechanism of action is inhibition of a particular gene, then a collaboration could be built by engaging researchers studying that gene pathway, irrespective of the disease indication. Using FoX capabilities, we now can identify different avenues of collaborative research that may not have been obvious initially, and may not have appeared by simply seeking other researchers working in similar areas. One can begin a search based on a compound’s mechanism of action (target) and subsequently expand the results via disease and/or author searches. In this manner, all target-disease and target-author associations for a given target can be readily identified. For example, expanding an initial FoX query for the phosphodiesterase 5 (PDE5) target identifies 87 diseases. By comparison, a PubMed search identifies 1986 publications on PDE5, but the results are not grouped by disease. If one does not know a priori what disease to look for, FoX offers easily identifiable disease groupings shown as clickable nodes on a graph (Figure 2). Out of 87 diseases, Pfizer researchers were interested in Raynaud’s Phenomenon as a potential indication for the drug PF-00489791, a long-acting PDE5 inhibitor. Nine authors published on both PDE5 and Raynaud’s Phenomenon. The FoX tool clearly provided a unique avenue for facile identification of potential collaborators to explore the use of PF-00489791 for Raynaud’s Phenomenon. FoX queries based on a compound’s primary mechanism of action may thus identify other, less obvious, indications for company compounds. Importantly, the tool also provides quick hyperlink access to supporting publications.
Figure 2.
Using FoX for finding collaborations. (a) Expansion of PDE5 targets identifies 87 diseases. The strength of the connection between PDE5 and each disease is shown by line thickness; thicker lines represent more publications about both PDE5 and the given disease. (b) Expansion of the ‘Raynaud’s Phenomenon’ node reveals researchers who have published on both PDE5 and Raynaud’s Phenomenon. Dr. Catherine M. Stein appears twice because of her association with two universities, Weill Cornell and Case Western Reserve.
Industry perspective
Over the last several years, the pharmaceutical industry has directed considerable effort into implementing novel business models that aim to address the diminishing return on R&D investments. Previously, companies focused almost exclusively on internal R&D to drive opportunities from conception to therapeutics. More recently, this model has rapidly evolved to recognize and intentionally seek to leverage expertise outside the company. In addition, the recognition that a single biological target may be important in multiple disease pathologies has spurred interest at some companies to explore the full medical potential of each clinical candidate.
The interests of Pfizer’s Indications Discovery Unit (IDU) in leveraging scientific expertise beyond Pfizer closely matched the Pharma Portal’s goals, resulting in significant contributions by the IUD to the Portal strategy. To enable the collaborative dialog, academic researchers (Pharma Portal members) were provided a mechanism to inquire whether the IDU had a compound for a specific target of interest. The inquiries were received by the IDU via submission of short proposals through the Pharma Portal website. This open-ended strategy proved to be of limited value, as investigators had no context and little guidance regarding what would constitute a successful submission. Requests were denied because of lack of a suitable compound; because access was encumbered by business agreements; or investing in the proposed development path could not be justified.
As pharmaceutical companies are key players in the success of the public-private partnerships in drug repositioning, the Portal aimed to understand factors affecting companies’ willingness to participate in the project. We found that different companies had vastly different perspectives on compound repositioning. Some disagreed with the perception that many clinical compounds were “sitting on the shelf”, and therefore, did not pursue relationships with the Portal. Not all companies saw compounds “sitting on the shelf” as a significant opportunity for drug development. Some refused to repurpose compounds that had been stopped for safety-related concerns, even though the risk in other patient populations might be acceptable. Others chose an out-partnering approach for compounds shelved for strategic reasons (i.e., priority or funding); these compounds were not considered for in-house efforts to identify new indications. Compounds shelved for lack of efficacy might be considered for repurposing, although many were also out-licensed or even sold at open auction. Some companies chose a narrow repurposing strategy only in specific disease areas. Still, others have not yet developed a repositioning strategy and did not feel ready to engage in such discussions. Clearly, striking the appropriate balance between aggressively embracing new, collaborative models and ensuring appropriate attention to the company’s bottom line continues to be a key consideration that will shape the future of this project.
