Abstract
Food intolerance in irritable bowel syndrome (IBS) is increasingly being recognized, with patients convinced that diet plays a role in symptom induction. Evidence is building to implicate fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) in the onset of abdominal pain, bloating, wind and altered bowel habit through their fermentation and osmotic effects. Hypersensitivity to normal levels of luminal distension is known to occur in patients with IBS, with consideration of food chemical intolerance likely to answer many questions about this physiological process. This paper summarizes the evidence and application of the most common approaches to managing food intolerance in IBS: the low-FODMAP diet, the elimination diet for food chemical sensitivity and others including possible noncoeliac gluten intolerance.
Keywords: FODMAPs, food chemicals, food intolerance, fructose, gluten, irritable bowel syndrome, salicylates
Introduction
The role of dietary components in inducing gastrointestinal symptoms of abdominal pain, bloating, flatus and altered bowel habit in irritable bowel syndrome (IBS) is difficult to explore. Food intolerances have been considered but issues surrounding diagnostic tools and well designed dietary trials result in questionable outcomes. Historically, patients have identified caffeine, alcohol, fibre and fats as symptom triggers, although strong evidence is conflicting in some and lacking in most [Francis and Whorwell, 1994; Olesen and Gudmand-Hoyer, 2000; Rao et al. 1998; Simren et al. 2001, 2007]. Asking patients to identify which foods contribute to symptoms is fraught with inaccuracies given meals are complex mixtures of dietary components, and the timing of symptom onset following a trigger food can vary.
There is consistently a lack of evidence for food allergy in IBS, with no change in reliable immunological markers following rechallenge of suspect trigger foods [Jones et al. 1982]. Evidence encourages researchers to further investigate the role of food intolerance as a major contributor to IBS symptoms. Foods are not the cause of the condition. Rather, in the presence of IBS, patients can begin to experience symptoms of bloating, abdominal pain and motility changes to specific food components. This is due to the IBS condition, which causes altered microflora, potential small intestinal bacterial overgrowth (SIBO), and visceral hypersensitivity. Food intolerance reactions can be delayed and the severity of symptoms can be dose dependent [Shepherd et al. 2008]. It is much more difficult to pinpoint trigger foods in the case of food intolerance. Many published studies report specific food intolerances according to patient questionnaires [Ballegaard et al. 1997; Niec et al. 1998]. This is unreliable methodology given the mix of foods included in meals and snacks and the likelihood of pinpointing the wrong culprit.
In recent decades, the role of dietary components in inducing IBS symptoms has been explored. There is evidence that certain food components can contribute to symptoms through the effects of malabsorption of carbohydrates [Barrett et al. 2010; Shepherd et al. 2008], and stimulation of hypersensitivity through food chemical ingestion [Niec et al. 1998]. Noncoeliac gluten intolerance also exists [Biesiekierski et al. 2010] and may be important in a subgroup of patients with IBS. This paper summarizes the evidence and application of the most common approaches to managing food intolerance in IBS: the low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diet, the elimination diet for food chemical sensitivity and others including possible noncoeliac gluten intolerance.
The low-FODMAP diet
Throughout the 1980s and 1990s, evidence was building for the role of poorly absorbed, short-chain carbohydrates (lactose, fructose and sorbitol) in the induction of IBS symptoms, with dietary restriction providing symptomatic relief [Goldstein et al. 2000; Nelis et al. 1990; Rumessen and Gudmand-Hoyer, 1988; Symons et al. 1992]. It was clear, however, that these sugars were not the only answer. Examination of the literature and the biochemistry and physiology of digestion of other carbohydrates suggested involvement of fructo-oligosaccharides (fructans) and galacto-oligosaccharides (GOS) because they are also short-chain carbohydrates and are incompletely absorbed in the human gastrointestinal tract. The incompletely absorbed sugar polyols, sorbitol and mannitol, used as artificial sweeteners but also found naturally in foods, were also potential culprits. Grouping of these poorly absorbed, short-chain carbohydrates according to their chain length resulted in the acronym FODMAP.
