Abstract
To evaluate the feasibility and advantages of constructing a novel tissue engineering bone, using β-tricalcium phosphate (β-TCP) and rat adipose-derived stem cells (ADSCs), modified with BMP2 and BMP7 by lentivirus. In the present study, ADSCs transfected with Lv-BMP2 and Lv-BMP7, alone or together, were seeded on β-TCP scaffold and cultured in vitro. Based on the results of DNA assay, alkaline phosphatase (ALP) activity, alizarin red staining and osteogenic marker genes expression analysis, the BMP2 and BMP7 genes cotransfection group exhibited a higher degree of osteogenic differentiation in vitro. To investigate the in vivo osteogenesis of the tissue engineering bone, the ADSCs/β-TCP constructs were implanted in rat femurs defects for 6 weeks and studied histomorphology and radiography. The results showed that BMP2 and BMP7 genes cotransfection group dramatically enhanced the efficiency of new bone formation than BMP2 group and BMP7 group in vivo. These results demonstrated that it was advantageous to construct tissue engineering bone using ADSCs cotransfected with BMP2 and BMP7 on β-TCP, providing a potential way for treating bone defects.
1. Introduction
The development of gene-modified tissue engineering bone has made it an attractive approach with great potential for repairing bone defects resulted from trauma, surgical resection, and congenital deformity corrections [1–3]. Many of these studies have focused on bone morphogenetic proteins (BMPs). They are multifunctional growth factors that belong to the transforming growth factor beta (TGFbeta) superfamily. BMP2 has the highest osteoinduction activity in vivo among the BMP family members [4–7]. In addition, many studies have demonstrated that local implantation of BMP7 results in the repair of critical size defects in long bones, craniofacial bones, and the formation of bony fusion masses in spinal fusions [8, 9]. Laflamme's result showed that the BMP2, BMP7, and a mixture of BMP2/BMP7 all promoted osteoblast growth on the collagen scaffold, with the mixture of BMP2/BMP7 enhancing the most growth [10]. Therefore, we speculate that combined gene therapy of BMP2 and BMP7 in tissue engineering bone might have more significant effect on bone regeneration than single gene alone.
The other important aspect of tissue engineering bone is the introduction of bioactive cells into the three-dimensional porous scaffold. Stem cells hold great promise for future translational research and clinical applications in many fields. Much research has focused on mesenchymal stem cells isolated from bone marrow in vitro and in vivo however, bone marrow procurement causes considerable discomfort to the patient and yields a relatively small number of harvested cells. By contrast, adipose tissue represents an abundant and easily accessible source of adult stem cells, termed adipose-derived stem cells (ADSCs), with the ability to equally differentiate along multiple lineage pathways, such as fat, bone, cartilage, skeletal, smooth, and cardiac muscle, endothelium, hematopoietic cells, hepatocytes, and neuronal cells [11, 12]. Animal and clinical studies have shown that ADSCs are capable of repairing damaged skeletal tissue or large-bone segmental defects [13, 14].
Therefore, in the present study, we further explore the osteogenic potential of tissue engineering bone using β-TCP combined with ADSCs lentivirally transfected with human BMP2 gene and human BMP7 gene, alone or together in vitro and in vivo.
2. Materials and Methods
2.1. Osteogenesis In Vitro
2.1.1. ADSCs Culture and Infection
ADSCs were obtained from subcutaneous adipose tissue in the inguinal groove of 4-week-old Sprague-Dawley rats as previously described [15]. Briefly, the adipose tissue was washed three times with 0.1 mol/L phosphate-buffered saline (PBS, pH = 7.4), and then minced finely and digested with 0.1% collagenase type I at 37°C for 60 min with vigorous shake for 15 seconds in 20 minute intervals. After centrifugation, the resulting pellet, which is defined as the adipose tissue stromal-vascular fraction (SVF), was exposed to lysis buffer for 10 minutes to remove red blood cells. The remaining cells were suspended in DMEM media supplemented with 10% FBS and 1% penicillin/streptomycin, filtered through 40 μm cell strainer, and plated at 1 × 104 cells/cm2 on tissue culture dishes. After reaching approximately 80% confluence, the cells were separated and cultured. ADSCs at passage 2 were transfected with human BMP2 and human BMP7, alone or together by lentivirus vector. After infection for 12 days, the medium was exchanged every 2 days. The cells at passage 3 were used for experiments.
