Abstract
The wireless motility capsule (WMC) is an ambulatory noninvasive and nonradioactive diagnostic sensor that continuously samples intraluminal pH, temperature, and pressure as it moves through the gastrointestinal (GI) tract. This review summarizes the data obtained in clinical trials with the WMC and discusses its role in clinical practice. The United States Food and Drug Administration has approved the SmartPill GI monitoring system for the evaluation of gastric emptying time in patients with suspected gastroparesis, the evaluation of colonic transit time in patients with suspected chronic constipation, and for the characterization of pressure profiles from the antrum and duodenum. Clinical studies have shown that WMC-measured GI transit times can distinguish patients with motility abnormalities similarly to conventional testing. However, the WMC offers the advantage of providing a full GI-tract profile, enabling the detection of multiregional GI transit abnormalities in patients with suspected upper or lower GI dysmotility. The WMC also characterizes pressure profiles of the GI tract and impaired pressure profile limits are reported for the antrum and duodenum. In comparison with manometry, interpretations of pressure measurements obtained by the WMC are limited by an inability to detect a peristaltic pressure wave front, and further investigation is required to develop clinical applications. Clinical studies with the WMC indicated that it should be considered for the evaluation of regional and whole gut transit time in patients with suspected upper or lower dysmotility, particularly if there are concerns about multiregional dysmotility.
Keywords: constipation, diffuse gastrointestinal dysmotility, gastroparesis, transit time, wireless motility capsule
Introduction
Functional gastrointestinal (GI) disorders such as functional dyspepsia, chronic constipation, and irritable bowel syndrome are multifactorial in etiology and can include an association with visceral hypersensitivity or dysmotility of the GI tract. After evaluating for alarm conditions and failure of empiric therapy, motility testing is often recommended, which has traditionally been focused on the specific region of the GI tract consistent with the chief complaint [Abell et al. 2008; Rao, 2009; Sarosiek et al. 2010]. However, studies show a significant overlap between functional motility disorders of the upper and lower GI tract, for example, patients with slow transit constipation were found to have delayed gastric emptying and abnormal functioning of the esophageal body and sphincters [Hasler, 2007; Agrawal et al. 2009]. A comprehensive assessment of upper and lower GI motility can provide critical objective data to aid in diagnosis and the planning of optimal therapeutic and feeding strategies.
The wireless motility capsule (WMC) provides a method of measuring regional and whole gut transit time in a single standardized ambulatory test without radiation. Sensors housed within the WMC continuously measure pH, pressure, and temperature for up to 5 days after ingestion as the WMC moves through the GI tract to provide transit and pressure information. Current methods for the assessment of GI tract function routinely involve radiation, are mostly limited to one GI tract region assessment, require multiple tests, and are often not available in a standardized protocol.
The American and European Neurogastroenterology and Motility Societies have recommended WMC testing in the assessment of: (a) gastric emptying and regional and whole gut transit time in individuals with suspected gastroparesis, symptoms of upper GI dysmotility, or suspected alterations of GI motility in multiple regions; (b) small bowel transit time (SBTT) and for clinical use in facilitating detection of small bowel dysfunction in patients with generalized motility disorders; (c) assessment of colonic transit time (CTT) in subjects with symptoms of chronic constipation while providing measurements of regional and whole gut transit [Rao et al. 2011]. The United States Food and Drug Administration (FDA) approved the SmartPill GI Monitoring System for: (a) the evaluation of gastric emptying time in patients with suspected gastroparesis; (b) the evaluation of CTT in patients with suspected slow transit constipation; (c) measurement of pH, temperature, and pressure throughout the GI tract with characterization of pressure profiles from the antrum and duodenum [FDA, 2009]. The aim of this review is to summarize objective data accumulated from WMC clinical trials, and to discuss the role of WMC testing in clinical GI practice.
Description of the test
Description of the WMC
The WMC system (SmartPill Corporation, Buffalo, NY, USA) consists of an ingestible single-use capsule, a receiver, and display software. Both the capsule and receiver have a battery life rated for 5 days of use once activated. The indigestible capsule measures 26 mm × 13 mm and houses sensors for pH, temperature, and pressure. The pH measurement is accurate to 0.5 pH units and pressure measurement is accurate to +/- 5 mmHg below 100 mmHg [SmartPill Corporation, 2009].