Material Transfer Agreements for compound transfers
One of the Portal’s specific goals was to evaluate potential barriers to technology transfer. Material Transfer Agreements (MTAs) for pharmaceutical compounds are often exceptionally challenging and time-consuming for both parties to draft, negotiate and reach consensus on compared to other types of MTAs. For example, from 2001 to 2010, the UC Davis technology transfer office successfully executed nearly 200 drug transfer MTAs with about two dozen pharmaceutical companies. Most of these MTAs were several pages long and required more than four months each to negotiate and execute, approximately three to four times longer than the time required for other MTAs. Commonly, negotiations are most heated around issues of limiting the use of the compound to non-commercial research purposes; limiting company liability; delaying academic publications or presentations to protect confidential information and IP; defining confidential information; reporting research results to the company; and IP provisions.
Historically, IP terms are by far the most difficult to negotiate. Companies invest considerable resources in developing proprietary drugs, and want to protect their freedom to operate using their own compounds. Universities, by contrast, want to maintain ownership of their discoveries and inventions, make them available for the benefit of the public, and receive fair consideration for those discoveries and inventions. As a compromise, and in a university-specific way, technology transfer offices may agree to grant companies nonexclusive, royalty-free commercial licenses for certain discoveries and inventions. As part of the agreement for such a license, the university must keep a close nexus between the patentable invention and the drug; avoid involvement in past and future inventions; ensure that the university will be reimbursed for any out-of-pocket patent prosecution costs; and be satisfied that provision of the drug is fair consideration for such a license. Importantly, when these challenging and often time-consuming issues are carefully addressed, companies have regularly accepted the terms in which the university grants the company a nonexclusive, royalty-free commercial license and a first right to negotiate an exclusive license for a patentable invention or discovery that uses or incorporates the proprietary compound.
Having a better appreciation of the potential IP restrictions, the Portal chose to initially focus on compounds that were nearing or had lost patent protection, anticipating diminished IP barriers for this set of compounds. The Portal’s leading partner, the Center for World Health and Medicine (CWHM) at St. Louis University, took a lead in developing the first comprehensive MTA template to negotiate transfers. Under the proposed structure, the CWHM would become a shared resource for housing, maintaining and distributing the discontinued compound library. By consolidating all donated compounds into one shared resource, the CWHM relieves companies of the burden of negotiating MTAs with individual compound seekers. Companies would only need to negotiate one MTA with the CWHM, which in turn would distribute donated compounds to other universities. Staffed with highly skilled ex-industry scientists, the CWHM offers comprehensive drug development expertise to the international academic community and runs multiple drug discovery and repurposing projects for neglected diseases. During the last six months, the CWHM has achieved significant progress in defining the IP provisions of a template MTA. Based in part on this progress, in 2010, the University-Industry Demonstration Partnership (UIDP), a group of 35 selected individuals brought together by the Industrial Research Institute (IRI) and the National Council of University Research Administrators (NCURA), chose the Pharma Portal as one of its projects. The UIDP plans to build upon the Portals’ initial accomplishments to ultimately define mutually agreeable conditions specifically related to drug repositioning.
Conclusions
The advantages of a global public–private partnership for drug repositioning are well understood by all parties. Most pharmaceutical companies have publicly expressed a desire to balance commercial viability with social responsibility, an objective that can be facilitated through the Pharma Portal. Providing a mechanism to access compounds by the academic network, while retaining certain safeguards, is a form of R&D outsourcing. Whereas some companies have initiated internal drug repositioning programs, the scope of such programs is guided by the overall company strategy/priorities. Providing public access to deprioritized assets represents a novel and productive collaborative approach that can be tailored to complement internal efforts. Pharmaceutical companies would gain access to a vast and diverse community of global researchers with expertise in many disease areas, potentially leading to newfound utility for discontinued candidates that would otherwise remain dormant.
Although many challenges to taking full advantage of this opportunity remain, the most significant achievements of the Portal were identifying and systematically addressing the barriers to collaborative drug repositioning, such as pharmaceutical companies’ hesitation to release compounds to outside entities; difficulties in identifying mutually beneficial matches between academic and industry investigators; and prolonged MTA negotiations (Table 4).