2.1.2. Cell Seeding
BMP2/ADSCs, BMP7/ADSCs, BMP2 + BMP7/ADSCs, and ADSCs as control were collected and seeded at a density of 1 × 106 in 30 μL on the β-TCP scaffolds and incubated at 37°C for 2 h to allow cells to attach to the scaffolds, respectively. Then the scaffolds were transferred into a 96-well plate. Each well was cultured with the normal culture medium to form tissue engineering bone. The medium was changed every 2-3 days after cell seeding on β-TCP.
2.1.3. DNA Assay
Cell proliferation of each group was determined every day by PicoGreen dsDNA reagent and Kits (Molecular Probes, OR, USA) for up to two weeks after cell seeding. A DNA standard was prepared by lysing serial dilutions of a known concentration of ADSCs. The cell scaffolds and standards were rinsed three times with PBS and incubated in 1 mL-digestive solution (50 mM Na3PO4, 20 mM N-acetyl cysteine, and 28 mg/mL papain). The specimens were vortexed for 10 min, then 10 mL supernatant was mixed with 10 mL PicoGreen dsDNA quantitation reagent in darkroom for 5 min, and the DNA content was quantified using a fluorospectrometer.
2.1.4. Alkaline Phosphatase Enzyme Activity (ALP)
Each group construct was analyzed at 14 days after cell seeding as previously described [16]. The scaffolds were homogenized with 1 mL Tris buffer (pH 7.4, Sigma, USA), and sonicated. The cell lysate (0.1 mL) was mixed with 0.5 mL p nitrophenol phosphate substrate solution (Sigma, USA) and 0.5 mL alkaline phosphatase buffer solution (Sigma, USA). After incubation at 37°C for 15 min, 10 mL of 0.05 N NaOH were added to stop the reaction. The production of p nitrophenol in the presence of ALP was measured by monitoring light absorbance of the solution at 405 nm at 1 min increments. The slope of the absorbance versus time plot was used to calculate the ALP activity.
2.1.5. Alizarin Red S Staining
Alizarin red S staining was used to evaluate ADSCs mineralization on β-TCP scaffolds. At 14 days after cell seeding, the constructs were fixed in 4% paraformaldehyde at 4°C for 24 h, and frozen at −20°C, then sectioned at 10 μm in thickness. After being washed with 0.01 M PBS, sections were stained with the Alizarin Red Solution (2%, pH 4.2) for 10 min, followed by extensive washing with distilled water to remove the remaining stain.
2.1.6. Real-Time Quantitative PCR Analysis (qPCR)
At 14 days, total RNA was extracted from each group by the TRIzol method strictly following the manufacturer's protocol (Invitrogen, USA). The first-strand cDNA was synthesized from 1 μg RNA with virus reverse transcriptase (TaKaRa, Japan), and used for quantitative real-time PCR. Expression levels of representative genes, including ALP, osteopontin (OPN), and osteocalcin (OC), were quantified with an ABI 7300 real-time PCR system (Applied Biosystems, USA) and SYBR green PCR reaction mix (TaKaRa, Japan). Primers and annealing temperature for each gene are listed in Table 1. The program used was 95°C for 5 minutes, 40 cycles of 95°C for 15 seconds, annealing temperature for 1 minute, and 72°C for 30 s. Melting analysis and agarose gel electrophoresis were performed to confirm the specificity of the PCR products. The relative expression levels of genes were analyzed using the 2−ΔΔCt method by normalizing with GAPDH housekeeping gene expression, and presented as fold increase relative to the control group.
Table 1.