Wireless motility capsule test
The test starts with the ingestion of a meal to initiate the postprandial motility pattern following an overnight fast. The meal consists of a standardized egg sandwich (255 kcal, 2% fat, 1 g fiber), or the nutritionally equivalent SmartBar (260 kcal, 2% fat, 2 g fiber), followed by 120 ml water [Kuo et al. 2008]. Immediately after the meal, the patients swallow the WMC with 50 ml water. Patients are then released from the clinical setting once absence of any complications from the ingestion is confirmed. They are given the data receiver and a diary for recording bowel movements, food intake, sleep, and GI symptoms. Physical restrictions include no strenuous activities such as sit-ups, abdominal crunches, and prolonged aerobic activity (> 15 min), which can affect pressure measurements. Additionally, patients refrain from alcohol, smoking, and the use of GI medications that could affect motility.
Patients are asked to fast for 6 h after capsule ingestion, after which they ingest a 250 ml Ensure® meal (250 kcal, protein 9 g, carbohydrates 40 g, fat 6 g, fiber 0 g) (Abbott Laboratories, Abbott Park, Il, USA). This meal allows for the evaluation of the fed response, which is the change in contractile pattern of the small bowel from a fasting to postprandial pattern. On WMC tracings, the fed response manifests as an increase in contraction frequency and/or average amplitude [Brun et al. 2010]. Patients are then instructed to resume their regular diet and routine 2 h after ingestion of the Ensure meal, and to return the data receiver and diary to the medical facility after 5 days. Downloaded data are analyzed using the display software (MotiliGI, SmartPill Corporation).
Motility measurements by WMC
Transit time/anatomical landmarks
pH, temperature, and pressure are used to define anatomic landmarks for measuring regional gut transit time. Capsule ingestion is marked by an abrupt rise in the temperature profile. Gastric emptying time (GET) is defined as the time from ingestion of the WMC to the abrupt pH rise (> 3 pH units) from gastric baseline to a pH > 4, marking the passage of the capsule from the acidic antrum to the alkaline duodenum [Mojaverian et al. 1985, 1989; Evans et al. 1988; Miner et al. 2003]. The ileocecal junction (ICJ) is defined as the abrupt pH drop of at least 1 pH unit, observed at least 30 min after GET and persisting for a minimum of 10 min [Zarate et al. 2010; Evans et al. 1988]. Body exit time is usually seen as a significant temperature drop when the ambient environmental temperature is sensed rather than body temperature (Figure 1). Compared with conventional GI transit time tests, the WMC has the advantage of being ambulatory, noninvasive, standardized, more widely available, and with less radiation exposure.
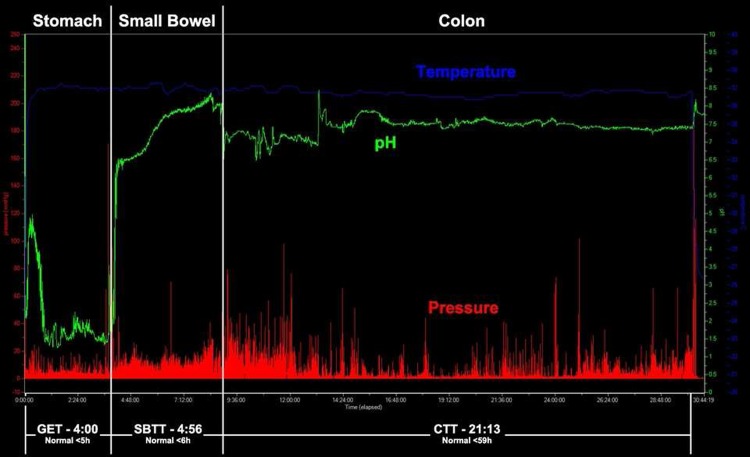
Figure 1.
Normal gastrointestinal motility tracing using the wireless motility capsule, with a normal GET, SBTT, and CTT. Transit times are in hh:mm. CTT, colonic transit time; GET, gastric emptying time; SBTT, small bowel transit time.
Pressure
The WMC also measures intraluminal pressure, functioning as a free-floating single pressure sensor device that records and transmits the amplitude and frequency of contractions to produce a standardized pressure profile characterization. The pressure data provide the following contractility measurements: frequency of contractions (Ct), amplitude of contractions, presented as an absolute value or area under the curve (AUC), and motility index (MI), which is calculated as MI = Ln(sum of amplitudes × number of contractions + 1). Normal contractility reference values have been published for the stomach and proximal small bowel [Kloetzer et al. 2010]. The WMC cannot detect peristaltic wave propagation and this remains a major limitation until new standards are developed to aid in the identification of various motility patterns.
