Abstract
The Normal Hematocrit Trial (NHT) was the largest trial of epoetin randomizing 1265 hemodialysis patients with cardiac disease to lower (9–11 g/dl) or higher (13–15 g/dl) hemoglobin (Hgb), hypothesizing that higher Hgb would reduce mortality, and improve survival and quality of life. The trial was terminated early, and a 1998 publication reported that targeting higher hematocrit levels led to an insignificant increase in the primary end points (death or myocardial infarct), or risk ratio 1.3, 95% confidence interval (CI), 0.9–1.90, but the P-value was not given, and all-cause death risk was not reported. A higher target reportedly did not increase hospitalization rates, but did significantly improve the ‘physical function' domain of quality of life. Comparing the 1996 Food and Drug Administration (FDA)-filed clinical trial report to the 1998 publication, however, found several discrepancies. Among these, the 1998 article reported interim trial results with only the adjusted CI but did not state that the unadjusted CIs were 99.912th percentile, and despite being a secondary end point, reported only the association of achieved Hgb with higher quality of life score. Randomization to the higher target had actually increased the risk for the primary end point (risk ratio 1.28, 95% CI=1.06–1.56; P=0.0112; 99.92% CI=0.92–1.78), the risk of death (risk ratio 1.27, 95% CI=1.04–1.54), non-access thrombotic events (P=0.041), and hospitalization rate (P=0.04), while ‘physical function' did not improve (P=0.88). Hence, disclosure of these results in the 1998 publication or access to the FDA-filed report on the NHT in the late 1990s would likely have led to earlier concerns about epoetin safety and greater doubts about its benefits.
Keywords: anemia, epoetin, hemodialysis, quality of life
There has been controversy over the risks and benefits of erythropoietic-stimulating agent (ESA) therapy since the completion of the Normal Hematocrit Trial (NHT) in 1996 and its publication in 1998.1 The NHT remains the largest randomized trial of ESA in hemodialysis patients. It treated patients with clinical evidence of heart failure or ischemic heart disease with epoetin (an ESA) to a target hematocrit of 30±3% vs. 42±3%. Hematocrit was calculated by multiplying the measured hemoglobin (Hgb) level by three. A majority of the US dialysis patients have ischemic heart disease or heart failure.2, 3, 4 Therefore, the NHT represented an important assessment of the risks and benefits of higher Hgb for the majority of dialysis patients.
The NHT was stopped early because of a trend toward increased deaths and nonfatal MI in the high-Hgb target arm.1 Although the NHT trial results demonstrated satisfactory outcomes with a Hgb target of 9–11 g/dl, subsequent nephrology anemia guidelines in 1997 and 2001 recommended a Hgb target of 11–12 g/dl when prescribing an ESA.5, 6 This was largely based on studies suggesting other benefits from somewhat higher Hgb, including improvements in health-related quality of life (HRQOL). The NHT publication stated that higher Hgb had significantly increased scores in the ‘physical function' domain of HRQOL. As the NHT is the largest trial of ESAs in hemodialysis, the report of improvements in physical function via higher Hgb had a great impact on clinical guidelines that attempted to balance the risks and benefits of ESA therapy.
By 2006, the improvement in HRQOL observed in the NHT was of central importance, as the US and European Renal guidelines recommended that all patients with chronic kidney disease (CKD) should have Hgb maintained at 11 g/dl or higher.7, 8 The 2006 US Kidney Disease Outcome Quality Improvement (KDOQI) guideline group concluded that ‘…QOL (quality of life) is a sufficient and, apparently, the sole determinant of treatment benefit.'7
Also in 2006, trials in non-dialysis CKD and other populations reported increased thrombovascular and cardiovascular events and death with ESA use, especially when targeting higher Hgb.9, 10, 11, 12 Consequently, the US regulatory authorities recommended lower Hgb goals and far more caution when using ESA.12, 13 In 2009, the TREAT trial, which compared placebo treatment to ESA therapy targeting a Hgb goal of 13 g/dl, demonstrated that ESA therapy afforded no reduction in cardiac events or deaths, a marginal improvement in quality of life, and an increased risk of strokes and arterial and venous thromboembolic events.14
Recently, the Food and Drug Administration (FDA) concluded that ‘Using ESAs to target a Hgb level of greater than 11 g/dl increases the risk of serious adverse cardiovascular events and has not been shown to provide additional patient benefit.'15 This is in conflict with the NHT results from 1998, which reported that a higher Hgb target improved HRQOL and insignificantly increased deaths.