Primers used for real-time PCR analysis.
| Gene | Primers | Annealing temperature (°C) |
|---|---|---|
| ALP | Forward: 5′-AGGCAGGATTGACCACGG-3′ | 58 |
| Reverse: 5′-TGTAGTTCTGCTCATGGA-3′ | ||
| OPN | Forward: 5′-GACGGCCGAGGTGATAGCTT-3′ | 63 |
| Reverse: 5′-CATGGCTGGTCTTCCCGTTGC-3′ | ||
| OC | Forward: 5′-AAAGCCCAGCGACTCTC-3′ | 59 |
| Reverse: 5′-CTAAACGGTGGTGCCATAGAT-3′ | ||
| GAPDH | Forward: 5′-AACCCATCACCATCTTCCAGG-3′ | 60 |
| Reverse: 5′-GCCTTCTCCATGGTGGTGAA-3′ |
2.2. Osteogenesis In Vivo
2.2.1. Preparation of Bone Defect Model and Implantation
Forty healthy Sprague-Dawley rats weighting about 250 g each were divided into four groups for the implantations of ADSCs/β-TCP, BMP2/ADSCs/β-TCP, BMP7/ADSCs/β-TCP, and BMP2 + BMP7/ADSCs/β-TCP. Rats were anesthetized with celiac injections of 10% chloral hydrate (3 mL/kg body weight), and approximately 2 mm diameter defect was created on the medial femur by an electric drill. The cell-scaffold constructs were placed into the defects via a press fit.
2.2.2. Radiography and Histological Detection
Radiographs and histomorphology of rat femurs were examined at 2, 4, and 6 weeks after the surgery. At each time point, rats were anesthetized and carried out X-ray radiography. Images were obtained by SPECT/CT (Philips, the Netherlands). After radiography examination, rats were sacrificed, and the scaffolds together with surrounding tissue were excised, stained with hematoxylin and eosin (H&E), and observed the healing condition of the defect by light microscope (Olympus, Japan).
2.3. Statistical Analysis
All data are expressed as means ± SD. SPSS 11.0 statistical software was used for repeated measurement variance analysis and ordinary ANOVA. Differences at P < 0.05 were considered statistically significant.
3. Results
3.1. Osteogenesis In Vitro
3.1.1. DNA Assay
The cell proliferation of BMP2/ADSCs, BMP7/ADSCs, and BMP2 + BMP7/ADSCs attached onto β-TCP scaffolds was determined by the PicoGreen dsDNA assay for 14 days, and the results were shown in Figure 1. The corresponding DNA quantity of each group showed a similar trend, and no significant difference could be found (P > 0.05). DNA quantity increased with time gradually during the first week of culture, peaked at day 7 and then leveled off, following a plateau phase. However, DNA quantity of viable cells still exhibited an increase of around 3-fold at day 14 compared to the initial quantity.
Figure 1.
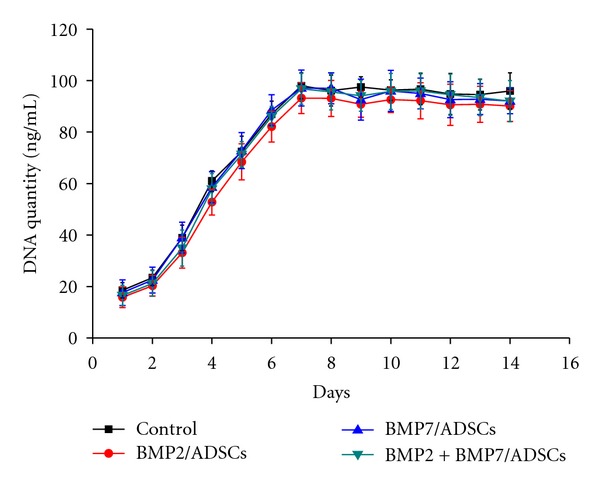
The proliferation curves of control group, BMP2/ADSCs group, BMP7/ADSCs group, and BMP2 + BMP7/ADSCs group on the β-TCP scaffolds for two weeks after cell seeding. Results are shown as mean ± SD (n = 3 for each group).
3.1.2. Alkaline Phosphatase Enzyme (ALP) Activity
The ALP activity of each group was assessed at 14 days after cell seeding. As shown in Figure 2, the ALP activity was enhanced respectively 4.9-fold and 5.3-fold in BMP2/ADSCs group and BMP7/ADSCs group compared to that in control group (P < 0.05). In BMP2 + BMP7/ADSCs group, the ALP activity was approximately 8-fold greater compared to the control group (P < 0.05).
Figure 2.