In 2005, the first paper describing FODMAPs was published [Gibson and Shepherd, 2005]. The first research trial confirming the role of a low-FODMAP diet in managing gastrointestinal complaints was a retrospective audit of patients with IBS and fructose malabsorption on a low-fructose/fructan diet [Shepherd and Gibson, 2006]. A total of 74% of patients reported symptomatic improvement on this dietary regimen. Confirmation of the efficacy of the diet was provided by a follow-up, randomized, placebo-controlled rechallenge trial in patients with IBS with fructose malabsorption [Shepherd et al. 2008]. All patients improved on a low-fructose/fructan diet, with significant exacerbation of symptoms by rechallenge of fructose or fructans, further exacerbated by a combination of fructose and fructans. Placebo response was minimal. Subsequent study of this dietary approach in the UK has shown it superior to a dietary approach previously considered as best practice [Staudacher et al. 2011].
The mechanism by which FODMAPs were exerting their effects was then studied via two separate trials. Using an ileostomy model [Barrett et al. 2010], it was confirmed that FODMAPs, consumed within meals, are poorly absorbed in the small intestine. Interestingly, delivery of FODMAPs to the stoma, correlated with increased water content of the output, suggesting an osmotic effect of the carbohydrates. This may well be the physiological mechanism that induces diarrhoea in some individuals. The second study [Ong et al. 2010] involved assessment of breath hydrogen during low- and high-FODMAP diets in patients with IBS and healthy volunteers. Ingestion of a low-FODMAP diet significantly reduced breath hydrogen production in healthy volunteers and patients with IBS with consequential reduction in gastrointestinal symptom scores in the IBS population. This confirms the fermentative nature of the short-chain carbohydrates and their role in the induction of bloating, distension, abdominal pain and excessive flatus.
These mechanistic insights are consistent with current understanding of the pathophysiological mechanisms that underlie IBS. Visceral hypersensitivity, the most important, renders the enteric nervous system to respond to normal distension of the gut by altering motility patterns and sending messages to the brain that may be interpreted as bloating, discomfort and pain. The low-FODMAP diet reduces fermentation and associated gas production, which is likely to minimize the distension induced by food thereby reducing symptom severity. Other potential factors include alterations in the number, composition, function and location of the microbiota. Some patients with IBS may have SIBO with fermentation of malabsorbed carbohydrates occurring in the narrow lumen of the small intestine, the location of which may be associated with abdominal pain and discomfort. They may have more predominant methane-producing bacteria which, when fermenting malabsorbed carbohydrates producing methane gas, is linked to delayed transit and constipation [Chatterjee et al. 2007; Fiedorek et al. 1990; Pimentel et al. 2003, 2006].
Since these initial studies, more details on food composition have become available to fine tune the FODMAP approach. This includes the consideration of a broader range of FODMAPs, including GOS, sorbitol and mannitol, in addition to fructose, lactose and fructans. These six carbohydrates make up the low-FODMAP diet as it is today, with published tables of food composition available on fruits and vegetables and breads and cereals [Biesiekierski et al. 2011; Muir et al. 2007, 2009]. Table 1 lists a summary of the richest FODMAP food sources compiled from these published food composition papers. Broader food composition has been completed for the use of the Monash University Comprehensive Nutrition Assessment Questionnaire (CNAQ).This Food Frequency Questionnaire has been validated and will be a useful tool for future investigation of FODMAPs and other dietary components in chronic disease and gastrointestinal disorders [Barrett and Gibson, 2010].
Table 1.
FODMAP carbohydrates and their richest food sources.