I sought to determine whether the results of the NHT published in 1998 were complete and supported the recommendations in renal anemia guidelines. I obtained the NHT report filed by Amgen (Thousand Oaks, CA) with the FDA in 1996 through a Freedom of Information Act request, and compared the primary and secondary predefined HRQOL end-point results in the Amgen clinical trial report (ACTR) with the 1998 publication.
RESULTS
The trial randomized 1265 patients, although the 1998 publication and most of the ACTR analyzed data on the 1233 patients who were part of the last interim analysis performed by the data safety monitoring board.1, 16 The clinical trial report states on page 4 that analyses on the entire 1265 patients did not change the results. The primary end-point results on the entire 1265-patient cohort were reported in a letter published in 2008.16
The differences in the major primary and secondary end points and adverse events between the 1998 publication and the ACTR are shown in Table 1. Table 1 also compares the ACTR and the FDA safety announcement on ESAs released in June 2011 with the 2008 letter containing final NHT information. The major differences regarding the primary end point are owing to the 1998 report and the 2008 letter exclusively reporting adjusted 95% confidence intervals (CIs), and not providing the P-value for the analysis, which would have been <0.05.1, 16 As shown in the ACTR on pages 54 and 58, the unadjusted CIs required for significance were 99.912% at the last interim analysis (P=0.00088) and 99.92% (P=0.0008) at study termination. As described in the methods of the original publication, these CIs were adjusted to account for repeated testing by the data safety monitoring board. Differences between the treatment groups with respect to both the primary end point and all-cause death are statistically significant by the unadjusted CIs, but fail to reach significance using the boundary rules adopted by the data safety monitoring board. Stopping the trial early before reaching the prescribed significance threshold created an insignificant difference between arms. In 2007 and 2011, the FDA reported only the unadjusted 95% CIs, and now classifies the trial results as showing significant harm.15
Table 1. Comparison of 1998 and 2008 New England Journal articles to the FDA-filed NHT Clinical Trial Report.
| 1998 New England Journal of Medicine publication | FDA-filed Clinical Trial Report | |
|---|---|---|
| Data reported | The last interim analysis performed on March 30, 1996 on 1233 patients | The last interim analysis performed on March 30, 1996 on 1233 patients |
| Primary end point (death+nonfatal MI) | RR 1.3; (95% CI 0.9–1.9). (P-value not given) 95 % CI adjusted for the previous interim analyses | RR 1.3 (99.912% CI=0.92–1.85) (P=0.0119)*,a |
| All-cause deaths | Not reported | Kaplan–Meier, log-rank test P=0.0188* RR 1.26 (95% CI=1.02–1.56)*,a |
| Hospitalization rate | 72% vs. 69% P=0.29 by Fisher's exact test | 72% vs. 69% P=0.04 by Kaplan–Meier curve, log-rank test. Adjustment for covariables, RR 1.14, 95% CI 0.994–1.30 (P=0.06) |
| Quality of life | Physical function domain ‘at 12 months increased by 0.6 point for each percentage-point increase in the hematocrit (P=0.03).' | None of seven domains showed any significant improvement over time or between groups |
| Thrombotic events | Vascular access 39% vs. 29%, P=0.001 Other thrombotic events, listed individually, P=NS | Vascular access 39% vs. 29%, P=0.001 Other thrombotic events, grouped, 22% vs. 18%, P=0.041 |
|
Final trial results |
2008 New England Journal of Medicine Letter to the Editor |
FDA-filed Clinical Trial Report |
| Data reported | Data reflect the final results on 1265 patients following trial termination on June 24, 1996. | Data reflect the final results on 1265 patients following trial termination on June 24, 1996. |
| Primary end point (death+nonfatal MI) | RR 1.28 (CI 0.92–1.78); P-value not given. The 95% CI were adjusted for the previous interim analyses with the use of the method of repeated confidence intervals. | Kaplan–Meier log-rank P=0.01138. Unadjusted OR 1.28 (99.92% CI=0.92–1.78) (0.0112)**,a |
Abbreviations: CI, confidence interval; FDA, Food and Drug Administration; MI, myocardial infarct; NS, not significant; RR, relative risk.
*The P-value required for significance at this interim analysis was set at 0.00088.
**The P-value required for significance at this analysis was set at 0.0008.
The final trial result for relative risk for the primary end point was 1.28, 95% CI 1.06–1.56, and for all-cause death was 1.27, 95% CI 1.04–1.54.