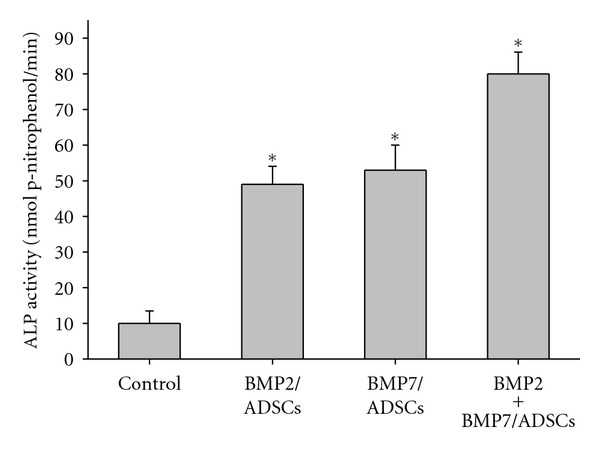
Detection of ALP activities for control, BMP2/ADSCs, BMP7/ADSCs, and BMP2 + BMP7/ADSCs groups at 14 day after cell seeding. Results are shown as mean ± SD (n = 3 for each group, *P < 0.05).
3.1.3. Alizarin Red S Staining
As shown in Figure 3, after cell seeding at 14 days, more obvious positive staining of Alizarin red S in BMP2 + BMP7/ADSCs group (Figure 3(d)) was observed than that in BMP2/ADSCs and BMP7/ADSCs groups (Figures 3(b) and 3(c)). The result indicated that ADSCs modified with both genes make much more mineralization than that modified with single gene.
Figure 3.
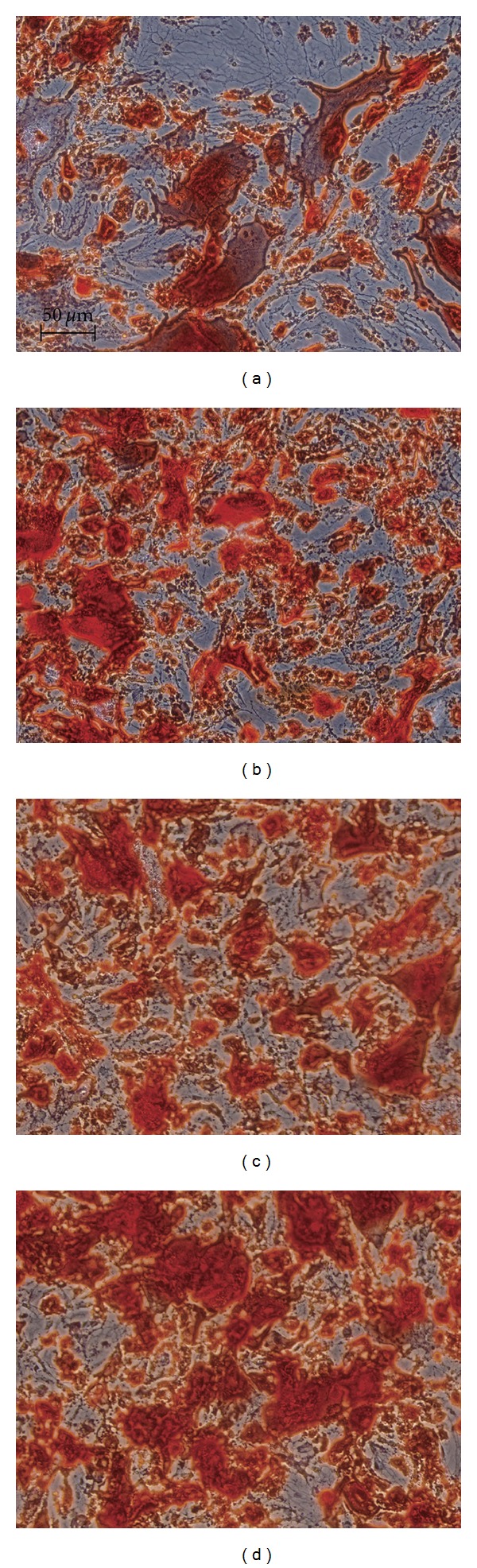
Alizarin red S staining for (a) control group, (b) BMP2/ADSCs group, (c) BMP7/ADSCs group, and (d) BMP2 + BMP7/ADSCs group at 14 days after cell seeding. Bar = 50 μm.