| FODMAP | Richest food sources |
|---|---|
| Fructo-oligosaccharides (fructans) | Wheat, rye, onions, garlic, artichokes |
| Galacto-oligosaccharides (GOS) | Legumes |
| Lactose | Milk |
| Fructose | Honey, apples, pears, watermelon, mango |
| Sorbitol | Apples, pears, stone fruits, sugar-free mints/gums |
| Mannitol | Mushrooms, cauliflower, sugar-free mints/gums |
Not all FODMAPs will be symptom triggers for all patients. Only those that are malabsorbed are likely to play a role. Importantly, fructans and GOS are always malabsorbed and fermented by intestinal microflora [Macfarlane et al. 2008; Rumessen and Gudmand-Hoyer, 1998; Saunders and Wiggins, 1981]. This results in gas production and associated flatulence in healthy people; however, with altered gut flora, motility disorders and hypersensitivity in IBS, the outcome can induce symptoms [Ong et al. 2010]. The remaining FODMAP carbohydrates will only induce symptoms in the proportion of patients with IBS that malabsorb them. The sugar polyols, sorbitol and mannitol, are incompletely absorbed [Evans et al. 1998; Fernandez-Banares et al. 1991; Langkilde et al. 1994]. The small levels found naturally in foods and in sugar-free products and medications can be well absorbed in most people. Hydrogen breath testing at a dose of 10 g of sorbitol and mannitol in patients with IBS suggests malabsorption in 57% and 20% of patients respectively [Yao et al. 2011]. The prevalence of fructose and lactose in white patients with IBS is 45% and 25% respectively [Barrett et al. 2009]. The prevalence of sorbitol, mannitol, fructose and lactose malabsorption in healthy people is greater than 18% for all sugars [Barrett et al. 2009; Yao et al. 2011]. A lack of symptoms in these people despite malabsorption is again explained by the absence of altered gut microflora and gut hypersensitivity.
Breath testing is a useful addition to the low-FODMAP diet application. In most areas of food intolerance, diagnosis is not possible. Breath testing provides a reliable measure of absorption of a test sugar by assessment of breath hydrogen levels. A significant rise in breath hydrogen following ingestion of the test sugar (e.g. fructose) demonstrates poor absorption with subsequent fermentation by intestinal microflora. In the presence of IBS, this may well contribute to symptoms, with restriction of the sugar useful in the management of gastrointestinal symptoms. Negative breath tests demonstrating complete absorption of the sugar suggests that the patient can continue to consume this sugar without impacting on their symptoms. Routinely, the breath tests that are offered to detect for FODMAP intolerances are fructose, lactose and sorbitol. It is vital to remember that, regardless of breath test results, there are three other FODMAP carbohydrates that need to be considered as potential triggers. Fructans and GOS are not breath tested as they are always malabsorbed. They are always fermented and should be considered as triggers in all patients with IBS. Mannitol is rarely offered as a breath test because it is not found widely in the diet and can be investigated as a trigger through simple dietary elimination and rechallenge.
When breath tests are undertaken, a low-FODMAP diet can be implemented without restricting sugars shown to be well absorbed. This individualizes the diet and avoids unnecessary restrictions. If breath testing is unavailable, a trial of a full low FODMAP diet can be conducted. This is usually recommended for 4–6 weeks, following which, rechallenge of any of the potentially well absorbed carbohydrates can be undertaken, that is, fructose, lactose, sorbitol and mannitol. Tolerance to fructans and GOS can then be tested. In large amounts these carbohydrates will always contribute to gas-associated symptoms. In extreme amounts this may even occur in healthy people [Ong et al. 2010]. However, small amounts of fructans and GOS may be tested to assess the level of tolerance of the patient. This is particularly important for vegetarians (legumes), but may also highlight to a patient that they can cope with garlic as a minor ingredient, or wheat products occasionally. This assists the nutritional composition of the diet longer term, as well as removing some of the social inhibitions that a special diet can endure. In addition, FODMAPs have prebiotic effects due to the production of short-chain fatty acids after fermentation. Therefore, all patients are encouraged to try and reintroduce FODMAPs to a level that they can comfortably tolerate.
The elimination diet
In 1978, the Australian pioneers in this field published a summary article on the application of an elimination diet for the management of chronic urticaria [Gibson and Clancy, 1978]. The basis for the elimination diet is still the same today: following a strict exclusion diet for 2–4 weeks, followed by dietary challenges to determine which of the components are contributing to symptoms. The diet involves restriction of common food allergens (eggs, fish, seafood, nuts, peas, beans), specific chemical substances in foods (naturally occurring or added; salicylates, benzoates, penicillin, yeast and tartrazine) including restrictions on the use of personal hygiene products and medications that contain these chemicals. A minimum of 2 weeks is suggested and up to 12 challenges can be required to determine which of these components are involved. Pin pointing which of the dietary components are involved at presentation is too difficult.