The 1998 publication states that ‘There were no differences between groups in the incidence of cerebrovascular accidents, transient ischemic attacks, peripheral gangrene, (or) intestinal ischemia….' Because of the concern that epoetin dosed to correct anemia could increase thrombotic events, the ACTR states on page 78 that there was a predefined category of ‘other thrombotic events', which included strokes, peripheral arterial thrombosis, and embolism but excluded myocardial infarcts and access thrombosis. According to the ACTR, page 79, patients randomized to the higher target experienced significantly more ‘other thrombotic events' (22.4 vs. 17.6%, P=0.041).
The HRQOL was measured at baseline and at 6-month intervals. Approximately 857 patients had data reported at any time point in the HRQOL results tables. In the 1998 publication, there were 961 and 849 patients in the trial at 6 months and 9 months, respectively. The 1998 publication reported the HRQOL changes as follows: ‘The physical-function score on the quality-of-life questionnaire at 12 months increased by 0.6 point for each percentage-point increase in the hematocrit (P=0.03). For example, an increase in the hematocrit from 30 percent to 42 percent was associated with a clinically meaningful increase of 7.2 points in the score on the physical-function scale. There were no significant changes in the scores on the other seven scales.'
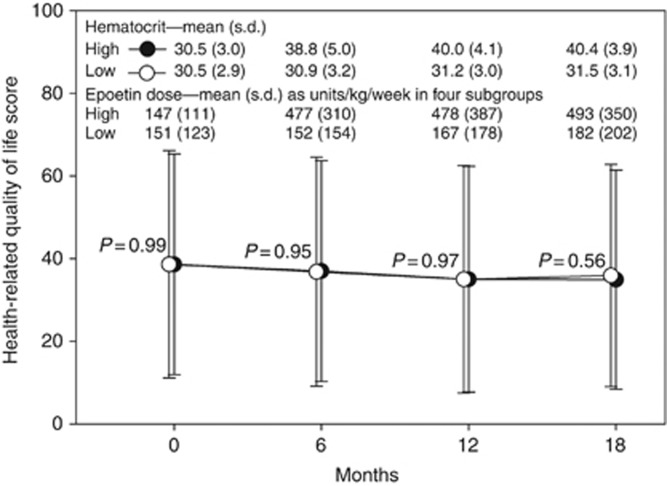
The ACTR reports on page 149 the 12-month physical function score as 35.0±27.3 in the high arm and as 35.0±27.5 in the low arm, P=0.97. Figure 1 graphically presents the ACTR mean scores for ‘physical function' domain at baseline, 6, 12, and 18 months along with the average hematocrit, epoetin dose, and P-values for group differences at each time point. The ACTR states that (page 70) ‘There were no statistically significant changes in quality-of-life scores between groups or over time.' Table 2 shows table A-11 of the ACTR report, the area under the curve results, which was the primary end-point analysis for HRQOL.
Figure 1.
Mean scale scores for physical function domain over time in high (black circle) and low (open circle) target arm, as mean±s.d. Hematocrit values, and mean epoetin doses (from intravenously treated patients), are also from the trial report. From Amgen clinical trial report pages 119, 120, 126, 127, and 149.
Table 2. The primary analysis of changes in HRQOL, the area under the curve results, for each scale determined over 18 months.
|
Group A (high target arm) |
Group B (low target arm) |
||||
|---|---|---|---|---|---|
| HRQOL scale | n | Mean±s.d. | n | Mean±s.d. | P-value |
| Primary end points | |||||
| Vitality | 424 | 45.1±19.6 | 427 | 43.6±19.8 | 0.25 |
| Physical function | 423 | 36.2±24.5 | 426 | 36.0±25.0 | 0.88 |
| Mental health | 424 | 69.3±19.6 | 426 | 69.8±18.9 | 0.72 |
| Secondary end points | |||||
| Body pain | 426 | 57.0±24.5 | 428 | 57.4±24.5 | 0.83 |
| Physical role | 422 | 34.6±34.8 | 429 | 35.3±34.7 | 0.78 |
| Emotional role limitations | 416 | 57.2±38.1 | 419 | 60.1±37.1 | 0.25 |
| Social function | 427 | 65.5±24.2 | 428 | 66.6±23.8 | 0.49 |
Abbreviation: HRQOL, health-related quality of life.
Death and missing values were assigned a last value carried forward. There are no significant differences in scores between groups in any scale. From ACTR pages 70 and 148.
Table 3 reproduces the results in the ACTR tables A-12 and A-13, which ‘show the mean scores for the seven scales at each 6-month time point, by treatment group.' The ACTR summarizes the HRQOL on page 70 as ‘There were no significant differences between Group A and Group B scores at any time, and scores did not change significantly over time.'