Table 4.
Barriers to collaboration: current status, future avenues, and the contribution of the Pharma Portal
| Barriers to collaborative initiatives in drug repositioning |
Current status | Possible future avenues | Pharma Portal contribution |
|---|---|---|---|
| Reluctance of pharmaceutical companies to release compounds for use as a shared resource |
|
|
|
| Difficulty identifying potential collaborators |
|
|
|
| Prolonged drug MTA negotiations |
|
|
|
| Lack of funding for drug repositioning research |
|
|
|
OSHU, Oregon Health and Sciences University; UW, University of Washington.
The lack of dedicated funding for conducting repositioning studies was another important challenge that the Portal overcame by identifying a novel, nimble funding mechanism, Partnership for Cures (http://www.4cures.org/). This non-profit organization sponsors Rediscovery Research™ or repurposing of “the existing science and medicine to create quick, safe and inexpensive treatments for patients...”. The organization provides 90% of a project’s direct costs, ranging from $100 to $250K, for one- to three-year projects. In early 2011, over 20 CTSA universities entered in memoranda of understanding (MOU) and became Charter Partners with the Partnership for Cures. Under the MOU, a Charter Partner agrees to establish the process for selecting the appropriate studies for funding by the Partnership for Cures, and to provide 10% of direct costs. This path enabled many investigators to obtain pilot funding for their drug repositioning projects.
Lack of information about discontinued compounds in particular, and the inconsistencies in nomenclature of molecular entities in general are other significant barriers. Such obstacle was clearly recognized by the NCGC, and led to creation of a new drug knowledge database now made available to the academic community [2]. NCGC accepts applications for screening of the compounds in house. If in the future NCGC is able to make some of these compounds available for distribution, we believe that the CWHM’s internal expertise and its experience with the Portal would make it a strong candidate as a compound distribution center for the NCGC’s pharmaceutical collection.
In conclusion, the Pharmaceutical Assets Portal brought to light many perceived and real barriers for global public–private partnerships in drug repositioning. The Pharma Portal deployed a bottom-up strategy, focusing its limited resources on factors directly affecting investigators, such as funding, agreements, and industry outreach. This project popularized the notion that it is, indeed, possible to bring a broad consortium of academic researchers into drug repositioning efforts. We hope that together with the NCGC, the Portal and other national collaborative resources may serve the common purpose of facilitating discovery of safe and effective therapies for unmet medical needs.
Acknowledgements
We thank the following people for contributing to this article: Aaron M. Cohen 5, Rafael A. Gacel-Sinclair6, Pakou Vang6, Peter G. Ruminski7, Bruce E. Bloom8, Anthony M. Boccanfuso9, and Pamela Nagasawa10
5 Department of Medical Informatics and Clinical Epidemiology, Oregon Health and Sciences University, 3181 S.W. Sam Jackson Park Road, Mail Code: BICC, Portland, OR 97239-3098, United States
6 Technology Transfer Services, a Unit of the Office of Research, University of California, Davis, 1850 Research Park Drive, Suite 100 Davis, CA 95618, United States
7 Center for World Health and Medicine, Doisy Research Center, Saint Louis University, St. Louis, MO 63104, United States
8 Partnership for Cures, 70 West Madison Street, Suite 1500, Chicago, IL 60602, United States
9 University Industry Demonstration Partnership (UIDP), the National Academies, 500 Fifth Street, NW, K 506, Washington, DC 20001, United States
10Department of Medical Education and Biomedical Informatics, University of Washington, 1959 Pacific Ave NE, Seattle, WA 98195, United States
This publication was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
References
- 1.Huang R, et al. The NCGC pharmaceutical collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001862. 80ps16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reis SE, et al. Reengineering the national clinical and translational research enterprise: the strategic plan of the National Clinical and Translational Science Awards Consortium. Acad. Med. 2010;85:463–469. doi: 10.1097/ACM.0b013e3181ccc877. [DOI] [PMC free article] [PubMed] [Google Scholar]