Stomach
Transit times
Gastric emptying scintigraphy (GES) is the current most commonly used method for measuring stomach transit time due to its ease of quantification and the use of physiologic test meals. The test is widely available and directly measures the emptying of a physiological meal, but is limited by variability in methodology, particularly the length of the study and meal type. In addition, adoption of consensus guidelines remains limited primarily to tertiary centers [Abell et al. 2008]. It does represent the most physiologic method for measuring the gastric emptying of a meal. Gastric emptying breath testing is another viable option, but is not available for clinical use in the USA.
The WMC offers an alternative method to measure gastric emptying, albeit providing an indirect rather than direct measure of meal emptying due to the indigestible nature of WMC. The relationship of WMC emptying to meal emptying is based on a predictable sequence of physiologic motor events that include meal trituration and emptying followed by gastric housekeeping activities to remove indigestible meal remnants and objects such as the WMC [Guyton and Hall, 2010].
The relationship of the emptying of a meal to the emptying of WMC was studied by Kuo and colleagues who reported a strong correlation of r = 0.73 between the two events [Kuo et al. 2008]. Cassilly and colleagues studied the sequence and mechanism of emptying WMC compared with a meal from the stomach by recording simultaneously WMC, GES, and antroduodenal manometry (ADM) in healthy volunteers. On average, the WMC emptied from the stomach after 96.6% of the meal moved postpyloric. The authors reported a strong correlation of the WMC gastric residence time to the duration of a fed pattern on manometry (r = 0.813; p < 0.01) [Cassilly et al. 2008]. Further investigation with simultaneous WMC testing and ADM in diseased patients such as those with gastroparesis would be helpful in further clarifying the utility of the WMC in the diseased state.
The diagnostic utility of WMC compared with GES to distinguish healthy from gastroparetic patients was reported by Kuo and colleagues. Simultaneous comparisons were made between WMC GET and GES using the Pearson correlation coefficient as a measure of agreement in a study comparing 87 healthy patients and 61 gastroparetics. The gastroparetics were defined by both a history of typical gastroparesis symptoms and a documented delayed scintigraphy test within the past 2 years. Receiver operator characteristic curve profiles for the two measures were equivalent (AUC(GET) = 0.83 versus AUC(GES) = 0.82) and, as noted above, a strong correlation of WMC GET to GES at 4 h was observed (Table 1) [Kuo et al. 2008].
Table 1.
Correlation of gastric emptying scintigraphy at 2 h and 4 h and gastric emptying time together with sensitivity and specificity values (n = 125).
| Gastric emptying parameter | SP–GET correlation (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
|---|---|---|---|---|
| GES 2 h | 0.63 (0.50–0.75) | 0.34 | 0.93 | 0.79 (0.71–0.88) |
| GES 4 h | 0.73 (0.61–0.82) | 0.44 | 0.93 | 0.82 (0.77–0.91) |
| GET | NA | 0.65 | 0.87 | 0.83 (0.74–0.90) |
Adapted from Kuo et al. [2008]. AUC, area under the curve; CI, confidence interval; GES, gastric emptying scintigraphy; GET, gastric emptying time; NA, not applicable; SP, Smart Pill.
Of note, 65% of the symptomatic subjects demonstrated a delay by GET while 44% demonstrated a delay by GES. The Tougas 4 h cutoff, defined as gastric emptying < 90% of the meal emptied at 4 h, was used for scintigraphy. This cutoff is defined by the 95th percentile in a healthy subject cohort [Tougas et al. 2000]. The WMC used a cutoff point of 5 h to mark delayed gastric emptying based on an analysis of the dataset from Kuo and colleagues providing values for sensitivity and specificity of 0.65 and 0.87, respectively [Kuo et al. 2008]. The increased detection of delayed emptying by WMC may be due to the fact that WMC GET reflects the function of the fasted state in addition to the fed state, whereas gastric scintigraphy typically measures fed state emptying only. It is worth noting that some normal transit results observed in gastroparetics with GES may be due to variability in gastric emptying with time, especially in idiopathic gastroparesis.