Anecdotally, salicylates are said to be a more common trigger compared with other components of the elimination diet. However, the frequency of salicylate intolerance is reported to be as low as 0.6–2.5% of the population [Togo et al. 2009]. Confusingly, reactions to salicylates can affect different systems and consequently cause different symptoms. Salicylates have been shown to be a trigger in 2–4% of patients attending an allergy clinic and 15–20% of those with chronic rhinitis, nasal polyps, sinusitis and nonallergic asthma [Slepian et al. 1985]. The prevalence of salicylate intolerance in gastrointestinal disorders was investigated in 2005 by oral, single-blind, placebo-controlled challenge: 2% Crohn’s disease, 7% ulcerative colitis, 6% gastrointestinally mediated allergy and 0.6% IBS [Raithel et al. 2005]. This may be an overestimate, considering this was not a consecutive cohort but testing of those with suspected salicylate intolerance.
The varying effects of food chemical intolerance complicate the pathophysiology, however it is theorized that chemicals induce their effects through stimulation of nerve endings in hypersensitive people [Raithel et al. 2005]. Hypersensitivity in IBS is now understood to occur through increased expression of transient receptor potential vanilloid type-1 (TRPV1 or VR1) nerve fibres [Akbar et al. 2008]. Research into this area needs to be continued to assess whether food chemicals stimulate this process and to confirm that exclusion of food chemicals can alleviate symptoms of pain and discomfort. The evidence that exists, specifically for food chemicals and their mechanism of action, includes examination of salicylate-induced asthma, with rechallenge of salicylates shown to activate mast cells which lead to overproduction of cysteinyl leukotrienes [Togo et al. 2009]. These are potent proinflammatory mediators and cause smooth muscle contraction [Worm et al. 2001]. Production of sulphur dioxide and hydrogen sulphide occurs when preservatives such as metabisulphites reach the acid environment of the stomach. This induces nonallergic hypersensitivity reactions in sensitive people [Zopf et al. 2009].
Most of the evidence for the use of the elimination diet is in conditions affecting the nose (rhinitis) [Juhlin, 1981], respiratory tract (asthma) [Slepian et al. 1985], skin (eczema and urticaria) [Juhlin, 1981] and behaviour [attention deficit hyperactivity disorder (ADHD)] [Wender, 1986]. Evidence for the use of the elimination diet and prevalence of food chemical sensitivities in gastrointestinal conditions is scarce.
The elimination diet for food chemicals includes restriction of salicylates (widely found in fruits, vegetables, herbs, spices, nuts, tea, coffee), amines (chocolate, canned/smoked fish, sauces, stock, nuts, seeds, vinegar, and some fruit and vegetables), monosodium glutamate (MSG: found in strong cheeses, soy sauce, and used as a flavour enhancer) as well as preservatives benzoates, propionate, sulphites, nitrites, sorbic acid, plus added antioxidants and colours [Swain et al. 2009]. In addition to these components, the initial exclusion period requires a restriction of common food allergens plus any other dietary components suspected to play a role, for example, wheat, dairy and soy. This complete elimination diet is highly restrictive in its initial phase. It can generally be undertaken for a period of 2–4 weeks, with patients advised to wait for several days once symptom free, before embarking on rechallenges. Placebo-controlled, blinded challenges are undertaken in some clinics and in research, with food challenges being used in practice. Unfortunately, diagnostic tests are unavailable for food chemical sensitivities.
Challenges are undertaken on each food chemical component, by choosing food sources of each chemical and reintroducing them group by group and assessing response. Induction of symptoms suggests involvement of that chemical in symptom onset and level of tolerance can then be tested to assess if small amounts can be included occasionally. The theory behind food chemical intolerance is a build-up effect, when foods can be included below the threshold. With a trigger food consumed once the threshold has been reached, symptoms are induced (see Figure 1).
Figure 1.