Table 3. The mean scale scores for primary end points (vitality, physical function, and mental health) and secondary end points over time.
|
Group A (high target arm) |
Group B (low target arm) |
||||
|---|---|---|---|---|---|
| HRQOL scale | n | Mean±s.d. | n | Mean±s.d. | P-value |
| Vitality | |||||
| Baseline | 425 | 44.5±22.0 | 429 | 44.7±21.8 | 0.90 |
| 6 months | 425 | 46.0±22.3 | 429 | 44.3±22.3 | 0.26 |
| 12 months | 425 | 44.8±21.9 | 429 | 43.0±22.2 | 0.23 |
| 18 months | 424 | 44.3±21.8 | 427 | 42.0±21.8 | 0.13 |
| Physical function | |||||
| Baseline | 424 | 38.6±26.7 | 428 | 38.6±27.5 | 0.99 |
| 6 months | 424 | 37.0±26.7 | 428 | 36.8±27.7 | 0.95 |
| 12 months | 424 | 35.0±27.3 | 428 | 35.0±27.5 | 0.97 |
| 18 months | 423 | 34.9±26.5 | 426 | 35.9±26.9 | 0.56 |
| Mental health | |||||
| Baseline | 425 | 70.3±20.7 | 428 | 69.9±20.6 | 0.78 |
| 6 months | 425 | 69.1±21.2 | 428 | 70.3±20.9 | 0.40 |
| 12 months | 425 | 69.0±21.9 | 428 | 69.5±21.3 | 0.73 |
| 18 months | 424 | 68.8±21.4 | 426 | 68.8±21.3 | 0.98 |
| Body pain | |||||
| Baseline | 427 | 58.3±27.4 | 430 | 58.7±28.2 | 0.85 |
| 6 months | 427 | 57.6±27.5 | 430 | 58.7±27.6 | 0.57 |
| 12 months | 427 | 56.2±27.9 | 430 | 56.7±27.9 | 0.79 |
| 18 months | 426 | 56.0±28.2 | 428 | 55.4±27.7 | 0.74 |
| Physical role limitations | |||||
| Baseline | 423 | 33.1±38.9 | 431 | 33.3±38.5 | 0.95 |
| 6 months | 423 | 36.5±41.0 | 431 | 36.0±41.4 | 0.87 |
| 12 months | 423 | 33.9±41.0 | 431 | 34.9±40.5 | 0.72 |
| 18 months | 422 | 33.2±41.1 | 429 | 35.7±40.3 | 0.38 |
| Emotional role limitations | |||||
| Baseline | 417 | 56.4±44.3 | 421 | 59.7±43.5 | 0.28 |
| 6 months | 417 | 57.7±44.6 | 421 | 60.1±43.6 | 0.43 |
| 12 months | 417 | 56.8±44.4 | 421 | 59.1±44.6 | 0.44 |
| 18 months | 416 | 57.0±44.8 | 419 | 61.6±43.6 | 0.14 |
| Social function | |||||
| Baseline | 428 | 65.0±27.8 | 430 | 66.4±27.5 | 0.48 |
| 6 months | 428 | 65.5±27.2 | 430 | 67.7±28.0 | 0.23 |
| 12 months | 428 | 65.3±28.2 | 430 | 65.9±27.9 | 0.74 |
| 18 months | 427 | 66.1±27.7 | 428 | 65.5±27.8 | 0.76 |
Abbreviation: HRQOL, health-related quality of life.
There are no significant differences in scores between groups at any time, and scores did not change significantly over time. Death and missing values were assigned a last value carried forward. From ACTR pages 70, 149, and 150.
According to the ACTR on page 38, differences in hospitalization rates between groups were to be compared using the Kaplan–Meier curve and the log-rank test and fitting Cox proportional hazards regression models. Instead, the 1998 publication reported no difference in hospitalization rates between groups when compared using Fisher's exact test (Table 1). The ACTR provides hospitalization rates identical to the 1998 publication, but on pages 66 and 67 reports the time to first hospitalization results in the following manner: ‘Kaplan-Meier curves for time to first hospitalization, show(ed) that Group B (lower hematocrit target) patients experienced higher hospitalization-free ‘survival' compared to Group A (higher hematocrit target) patients (P=0.04; log rank test)…'. After adjusting for multiple covariables ‘the RR of hospitalization…was 1.14, but the CI spanned 1.00 (0.99 to 1.30).'