Pressure
ADM is the current standard in measuring gastric and small intestinal pressure and contractility. An ADM device has six or more pressure transducers spaced at fixed locations along a catheter. This allows for simultaneous recording of pressure waves at multiple sites and detection of peristaltic wave propagation. Its utility is limited by the invasive nature of the test, the expertise required to perform and interpret the test, and its lack of availability in most clinical settings.
The WMC provides a standardized gastric pressure-profile characterization, though the clinical utility requires further refinement. Kloetzer and colleagues used the WMC to compare antroduodenal pressure profiles, using Ct, AUC, and MI, between 71 healthy and 42 gastroparetic subjects (Table 2) [Kloetzer et al. 2010]. Overall, gastroparetics had a 35% reduction in Ct in both the gastric and small bowel windows compared with healthy subjects. In diabetic subjects with gastroparesis, the Ct was reduced by 50%. Furthermore, 33% of the gastroparetic subjects had gastric motor function below the fifth percentile of the normal population. This prevalence increased to 73% for subjects with a very delayed GET > 12 h [Kloetzer et al. 2010]. One hypothesis is that significantly lower pressure parameters in patients with severely delayed GET may be an indicator of an inability to generate effective high amplitude contractions/phase III migrating motor complexes to achieve the emptying of the indigestible capsule.
Table 2.
Median values of motility parameters for healthy subjects versus gastroparetic idiopathic subjects and gastroparetic diabetic subjects.
| Gastric window |
Small bowel window |
|||||
|---|---|---|---|---|---|---|
| Ct/h | AUC (mmHg/s) | MI | Ct/h | AUC (mmHg/s) | MI | |
| Normal subjects n = 71 | 72 | 4789 | 11.83 | 145 | 5182 | 12.78 |
| Idiopathic gastroparetics | 48 | 4048 | 11.46 | 109 | 4763 | 12.31 |
| n = 26 | p = 0.07 | p = 0.23 | p = 0.09 | p = 0.14 | p = 0.88 | p = 0.21 |
| Diabetic gastroparetics | 37 | 3032 | 10.84 | 62 | 4325 | 11.6 |
| n = 16 | p = 0.04 | p = 0.26 | p = 0.03 | p = 0.02 | p = 0.55 | p = 0.04 |
Adapted from Kloetzer et al. [2010]. AUC, area under the curve; Ct, frequency of contractions; MI, motility index.
pH
The widespread use of acid suppression may affect the ability of the capsule to discriminate between stomach and small bowel pH. The study by Michalek and colleagues comparing 20 healthy volunteers on high dose proton pump inhibitor (PPI) treatment (esomeprazole 40 mg twice a day) with 50 healthy volunteers not on acid suppression demonstrated that while patients on PPI did have a reduced magnitude of pH change at GET, an abrupt pH increase of > 0.5 pH units remains clear with sustained elevation of pH into the small bowel [Michalek et al. 2011]. They also noted that the frequency of gastric contractions measured by the WMC during the hour prior to gastric emptying is doubled regardless of acid suppression therapy. This doubling in contraction frequency, the persistence of the pH increase as the WMC moves into the small bowel, and the lack of temperature and pH change in response to further meal ingestion serve as additional markers in determining GET in those who need to remain on acid suppression therapy or have unclear pH data. Additional research is required to evaluate the utility of the WMC in other hypoacidic states such as achlorhydria.
Small bowel
Transit time
There are several tests available to study SBTT. A small bowel series provides some indirect information regarding transit time, typically in cases of severely abnormal transit such as ileus. However, the technique remains limited by nonphysiologic conditions during the test (i.e. posture, fasting), and the absence of published limits of normality. Scintigraphy has also been used to evaluate SBTT, typically as a component of whole gut scintigraphy with some exposure to radiation. Other techniques such as breath testing are not widely adopted or standardized.
The WMC can identify SBTT with its ability to define gastric emptying and ileocecal transit based on changes in pH profile [Michalek et al. 2011; Zarate et al. 2010]. SBTT is marked by the capsule entering the duodenum from the antrum until the WMC passes through the ICJ. A study by Maqbool and colleagues compared SBTT values obtained from whole gut scintigraphy with WMC SBTT in 10 healthy individuals and found significant correlation (r = 0.69; p = 0.05) [Maqbool et al. 2009].