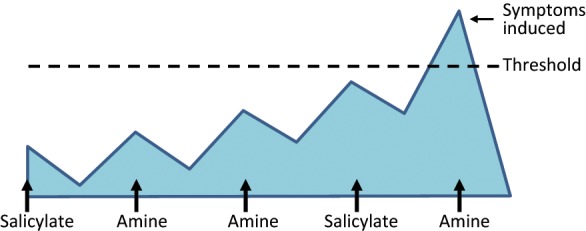
Accumulating effect of food chemical intolerance.
The strict elimination diet needs to be continued during the rechallenge period, with the exception of the challenge foods. With so many rechallenges to undertake (potentially 17 if all chemicals, allergens and other suspect groups are excluded), this can require continuation of a very strict diet for many months. There are no published trials using this approach in IBS management in a controlled manner, possibly due to the intensive dietary requirements which must impact on compliance.
Other considerations
Gluten, as a trigger for gastrointestinal symptoms, is well known and understood in the setting of coeliac disease. More and more frequently gluten restriction is being implemented by the general public for numerous reasons, including the management of IBS, ADHD, chronic fatigue syndrome and others. A gluten-free diet is often advised by alternative health practitioners, despite the lack of evidence for its use in any condition apart from coeliac disease. Many patients with IBS will report improvements in symptoms with a gluten-free diet, but until recently, this had not been investigated. Selecting and studying patients with IBS who feel better on a gluten-free diet is difficult because many have not been formally investigated for coeliac disease prior to implementation of the diet. This is concerning as detection of coeliac disease abnormalities is impaired by adoption of a gluten-free diet. Restriction of gluten prior to adequate medical investigations including blood tests and gastroduodenoscopy leads to the need for a 4–6-week gluten challenge, which can be distressing for patients who feel better on a gluten-free diet. It is vital that practitioners understand that formal coeliac investigations must be performed before implementation of a gluten-restricted diet.
Biesiekierski and colleagues were the first to publish a randomized, controlled trial to confirm the existence of noncoeliac gluten intolerance [Biesiekierski et al. 2010]. A total of 34 patients with IBS, whose symptoms were well controlled on a gluten-free diet, were challenged to bread and muffins containing gluten or placebo. A total of 68% of the patients had exacerbation of symptoms within 1 week of gluten challenge compared with 40% receiving placebo. This suggests the existence of gluten intolerance in IBS, but no mechanism was found. Further dietary trials are required to replicate these findings and uncover the physiology behind gluten intolerance in this patient group.
Coffee is commonly reported as a trigger for patients with IBS. The difficulty lies with identifying the component of coffee that is involved (salicylates versus caffeine), or if these patients react to milk, if this is taken with it. Certainly, coffee is confirmed to be a colonic stimulant that may well be involved in altered gastrointestinal motility [Rao et al. 1998]. Dietary fats have also been implicated due to their ability to increase colonic hypersensitivity [Simren et al. 2007].
Application of these dietary strategies in the clinical setting
Implementation of the food chemical elimination diet and the low-FODMAP diet requires close dietetic supervision. The elimination diet removes nutritionally important components, particularly during the initial baseline dietary phase. This can also occur during the low-FODMAP diet, if alternative food choices are not taken up. Vitamin and mineral supplementation may be required throughout the baseline and rechallenge phases, particularly during the elimination diet. Patients following these approaches without dietetic support are more likely to be noncompliant, resulting in insufficient improvement, or they may continue on an overly restricted approach unnecessarily. Educating patients on either approach requires the dietitian to have detailed knowledge in each area, understanding food composition and rechallenge processes. These are highly specialized areas of nutrition counselling.
Considering the differences between the two approaches, predicting which diet will provide improvement on a case-by-case basis, is important. Unfortunately, without clinical trials investigating the benefits of the elimination diet in an IBS population, it is impossible to compare the effectiveness of the two approaches. There is an urgent need for a randomized, crossover intervention study to compare the effectiveness of the diets and to ascertain whether response can be predicted based on baseline symptom profile or medical history. If baseline assessment can be directed towards predicting which diet will be most effective, it will be of great benefit to the practitioner, dietitian and the patient. For example, there is a suggestion that patients with IBS and a history of allergies or with other conditions suggestive of food chemical sensitivities – that is, asthma, eczema, rhinitis – may respond better to the elimination diet, although this has never been formally studied.