3.1.4. Real-Time Quantitative PCR Analysis
Figure 4 shows the relative expression of ADSCs on β-TCP scaffolds for osteogenic gene. At day 14 after cell seeding, the mRNA-relative expression of osteogenic marker genes ALP, OPN, and OC in BMP2 + BMP7/ADSCs group was higher compared to BMP2/ADSCs group and BMP7/ADSCs group, and respectively, 9.5 times, 5.2 times, and 3.8 times more than those of control group (P < 0.05).
Figure 4.
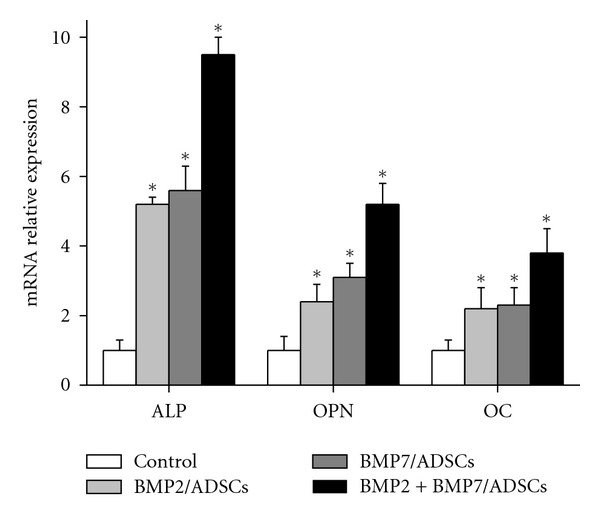
The mRNA-relative expression analysis of osteogenic marker genes ALP, OPN, and OC in control, BMP2/ADSCs, BMP7/ADSCs, and BMP2 + BMP7/ADSCs groups. Results are shown as mean ± SD (n = 3 for each group, *P < 0.05).
3.2. Osteogenesis In Vivo
3.2.1. Behavior of Animal Model after Surgery
A 2 mm-diameter defect was created on the femur middle. The constructs of cell and scaffold were placed into the defects. The dietary and behavior reduced at the first day after surgery, but the behavior returned to normal at the second day. No obvious infection phenomena was observed in all groups.
3.2.2. Radiography
Figure 5 shows the radiographical images of the femur defects after surgery. At 2 weeks, there was no markedly newly formed bone tissue in each group. At 4 weeks, there was partly newly formed high-density tissue in the defects of all groups, and the amount of the new tissue was more in the BMP2 + BMP7/ADSCs constructs treated femurs than those treated with either the BMP2/ADSCs or BMP7/ADSCs. At 6 weeks, there was incremental high-density tissue in BMP2/ADSCs and BMP7/ADSCs constructs treated femurs. But the bone defects were not healed completely. In BMP2 + BMP7/ADSCs group, the femur defect has been basically healed.
Figure 5.
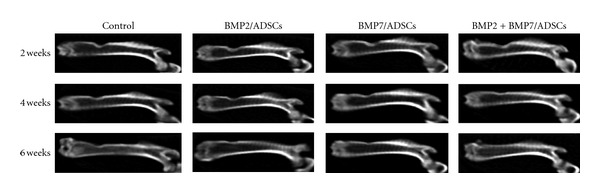
X-rays photograph of rat femurs defects in control, BMP2/ADSCs, BMP7/ADSCs, and BMP2 + BMP7/ADSCs groups at 2 weeks, 4 weeks, and 6 weeks after surgery.
3.2.3. Histological Observation
As shown in Figure 6, at week 2 after surgery, connective tissues filled the defects in all groups. Scaffold's pores were filled by inflammatory cells. At week 4 after surgery, new bone formation was observed in all implanted defects. In BMP2/ADSCs and BMP7/ADSCs group, a small amount of the inflammatory cells can still be seen inside the scaffolds. In BMP2 + BMP7/ADSCs group, the scaffold had been replaced with neogenetic tissue, including a large number of new trabecular bone and fibrous tissue. At week 6 after surgery, the inflammatory reaction disappeared in all groups. In BMP2/ADSCs and BMP7/ADSCs groups, a few bone trabecula, part cortical bone and bone marrow could be seen inside the bone defects. However, in BMP2 + BMP7/ADSCs group, normal cortical bone, and bone marrow can be observed. This indicated that the femur defect had recovered completely.