DISCUSSION
This report documents several differences between the original publication of the NHT trial result in 1998 and the ACTR filed with the FDA in 1996. The NHT was intended to assess the risk/benefit of targeting higher Hgb.1 Unadjusted mortality and the primary end point of mortality plus nonfatal myocardial infarcts were significantly higher in the high target arm. These results were not a complete surprise to the company, as the ACTR states on page 21 that a rationale for the trial was an ‘increased risk of mortality (was) considered among the potential adverse effects of full correction of anemia in dialysis patients'. The original publication presented these results as insignificantly different because of adjustment of the CIs for repeated reviews by the data safety board monitoring, and this was approved by the journal's editors.1, 17 Unadjusted statistical results were not reported in the 1998 publication on the last interim analysis, or in a published letter in 2008, which provided the final trial results.16 The FDA now reports in ESA package inserts that the NHT found significantly higher relative risk when targeting higher Hgb for both all-cause mortality (1.27, 95% CI 1.04–1.54) and the primary end point (1.28, 95% CI 1.06–1.56). These CIs are not adjusted.15
On the basis of the ACTR results, compared with a Hgb target of 9–11 g/dl, targeting 13–15 g/dl increases the risk of graft and fistula thrombosis (P=0.001) and other thrombotic events (P=0.04), accelerates the time to first hospitalization (P=0.04), and tends to increase all-cause mortality (P=0.0188; HR 1.26, 95% CI 1.02–1.56). The sole benefit was a reduction in the proportion of patients transfused (P=0.001), whereas HRQOL domains did not improve.
The NHT remains the largest ESA trial in the hemodialysis population. The trial population characteristics—42% black, 44% with diabetic nephropathy, and 28% with hypertensive nephropathy, with a mean age of about 65 years, and a mean time on dialysis of 3 years—are comparable to those of the prevalent US dialysis population.1, 18 In addition, a majority of the US dialysis patients have cardiac disease, as did all the patients in the NHT.2, 3, 4 As higher Hgb associates with improved survival, lower cardiac event rates, and higher HRQOL, it was hypothesized that raising Hgb would lead to improved outcomes. The NHT was designed to test this hypothesis.1 The surprising results suggesting harm from targeting higher Hgb represented the first major evidence of safety problems with the use of ESAs.
In 1997, following early termination of the NHT but before publication of the results, the National Kidney Foundation's Dialysis Outcomes Quality Initiative (DOQI, now KDOQI) guidelines recommended a Hgb goal of 11–12 g/dl when using an ESA in CKD patients, with physicians determining which patients warranted treatment.5 The 1997 KDOQI workgroup was aware that the NHT had been terminated early because the high target arm ‘was experiencing a greater incidence of nonfatal myocardial infarcts or death' than the control arm.5 The 1997 KDOQI guideline and 2001 update did not have the formal evidence rules and rigid evaluation techniques that are now commonly used to develop guidelines.5, 6 Consequently, both observational data and trials of varying quality were considered in support of guidelines. There was also no substantive emerging evidence of safety problems with ESAs in patients with renal disease or other populations until later. In addition, there was a prevailing viewpoint that higher Hgb was conferring distinct benefits in cardiovascular health and patient stamina based on observational data and small trial results. Similarly, the January 2001 updated guidelines used very similar logic and cited almost identical strands of evidence to again recommend a Hgb target of 11–12 g/dl when using epoetin.6 The 2001 update additionally cited a lower rate of hospitalization when targeting higher Hgb, which would have been undermined by publication of the ACTR results noted here.6 Regardless, it is unlikely that knowledge of the results outlined in this paper would have significantly altered either the 1997 or the 2001 KDOQI guidelines because of the lack of corroborating evidence of harm from ESAs.
In contrast, by 2006, there was evidence that ESAs increased thrombovascular risks and increased deaths in some cancer trials.9 In addition, some of the earlier proposed benefits from targeting normal Hgb, such as further regression of left ventricular hypertrophy, had failed to materialize in randomized trials.19 Most importantly, the 2006 KDOQI Anemia guidelines were far more rigorous evaluations of evidence.7 Only clinical trials were considered evidence, the quality of each trial was assessed, and pertinent results were collected into evidence tables.