The different physiological mechanisms behind each of the strategies discussed suggests that combining approaches may be helpful, albeit complicated, in many patients. Patients experiencing symptoms of bloating, excessive flatulence, pain or diarrhoea are likely to benefit from a restriction of FODMAPs, known to produce large amounts of gas and to exert a natural laxative effect. If these symptoms continue despite significant restriction of FODMAPs and other simple changes (e.g. caffeine restriction), it suggests hypersensitivity to normal levels of luminal content, with the elimination diet and restriction of dietary fats likely to benefit.
Conclusion
There is emerging evidence for the role of food intolerance in the management of IBS symptoms. This does not present a cure, rather suggested dietary modifications to improve symptoms and quality of life. The greatest body of evidence is for the low-FODMAP diet, which improves symptoms in at least 74% of patients with IBS. There is potential for a low food chemical diet to improve IBS symptoms by impacting on the level of hypersensitivity to luminal distension, but further work is needed. Elimination of food chemicals is more difficult to introduce, more challenging for the dietitian and patient alike, and is largely based on hypothetical constructs rather than high-level evidence. For these reasons, elimination diet techniques should remain a second-line dietary therapy to be instituted only by those trained in the techniques. Noncoeliac gluten intolerance appears to exist, but further research is needed to confirm this and to examine the mechanism of action. Caffeine and fats may well play a role in inducing IBS symptoms in some patients, a reduction of which can be trialled to assess response. The considerable evidence supporting the low-FODMAP diet for IBS, and the fact that it is relatively easy to implement without significant nutritional concerns, supports the suggestion that this should be the first dietary manipulation trialled in patients presenting with IBS.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest statement: PG has registered the term ‘FODMAP’ as a trademark.
Contributor Information
Jacqueline S. Barrett, Eastern Health Clinical School, Monash University, Level 2, 5 Arnold Street, Box Hill, Victoria 3128, Australia
Peter R. Gibson, Eastern Health Clinical School, Monash University, Box Hill, Victoria, Australia
References
- Akbar A., Yiangou Y., Facer P., Walters J.R.F., Anand P., Ghosh S. (2008) Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57: 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballegaard M., Bjergstrom A., Brondum S., Hylander E., Jensen L., Ladefoged K. (1997) Self-reported food intolerance in chronic inflammatory bowel disease. Scand J Gastroenterol 32: 569–571 [DOI] [PubMed] [Google Scholar]
- Barrett J.S., Gearry R.B., Muir J.G., Irving P.M., Rose R., Rosella O., et al. (2010) Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther 31: 874–882 [DOI] [PubMed] [Google Scholar]
- Barrett J.S., Gibson P.R. (2010) Development and validation of a comprehensive semi-quantitative food frequency questionnaire that includes FODMAP intake and glycemic index. J Am Diet Assoc 110: 1469–1476 [DOI] [PubMed] [Google Scholar]
- Barrett J.S., Irving P.M., Shepherd S.J., Muir J.G., Gibson P.R. (2009) Prevalence of fructose and lactose malabsorption in patients with gastrointestinal disorders. Aliment Pharmacol Therapeut 30: 165–174 [DOI] [PubMed] [Google Scholar]
- Biesiekierski J.R., Newnham E.D., Irving P.M., Barrett J.S., Haines M., Doecke J.D., et al. (2010) Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. AmJ Gastroenterol 106: 508–514; quiz 515 [DOI] [PubMed] [Google Scholar]