The mean SBTT of WMC is 4.1 h based on testing in 87 healthy volunteers. The normal limits for SBTT defined by the 5th and 95th percentile are 2.5–6 h [Brun et al. 2011a]. The same report also retrospectively reviewed WMC data from 77 patients with suspected GI dysmotility at a single center over a 3-year period. They found that 29 (37.6%) patients presenting with symptoms of upper or lower motility disorders had a slow SBTT compared with controls with respective means of 447 min versus 301 min (p < 0.001) [Brun et al. 2011a]. The clinical significance of SBTT and its correlation with symptoms requires further investigation.
Pressure
Unlike SBTT, evaluation of small bowel contractility by ADM is more established. ADM is clinically useful, particularly in the assessment of gastroparesis and differentiating between myopathy and neuropathy, but as discussed earlier has limitations.
The clinical utility of WMC to differentiate myopathic from neuropathic small bowel disorders is now only being explored. Urma and colleagues first reported the observation of a fed response in the small bowel pressure profile in healthy subjects [Urma et al. 2005]. Brun and colleagues recently noted this fed response was delayed in patients with gastroparesis, and blunted and shorter in patients with constipation [Brun et al. 2010]. These findings may suggest an underlying neuropathy contributing to postprandial symptoms.
Preliminary data also showed that patients found to have delayed SBTT also had significantly lower proximal small bowel pressure parameters of mean contractions (p = 0.003), mean AUC (p = 0.025), and MI (p < 0.001), compared with healthy individuals [Brun et al. 2011a]. Additional investigation is needed to determine the clinical significance of these findings.
Colon
Transit time
Evaluating CTT is key in the evaluation of chronic constipation. Methods include whole gut scintigraphy, which has the advantage of being able to assess segmental transit time, but is hindered by its limited availability, expense, and use of radiation [Lundin et al. 2007]. Radiopaque marker (ROM) tests are the most widely used to evaluate CTT, but inherent limitations include the need for serial X-rays, multiple visits, radiation exposure, and lack of standardization [Rao, 2005; Rao et al. 2009].
The WMC has been demonstrated to be comparable to ROM in the evaluation of CTT [Camilleri et al. 2010]. In a study of 78 constipated and 87 healthy subjects, the simplified or Hinton ROM test and the WMC were simultaneously administered to compare the sensitivity, specificity, and AUC or receiver operating characteristic curve of each technique. Constipated subjects had a significantly slower day 2 and day 5 ROM and WMC transits than the controls (p < 0.001) with a good correlation of CTT of the WMC to the number of ROMs remaining (Table 3). Furthermore, the WMC had comparable specificity (0.95) and sensitivity (0.46) to ROM for identifying abnormal transit time in patients with constipation symptoms (Table 4) [Rao et al. 2009].
Table 3.
Correlation of colonic transit time and whole gut transit time as measured by the SmartPill with the number of retained ROMs.
| SmartPill parameter | Overall group day 2 ROM (95% CI) | Day 2 ROMs in healthy subjects | Day 2 ROMs in constipated subjects | Overall group day 5 ROM (95% CI) | Day 5 ROMs in healthy subjects | Day 5 ROMs in constipated subjects |
|---|---|---|---|---|---|---|
| Colonic transit time | 0.78 (0.70–0.84) | 0.70 | 0.74 | 0.59 (0.46–0.69) | 0.40 | 0.69 |
| Whole gut transit time | 0.77 (0.68–0.84) | 0.74 | 0.67 | 0.58 (0.45–0.69) | 0.39 | 0.66 |
Adapted from Rao et al. [2009]. CI, confidence interval; ROM, radiopaque marker.
Table 4.
Area under the curve of the receiver operating characteristics curve, sensitivity and specificity for the SmartPill colonic transit time and whole gut transit time, and day 5 radiopaque markers.
| Parameter | Area under the curve (95% CI) | Cutoff value | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Colonic transit time | 0.73 (0.65–0.82) | All subjects | 59 h | 0.46 | 0.95 |
| Men | 44 h | 0.50 | 0.90 | ||
| Women | 59 h | 0.46 | 0.92 | ||
| Whole gut transit time | 0.76 (0.68–0.84) | All subjects | 73 h | 0.42 | 0.95 |
| Men | 52 h | 0.63 | 0.90 | ||
| Women | 73 h | 0.41 | 0.92 | ||
| Day 5 radiopaque marker | 0.71 (0.63–0.78) | All subjects | >5 markers | 0.37 | 0.95 |
Adapted from Rao et al. [2009]. CI, confidence interval.