On the basis of review of the evidence tables, the 2006 workgroup wrote a Clinical Practice Guideline that ‘In patients with CKD, Hb (hemoglobin) should be 11.0 g/dl or greater.'7 This was a substantive change from previous guidelines, which had physicians determine whether anemia warranted treatment. A Clinical Practice Guideline, unlike practice recommendations, can form the basis for clinical performance measures to evaluate physicians.20 The workgroup stated that ‘In developing the statement Hb level should be 11.0 g/dl or greater, the Work Group concluded that—when comparing higher with lower Hb targets—QOL is a sufficient and, apparently, the sole determinant of treatment benefit' (italics in the original).7
The 2006 KDOQI Hgb target guideline was based solely on review of interventional trial results. The workgroup cited five high-quality trials in support of HRQOL claim; by far the largest was the 1998 NHT publication (Table 4).7 The NHT results were described as ‘Physical: + (better with higher Hgb), Increased by 0.6 point for each percentage point increase in Hct', as quoted from the 1998 publication.7 Two of the other trials reported no significant improvement in HRQOL when targeting higher vs. lower Hgb.21, 22 The other two high-quality trials, limited to healthier hemodialysis patients, had conflicting results regarding improvement in HRQOL.19, 23 One found no improvement in any SF-36 measure with higher Hgb targets, but did find improvements on other HRQOL instruments,23 whereas the other trial found an improvement in Vitality domain scores that persisted for the first 72 of the 96 weeks.19 This last trial also reported a higher stroke rate (4% vs. 1%, P=0.048) with targeting higher Hgb.19 Four lower-quality trials of 253, 35, 14, and 10 patients were also cited, but none used the SF-36; two of these did not use active treatment in one arm, further limiting their utility.7 The workgroup concluded that ‘This evidence supports the conclusion that patients treated to a Hb target greater than 11.0 g/dl likely will experience measurable QOL benefits with little or no increase in AEs compared with treatment at lower Hb levels.'7 The ACTR results greatly undermine this conclusion (see Table 4) and consequently it is likely that the 2006 KDOQI recommendations would have been more conservative, and would certainly not have qualified as a Clinical Practice Guideline.
Table 4. Results from high-quality trials assessing effect of hemoglobin target on HRQOL.
| Trial | Population, patient characteristics | Numbera | High vs. low hemoglobin target (g/dl) | Positive HRQOL differences between arms | Support 2006 hemoglobin target of 11 g/dl or higher? |
|---|---|---|---|---|---|
| Besarab et al.1 | Hemodialysis, all had heart failure or CAD | 1233 | 9–11 vs. 13–15 | None (improved physical function according to 1998 publication) | No No significant difference between groups |
| CanEPO21 b | Hemodialysis, all had LVD or LVH | 78 | 9.5–11 vs. 11.5–13 | None | No |
| Foley et al.23 | Hemodialysis, free of marked comorbidity | 94 | 9.5–10.5 vs. 13–14 | Fatigue, depression, relationships | Possibly in healthier subjects |
| Parfrey et al.19 | Hemodialysis, no CAD or LVD | 324 | 9.5–11.5 vs. 13.5–14.5 | Vitality | Possibly; significantly increased risk of stroke |
| Roger et al.22 | CKD stage 3 and 4, excluded uncontrolled angina, class III or IV HF, severe chronic respiratory disease, symptomatic peripheral vascular disease, or fistula placement | 155 | 9–10 vs. 12–13 | None | No |
Abbreviations: CAD, coronary atherosclerotic disease; CKD, chronic kidney disease; HF, heart failure; HRQOL, health-related quality of life; LVD, left ventricular dilation; LVH, left ventricular hypertrophy.
Data are extracted from the 2006 KDOQI Anemia guideline evidence tables 13 and 17. Data from only high-quality trials are shown.
This trial had a 40-patient third arm, which received placebo; both treatment arms had improvements in HRQOL compared with placebo.
In retrospect, it is apparent how a guideline group could be misled by the 1998 publication. In the methods, the 1998 publication stated that HRQOL would be assessed as a secondary end point, but the results section provides a description of the association of achieved hematocrit to improved ‘physical function' within a treatment arm. In describing the association of higher Hgb with higher ‘physical function', the 1998 publication offers as an example of the magnitude of the improvement a comparison of hematocrit values of 42% and 30%, the two hematocrit values that correspond to the two targets in the study. This gives the incorrect impression that being randomized to a hematocrit of 42%, rather than 30%, increases the ‘physical function' score by 7.2, and that the P-value (0.03) refers to the comparison between treatment groups. The results from the ACTR presented here show that randomization to the higher target did not improve any parameter of HRQOL.
Targeting an intermediate Hgb of 11–12 g/dl in hemodialysis patients with cardiac disease, as KDOQI recommended in a 2007 update, would lead to a smaller or insignificant reduction in transfusion rates than those observed in the NHT. We lack trial data on whether an intermediate target with ESAs mitigates the harm observed with higher targets. The CHOIR trial in patients with Stage 3 and 4 CKD used ESA treatment to target Hgb to 11.3 vs. 13.5 g/dl, and found an increased risk of cardiovascular events and death with the higher target.10 The placebo-controlled TREAT trial reported a significant increase in thrombotic events and strokes with ESA use, and case–control studies support the fact that ESA use increases strokes.14, 24 Therefore, it is unclear whether lowering the Hgb target while using ESA reduces stroke risk. Consequently, a target above 11 g/dl but below 13 g/dl appears to provide marginal benefits and poses indeterminate risks.