- Biesiekierski J.R., Rosella O., Rose R., Liels K., Barrett J.S., Shepherd S.J., et al. (2011) Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet 24: 154–176 [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Park S., Low K., Kong Y., Pimentel M. (2007) The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol 102: 837–841 [DOI] [PubMed] [Google Scholar]
- Evans P.R., Piesse C., Bak Y.T., Kellow J.E. (1998) Fructose-sorbitol malabsorption and symptom provocation in irritable bowel syndrome: relationship to enteric hypersensitivity and dysmotility. Scand J Gastroenterol 33: 1158–1163 [DOI] [PubMed] [Google Scholar]
- Fernandez-Banares F., Esteve-Pardo M., Humbert P., de Leon R., Llovet J.M., Gassull M.A. (1991) Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology 101: 1453–1454 [DOI] [PubMed] [Google Scholar]
- Fiedorek S.C., Pumphrey C.L., Casteel H.B. (1990) Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr 10: 473–477 [DOI] [PubMed] [Google Scholar]
- Francis C.Y., Whorwell P.J. (1994) Bran and irritable bowel syndrome: time for reappraisal. Lancet 344: 39–40 [DOI] [PubMed] [Google Scholar]
- Gibson A.R., Clancy R.L. (1978) An Australian exclusion diet. Med J Aust 1: 290–292 [DOI] [PubMed] [Google Scholar]
- Gibson P.R., Shepherd S.J. (2005) Personal view: food for thought – western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther 21: 1399–1409 [DOI] [PubMed] [Google Scholar]
- Goldstein R., Braverman D., Stankiewicz H. (2000) Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. Isr Med Assoc J 2: 583–587 [PubMed] [Google Scholar]
- Jones V.A., McLaughlan P., Shorthouse M., Workman E., Hunter J.O. (1982) Food intolerance: a major factor in the pathogenesis of irritable bowel syndrome. Lancet 2: 1115–1117 [DOI] [PubMed] [Google Scholar]
- Juhlin L. (1981) Recurrent urticaria: clinical investigation of 330 patients. Br J Dermatol 104: 369–381 [DOI] [PubMed] [Google Scholar]
- Langkilde A., Andersson H., Schweizer T., Wursch P., Langkilde A., Andersson H., et al. (1994) Digestion and absorption of sorbitol, maltitol and isomalt from the small bowel. A study in ileostomy subjects. EurJ Clin Nutr 48: 768–775 [PubMed] [Google Scholar]
- Macfarlane G., Steed H., Macfarlane S. (2008) Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol 104: 305–344 [DOI] [PubMed] [Google Scholar]
- Muir J., Rose R., Rosella O., Liels K., Barrett J., Shepherd S., et al. (2009) Measurement of short chain carbohydrates (FODMAPs) in common Australian vegetables and fruit by high performance liquid chromatography. J Agric Food Chem 57: 554–565 [DOI] [PubMed] [Google Scholar]
- Muir J.G., Shepherd S.J., Rosella O., Rose R., Barrett J.S., Gibson P.R. (2007) Fructan and free fructose content of common Australian vegetables and fruit. J Agric Food Chem 55: 6619–6627 [DOI] [PubMed] [Google Scholar]
- Nelis G.F., Vermeeren M.A., Jansen W. (1990) Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology 99: 1016–1020 [DOI] [PubMed] [Google Scholar]
- Niec A.M., Frankum B., Talley N.J. (1998) Are adverse food reactions linked to irritable bowel syndrome? Am J Gastroenterol 93: 2184–2190 [DOI] [PubMed] [Google Scholar]
- Olesen M., Gudmand-Hoyer E. (2000) Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am J Clin Nutr 72: 1570–1575 [DOI] [PubMed] [Google Scholar]
- Ong D.K., Mitchell S.B., Barrett J.S., Shepherd S.J., Irving P.M., Biesiekierski J.R., et al. (2010) Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol 25: 1366–1373 [DOI] [PubMed] [Google Scholar]
- Pimentel M., Lin H.C., Enayati P., van den Burg B., Lee H.R., Chen J.H., et al. (2006) Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol 290: G1089–G1095 [DOI] [PubMed] [Google Scholar]