Figure 6.
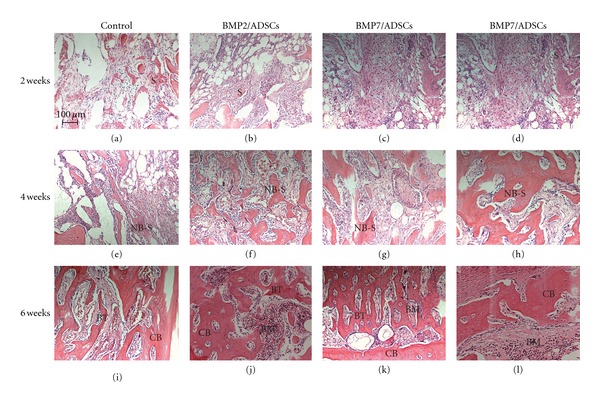
HE staining of the transverse bone defect sections in different groups at 2 weeks, 4 weeks, and 6 weeks post-surgery. Abbreviations used: scaffold (S); new bone from implanted scaffold (NB-S); bone marrow (BM), cortical bone (CB), bone trabecular (BT). Bar = 100 μm.
4. Discussion
Many researchers studied the possibility of using stem cells as donor cells. But few related reports on the adipose tissue-derived stem cells (ADSCs) are available [17]. Adipose tissue by virtue of its several advantages, such as abundance and easy acquirement, is becoming a promising seed cell source. Therefore, we constructed human BMP2 and human BMP7 lentiviral expression vectors and then infected the ADSCs to produce therapeutic seed cells. There are several choices of vectors for gene transfer, such as plasmids, liposomes, retrovirus, adenovirus, and adenoassociated virus [18]. However, viruses are the most efficient vectors for gene delivery [19]. Recently, lentiviral vectors-based ex vivo gene therapy has been developed for bone regeneration [20]. Lentiviral vectors can transduce both dividing and nondividing cells and incorporate into the host genome, allowing for prolonged target gene expression, high transfection efficiency, and low toxicity [21, 22]. It is feasible and efficient that ADSCs are transfected using lentiviral vector incorporating the foreign gene.
Bone tissue engineering methods consist of three elements: a donor cell source, a three-dimensional scaffold, or matrix and osteoinductive growth factors [23]. Therefore, a suitable scaffold for bone tissue engineering is also critical for the success of gene therapy strategy. β-TCP shows excellent osteoconductivity and biocompatibility, and its biodegradation rate could be easily controlled. The novel porous β-TCP scaffolds applied in this study has been proved to have prominent osteoconductive activity and exhibited good osteogenic activity both in vitro and in vivo with the aid of osteogenic medium in a previous study [24, 25]. In our study, the dsDNA assay confirmed that ADSCs can easily adhere to, and proliferate inside the β-TCP scaffolds. The ALP activity assay, Alizarin red S staining, and real-time PCR analysis results also indicated that the novel porous β-TCP scaffolds can support the proliferation and osteogenic differentiation of rat ADSCs in vitro.
ADSCs modified with BMP2 and BMP7 have emerged as an effective strategy for bone regeneration [26, 27]. Koh et al. evaluated the ability of BMP2 homodimer, BMP7 homodimer, or BMP2/7 heterodimers to stimulate SMAD, p38, ERK, and JNK pathways in C2C12 cells and have demonstrated that BMP2/7 heterodimers have enhanced ability to stimulate alkaline phosphatase and Smad 1,5,8 phosphorylation relative to equivalent amounts of BMP2 or BMP7 homodimers [28]. Zhu et al., confirmed that combination of BMP2 and BMP7 gene transfer led to a significant increase in osteoblastic differentiation compared with single BMP gene transfer in both C2C12 and MC3T3-E1 cells [29]. These investigations indicate that the mixture of BMP2 and BMP7 can induce bone formation significantly more effective and are consistent with our results.