Healthier dialysis patients have significantly higher baseline vitality scores on the SF-36 than reported in the NHT, and may experience some temporary improvement in vitality with higher Hgb.19, 25 In contrast, in the NHT even at 6 months, after a mean 3 g/dl increase in Hgb and before substantial separation of the mortality curves, there was no significant improvement in vitality or other HRQOL domains. In addition, although KDOQI guideline groups and many in the nephrology community consider the results of trials of higher Hgb targets as indicative of improving quality of life, the FDA considers the results unconvincing, noting that the three largest trials comparing Hgb targets showed that ‘…the overall quality-of-life effects were small and inconsistent'.26 These HRQOL improvements were seen in trial arms that significantly increased the risk of cardiovascular events or death.1, 10, 14 Consequently, the FDA has stated that ‘Using ESAs to target a Hgb level of greater than 11 g/dl … has not been shown to provide additional patient benefit.'6
Four of the authors of the 1998 publication were Amgen employees, and undoubtedly had access to the ACTR to prepare the article. Four other authors had only academic affiliations, and their knowledge of this information is unknown. To assure complete and balanced presentation of industry-funded randomized trial results, all authors should be required to review the complete clinical trial report and confirm in writing that the manuscript reflects a complete reporting of the safety and efficacy data. In addition, all clinical trial reports filed with the FDA on all approved drugs should be made freely available within a year or two of trial completion. Alternatively, guideline groups should obtain trial reports on all major studies under review from the FDA via the Freedom of Information Act. To assure impartial review, trial report data should be extracted and confirmed by external expert reviewers who have no connections to the pharmaceutical industry or the guideline organization.
On the basis of these complete results on the NHT, in hemodialysis patients with ischemic heart disease or heart failure, there are no compelling benefits from targeting Hgb to above 11 g/dl with ESAs.
MATERIALS AND METHODS
A Freedom of Information Act request was submitted on January 26, 2008 for the report on the NHT filed by Amgen with the FDA following termination of the trial in 1996. The ACTR was received 1260 days later on July 11, 2011. Statements that are verbatim from the ACTR are in quotes in this publication, followed by the ACTR page number or the ACTR's unnumbered five-page synopsis. The ACTR was available to this Journal's reviewers and editors throughout the review and approval process. Select extracts of the report are available by emailing DCoyne@dom.wustl.edu and placing ‘Amgen NHT Trial Report' in the subject line. The report may also be obtained through a Freedom Of Information Act request to the FDA for the 1996 Epoetin Alfa clinical study report EPO-930107.
I extracted information on the primary and secondary end points of the trial, and the final results as reported in the ACTR. Results were compared with the 1998 publication and references to the NHT in the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) anemia guidelines published in 1997, 2001, and 2006.
The trial design, baseline characteristics, and treatment goals are outlined in the original publication.1 Briefly, hemodialysis patients with clinical evidence of heart disease (history of heart failure or ischemic heart disease) who were already receiving epoetin and had stable Hgb of 10–11 g/dl (hemocrit 30–33%) were randomized to a higher or lower Hgb target. The higher target (Group A) received a 1.5-fold increase in epoetin dose, followed by further increases at 2-week intervals as needed to reach a target of 14±1 g/dl. Those randomized to the lower target (Group B) had epoetin doses adjusted to maintain a Hgb level of 10±1 g/dl. Contrary to the trial's name, Hgb—not hematocrit—was determined, and hematocrit was calculated by multiplying the Hgb level by three.
According to the ACTR, page 2, ‘The primary objective of this study was to assess the effects of the two hematocrit targets, 42 and 30%, on morbidity and mortality in dialysis patients with clinically evident cardiac disease who were receiving Epoetin alfa therapy. Quality of life was also evaluated.' The primary end point was death and nonfatal myocardial infarct. ‘Secondary end points included all-cause mortality alone, MI alone, all-cause hospitalization, congestive heart failure (CHF), unstable angina, coronary artery bypass graft, (CABG), percutaneous transluminal coronary angioplasty (PTCA), quality of life, and transfusions, as well as changes in cardiac medications.'(ACTR, page 38). ‘All-cause mortality alone and all-cause hospitalization were also compared between treatment groups using the log-rank test and fitting Cox proportional hazards regression models. Other secondary end points such as acute MI, CHD, unstable angina, CABG, PTCA, and transfusions were compared between groups using chi-square tests.' (ACTR, page 38).