- Pimentel M., Mayer A.G., Park S., Chow E.J., Hasan A., Kong Y. (2003) Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci 48: 86–92 [DOI] [PubMed] [Google Scholar]
- Raithel M., Baenkler H.W., Naegel A., Buchwald F., Schultis H.W., Backhaus B., et al. (2005) Significance of salicylate intolerance in diseases of the lower gastrointestinal tract. J Physiol Pharmacol 56 (Suppl. 5): 89–102 [PubMed] [Google Scholar]
- Rao S.S., Welcher K., Zimmerman B., Stumbo P. (1998) Is coffee a colonic stimulant? Eur J Gastroenterol Hepatol 10: 113–118 [DOI] [PubMed] [Google Scholar]
- Rumessen J.J., Gudmand-Hoyer E. (1988) Functional bowel disease: malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology 95: 694–700 [DOI] [PubMed] [Google Scholar]
- Rumessen J.J., Gudmand-Hoyer E. (1998) Fructans of chicory: intestinal transport and fermentation of different chain lengths and relation to fructose and sorbitol malabsorption. Am J Clin Nutr 68: 357–364 [DOI] [PubMed] [Google Scholar]
- Saunders D.R., Wiggins H.S. (1981) Conservation of mannitol, lactulose, and raffinose by the human colon. Am J Physiol 241: G397–G402 [DOI] [PubMed] [Google Scholar]
- Shepherd S.J., Gibson P.R. (2006) Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc 106: 1631–1639 [DOI] [PubMed] [Google Scholar]
- Shepherd S.J., Parker S.C., Muir J.G., Gibson P.R. (2008) Randomised, placebo-controlled evidence of dietary triggers for abdominal symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 765–771 [DOI] [PubMed] [Google Scholar]
- Simren M., Abrahamsson H., Bjornsson E.S. (2007) Lipid-induced colonic hypersensitivity in the irritable bowel syndrome: the role of bowel habit, sex, and psychologic factors. Clin Gastroenterol Hepatol 5: 201–208 [DOI] [PubMed] [Google Scholar]
- Simren M., Mansson A., Langkilde A.M., Svedlund J., Abrahamsson H., Bengtsson U., et al. (2001) Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 63: 108–115 [DOI] [PubMed] [Google Scholar]
- Slepian I.K., Mathews K.P., McLean J.A. (1985) Aspirin-sensitive asthma. Chest 87: 386–391 [DOI] [PubMed] [Google Scholar]
- Staudacher H.M., Whelan K., Irving P.M., Lomer M.C.E. (2011) Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet 24: 487–495 [DOI] [PubMed] [Google Scholar]
- Symons P., Jones M.P., Kellow J.E. (1992) Symptom provocation in irritable bowel syndrome. Effects of differing doses of fructose-sorbitol. Scand J Gastroenterol 27: 940–944 [DOI] [PubMed] [Google Scholar]
- Swain A., Soutter V., Loblay R. (2009) RPAH Elimination Diet Handbook. Sydney [Google Scholar]
- Togo K., Suzuki Y., Yoshimaru T., Inoue T., Terui T., Ochiai T., et al. (2009) Aspirin and salicylates modulate IgE-mediated leukotriene secretion in mast cells through a dihydropyridine receptor-mediated Ca(2+) influx. Clinical Immunology 131: 145–156 [DOI] [PubMed] [Google Scholar]
- Wender E.H. (1986) The food additive-free diet in the treatment of behavior disorders: a review. J Dev Behav Pediatr 7: 35–42 [DOI] [PubMed] [Google Scholar]
- Worm M., Vieth W., Ehlers I., Sterry W., Zuberbier T. (2001) Increased leukotriene production by food additives in patients with atopic dermatitis and proven food intolerance. Clin Exp Allergy 31: 265–273 [DOI] [PubMed] [Google Scholar]
- Yao C.K., Tan H.L., van Langenberg D.R., Barrett J.S., Gibson P.R., Muir J.G. (2011) Discordant absorption of sorbitol and mannitol in patients with irritable bowel syndrome. Am J Gastroenterol submitted [DOI] [PubMed] [Google Scholar]
- Zopf Y., Baenkler H.W., Silbermann A., Hahn E.G., Raithel M. (2009) The differential diagnosis of food intolerance. Dtsch Arztebl Int 106: 359–369; quiz 369–370 [DOI] [PMC free article] [PubMed] [Google Scholar]