Heterodimeric BMPs can increase biological activity [30] and facilitate more rapid bone regeneration in better quality [31]. In the present study, in vitro results indicated that ADSCs co-expressing BMP2 and BMP7 on β-TCP scaffolds exhibited a higher degree of osteogenic differentiation compared to those expressing individually BMP2 and BMP7. The results of in vitro experiments prompted us to investigate the osteogenesis of the constructs in vivo. In order to obtain defect repairing in a short period of time, we adopted a cavity defect model in a rat femur and investigated the tissue response to the cell-scaffold transplant. Radiographic and histological studies were performed on the specimens after different implantation periods. Active bone regeneration in the defects was found in all ADSCs/β-TCP constructs. However, the ADSCs modified with BMP2 and BMP7 constructs presented faster and more effective osteogenesis at the defect area than other groups in various implantation periods. Therefore, it was a promising approach using ADSCs cotransfected with BMP2 and BMP7 seeded on β-TCP to construct tissue engineering bone for repairing bone defects.
5. Conclusion
In the present study, the in vitro experimental results indicated that ADSCs cotransfected with human BMP2 and BMP7 grown on β-TCP exhibited good attachment and growth, and a higher degree of osteogenic differentiation than single gene group. The in vivo experiment demonstrated that the BMP2 + BMP7/ADSCs group implanting present faster and more effective osteogenesis than BMP2/ADSCs group or BMP7/ADSCs group. The results demonstrated that it was advantageous to construct tissue engineering bone using β-TCP combined with ADSCs co-transfected with BMP2 and BMP7, providing a new idea for treating bone defects.
References
- 1.Roldán JC, Detsch R, Schaefer S, et al. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. Journal of Cranio-Maxillofacial Surgery. 2010;38(6):423–430. doi: 10.1016/j.jcms.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Jiang XQ, Chen JG, Gittens S, Chen CJ, Zhang XL, Zhang ZY. The ectopic study of tissue-engineered bone with hBMP-4 gene modified bone marrow stromal cells in rabbits. Chinese Medical Journal. 2005;118(4):281–288. [PubMed] [Google Scholar]
- 3.Schek RM, Wilke EN, Hollister SJ, Krebsbach PH. Combined use of designed scaffolds and adenoviral gene therapy for skeletal tissue engineering. Biomaterials. 2006;27(7):1160–1166. doi: 10.1016/j.biomaterials.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Jeon O, Song SJ, Yang HS, et al. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochemical and Biophysical Research Communications. 2008;369(2):774–780. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 5.Xu XL, Lou J, Tang T, et al. Evaluation of different scaffolds for BMP-2 genetic orthopedic tissue engineering. Journal of Biomedical Materials Research—Part B. 2005;75(2):289–303. doi: 10.1002/jbm.b.30299. [DOI] [PubMed] [Google Scholar]
- 6.Dai KR, Xu XL, Tang TT, et al. Repairing of goat tibial bone defects with BMP-2 gene-modified tissue-engineered bone. Calcified Tissue International. 2005;77(1):55–61. doi: 10.1007/s00223-004-0095-z. [DOI] [PubMed] [Google Scholar]
- 7.Kiyozuka Y, Miyazaki H, Yoshizawa K, et al. An autopsy case of malignant mesothelioma with osseous and cartilaginous differentiation: bone morphogenetic protein-2 in mesothelial cells and its tumor. Digestive Diseases and Sciences. 1999;44(8):1626–1631. doi: 10.1023/a:1026627413715. [DOI] [PubMed] [Google Scholar]
- 8.Bong MR, Capla EL, Egol KA, et al. Osteogenic protein-1 (bone morphogenic protein-7) combined with various adjuncts in the treatment of humeral diaphyseal nonunions. Bulletin. 2005;63(1-2):20–23. [PubMed] [Google Scholar]
- 9.Bilic R, Simic P, Jelic M, et al. Osteogenic protein-1 (BMP-7) accelerates healing of scaphoid non-union with proximal pole sclerosis. International Orthopaedics. 2006;30(2):128–134. doi: 10.1007/s00264-005-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laflamme C, Rouabhia M. Effect of BMP-2 and BMP-7 homodimers and a mixture of BMP-2/BMP-7 homodimers on osteoblast adhesion and growth following culture on a collagen scaffold. Biomedical Materials. 2008;3(1) doi: 10.1088/1748-6041/3/1/015008.015008 [DOI] [PubMed] [Google Scholar]