The ACTR stated on pages 44 and 45, ‘Health related Quality of Life (HRQOL) Study': ‘Patients completed a quality-of-life questionnaire (the Medical Outcomes Study 36-Item Health Survey [SF-36]) at baseline and every 6 months. The SF-36 is a medical outcomes instrument that has been validated in prior studies of ESRD patients.' ‘The primary HRQOL end points chosen prospectively for this study were the vitality scale, the physical function scale, and the mental health index scale. Secondary end points were the bodily pain scale, the physical role limitation scale, the emotional role limitations scale, and the social function scale. Area under the curve was calculated for an 18-month period. The last known value was carried forward for missing data.'
The ACTR also stated on page 78 that a ‘general category of ‘other thrombotic events' was (prospectively) established because there was concern regarding the use of epoetin to correct anemia and these outcomes.'
DWC is a consultant to Abbott, Alnylam, Chiasma, and Sanofi Aventis. He is a speaker for Abbott and Watson. None of these entities make ESAs. He has participated in numerous trials of erythropoiesis-stimulating agents and intravenous iron products.
Footnotes
Author contributions
DWC solely developed the idea of this report, obtained and analyzed the clinical data report from the FDA, and wrote the manuscript.
References
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin [see comment] N Eng J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- Stack AG, Bloembergen WE. A cross-sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis. 2001;38:992–1000. doi: 10.1053/ajkd.2001.28588. [DOI] [PubMed] [Google Scholar]
- Stack AG, Bloembergen WE. Prevalence and clinical correlates of coronary artery disease among new dialysis patients in the United States: a cross-sectional study. J Am Soc Nephrol. 2001;12:1516–1523. doi: 10.1681/ASN.V1271516. [DOI] [PubMed] [Google Scholar]
- Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation-Dialysis Outcomes Quality Initiative NKF-DOQI clinical practice guidelines for the treatment of anemia of chronic renal failure. Am J Kidney Dis. 1997;30 (4 Suppl 3:S192–S240. [PubMed] [Google Scholar]
- National Kidney Foundation. KDOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease, 2000. Am J Kidney Dis. 2001;37 (1 Suppl 1:S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- KDOQI; National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47 (5 Suppl 3:S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19 (Suppl 2:ii1–ii47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Eng J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Eng J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Public health advisory: erythropoiesis-stimulating agents (ESAs) . http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm054721.htm . Accessed 19 March 2007.
- Early Communication about an Ongoing Safety Review Epoetin alfa http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm136211.htm . Accessed 8 May 2009.
- Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Eng J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- FDA Drug Safety Communication Modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm . Accessed 24 June 2011.
- Besarab A, Goodkin D, Nissenson AR. The Normal Hematocrit Study—follow-up. N Eng J Med. 2008;358:433–434. doi: 10.1056/NEJMc076523. [DOI] [PubMed] [Google Scholar]
- Goodkin DA. The normal hematocrit cardiac trial revisited. Semin Dial. 2009;22:495–502. doi: 10.1111/j.1525-139X.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- US Renal Data System . USRDS 2008 Annual Data Report: Atlas of End Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD; 2008. [Google Scholar]
- Parfrey PS, Foley RN, Wittreich BH, et al. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16:2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- Uhlig K, Macleod A, Craig J, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2006;70:2058–2065. doi: 10.1038/sj.ki.5001875. [DOI] [PubMed] [Google Scholar]
- Canadian Erythropoietin Study Group Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ. 1990;300:573–578. doi: 10.1136/bmj.300.6724.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger SD, McMahon LP, Clarkson A, et al. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): results of a randomized clinical trial. J Am Soc Nephrol. 2004;15:148–156. doi: 10.1097/01.asn.0000102471.89084.8b. [DOI] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000;58:1325–1335. doi: 10.1046/j.1523-1755.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- Seliger SL, Zhang AD, Weir MR, et al. Erythropoiesis-stimulating agents increase the risk of acute stroke in patients with chronic kidney disease. Kidney Int. 2011;80:288–294. doi: 10.1038/ki.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf DE, Goldfarb DS. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int. 2009;75:15–24. doi: 10.1038/ki.2008.414. [DOI] [PubMed] [Google Scholar]
- Unger EF, Thompson AM, Blank MJ, et al. Erythropoiesis-stimulating agents—time for a reevaluation. N Eng J Med. 2010;362:189–192. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]