- 11.Tong ML, Martina M, Hutmacher DW, Hui JHPO, Eng HL, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25(3):750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 12.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends in Biotechnology. 2006;24(4):150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Yao J, Wu L, et al. Osteogenic induction of adipose-derived stromal cells: not a requirement for bone formation in vivo. Artificial Organs. 2010;34(1):46–54. doi: 10.1111/j.1525-1594.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhao Q, Wang E, Zhang C, Wang G, Yuan Q. Transplantation of Cbfa1-overexpressing adipose stem cells together with vascularized periosteal flaps repair segmental bone defects. doi: 10.1016/j.jss.2011.12.011. Journal of Surgical Research. In press. [DOI] [PubMed] [Google Scholar]
- 15.Lin G, Garcia M, Ning H, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells and Development. 2008;17(6):1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang J, Fan CY, Zeng BF. Experimental construction of BMP2 and VEGF gene modified tissue engineering bone in vitro. International Journal of Molecular Sciences. 2011;12(3):1744–1755. doi: 10.3390/ijms12031744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X-Z, Liu G-H, Wang Z-Q, et al. Over-expression of VEGF165 in the adipose tissue-derived stem cells via the lentiviral vector. Chinese Medical Journal. 2011;124(19):3093–3097. [PubMed] [Google Scholar]
- 18.Wang WG, Lou SQ, Ju XD, Xia K, Xia JH. in vitro chondrogenesis of human bone marrow-derived mesenchymal progenitor cells in monolayer culture: activation by transfection with TGF-β2. Tissue and Cell. 2003;35(1):69–77. doi: 10.1016/s0040-8166(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama O, Sung An D, Kung SPK, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Molecular Therapy. 2005;11(3):390–398. doi: 10.1016/j.ymthe.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Hsu WK, Sugiyama O, Park SH, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40(4):931–938. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Mcmahon JM, Conroy S, Lyons M, et al. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells and Development. 2006;15(1):87–96. doi: 10.1089/scd.2006.15.87. [DOI] [PubMed] [Google Scholar]
- 22.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose FRAJ, Oreffo ROC. Bone tissue engineering: hope vs hype. Biochemical and Biophysical Research Communications. 2002;292(1):1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Zhao L, Cui L, Liu W, Cao Y. Tissue-engineered bone formation using human bone marrow stromal cells and novel β-tricalcium phosphate. Biomedical Materials. 2007;2(2, article 04):78–86. doi: 10.1088/1748-6041/2/2/004. [DOI] [PubMed] [Google Scholar]
- 25.Vindigni V, Cortivo R, Iacobellis L, Abatangelo G, Zavan B. Hyaluronan benzyl ester as a scaffold for tissue engineering. International Journal of Molecular Sciences. 2009;10(7):2972–2985. doi: 10.3390/ijms10072972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren Y, Han C, Jia Y, Yin H, Li S. Expression of human bone morphogenetic protein 7 gene in adipose-derived stem cells and its effects on osteogenic phenotype. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25(7):848–853. [PubMed] [Google Scholar]
- 27.Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS, Lee SH. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Engineering—Part A. 2008;14(8):1285–1294. doi: 10.1089/ten.tea.2007.0253. [DOI] [PubMed] [Google Scholar]
- 28.Koh JT, Zhao Z, Wang Z, Lewis IS, Krebsbach PH, Franceschi RT. Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. Journal of Dental Research. 2008;87(9):845–849. doi: 10.1177/154405910808700906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W, Rawlins BA, Boachie-Adjei O, et al. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. Journal of Bone and Mineral Research. 2004;19(12):2021–2032. doi: 10.1359/JBMR.040821. [DOI] [PubMed] [Google Scholar]
- 30.Aono A, Hazama M, Notoya K, et al. Potent ectopic bone-inducing activity of bone morphogenetic protein- 4-7 heterodimer. Biochemical and Biophysical Research Communications. 1995;210(3):670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Zheng Y, Zhao J, et al. Low-dose rhBMP2/7 heterodimer to reconstruct peri-implant bone defects: a micro-CT evaluation. Journal of Clinical Periodontology. 2012;39(1):98–105. doi: 10.1111/j.1600-051X.2011.01807.x. [DOI] [PubMed] [Google Scholar]