The upper limit of normal colonic transit defined in the Rao study [Rao et al. 2009] above (95th percentile of the normal population) was validated by Camilleri and colleagues in their prospective study comparing CTT by WMC and ROM, using the procedure described by Metcalf and colleagues in 157 chronically constipated patients [Camilleri et al. 2010; Metcalf et al. 1987]. For delayed transit, the positive percentage agreement between WMC and ROM was 79.6% (95% confidence interval (CI) = 0.67–0.98). The negative percentage agreement, representing normal transit, was 90.8% (95% CI = 0.83–0.96). Overall device agreement was 87%. There was a significant correlation (p < 0.001) of CTT between ROMs and the WMC (r = 0.707) [Camilleri et al. 2010].
Pressure
Colonic manometry is the conventional method for evaluating colonic pressure and contractility, yet it has similar drawbacks to ADM, and requires a stool cleanout making it difficult to assess the colon under normal physiologic conditions [Scott, 2003; De Schryver et al. 2002]. The WMC can obtain colonic pressure profiles and does not require a colonic preparation. However, the clinical application of WMC pressure data has yet to be firmly established, and the use of WMC to relate characterized pressure profiles of the colon to diseased states does not yet have FDA approval.
The most complete assessment of colonic pressure profiles characterized by WMC was reported by Hasler and colleagues in 53 healthy and 36 constipated subjects; 12 with the constipation subtype of irritable bowel syndrome (C-IBS). Colonic pressure activity was found to be greater distally than proximally with the number of contractions increasing from the first to fourth quartiles in healthy patients (p < 0.0001), along with those with moderate slow transit constipation (p = 0.002), C-IBS (p = 0.02), and normal transit constipation (p = 0.052). However, no differences were seen in pressure activity from the first to fourth quartile in patients with severe slow transit constipation. The authors considered that the greater contractile activity distally may serve a role in stool propulsion. The blunting of this increase in distal contractions may contribute to delayed colonic transit in those with severe slow transit constipation. The authors also noted elevated pressure amplitudes in patients with C-IBS [Hasler et al. 2009].
Safety of device
Through three multicenter clinical trials with 495 subjects involving healthy, gastroparetic (n = 61), or constipated (n = 269) subjects, there was one serious adverse event reported where the capsule failed to empty the stomach after 5 days in a gastroparetic patient. The capsule passed into the duodenum successfully with intravenous erythromycin. Endoscopic assessment showed the presence of a gelatinous bezoar in the patient’s stomach. All the remaining subjects enrolled in clinical studies were able to pass the WMC in the stool with the majority occurring within 5 days of ingestion and the longest retention time being 26 days. An inability to swallow the capsule was uncommon with only 3 (0.6%) out of a total of 495 subjects unable to ingest the WMC. Equipment malfunction (capsule, receiver and/or software) was more common with 36 (7.2%) incidences of equipment or software malfunctions [Kuo et al. 2011; Rao et al. 2011; Camilleri et al. 2010]. This failure rate is likely to be secondary to the use of prototypic equipment in the earliest studies, which has since been upgraded. In a postmarket analysis of approximately 6000 WMC ingestions, the incidence of equipment failure was 0.8–0.9% [SmartPill Corporation, 2009].
Bowel obstruction secondary to capsule retention represents the most serious potential adverse event of WMC testing. The manufacturer reports a WMC retention rate of 0.33% based on 20 reports of prolonged capsule retention in a postmarket analysis of 6000 capsules. Prolonged retention was defined by radiologic identification of the WMC in the body 2 weeks postingestion on kidneys, ureters, and bladder (KUB) imaging. Of the 20 patients, 5 underwent endoscopic capsule retrieval from the stomach. In the remaining cases, the capsule was monitored radiologically and ultimately exited the body without intervention. There were no surgical interventions associated with WMC retentions reported to date [SmartPill Corporation, 2009].
Clinical guidelines for the management of WMC retention are based on anatomic location. Upon return of the data receiver, body exit of the capsule can be confirmed by analyzing the temperature data. If unable to determine body exit, the location of the capsule (stomach, small bowel, or colon) can be determined using pH data. If the WMC is located in the stomach and small bowel, serial X-rays are indicated with potential endoscopy or medical intervention with a prokinetic agent. If located in the colon, follow up beyond symptom monitoring is not recommended due to a low risk of obstruction and experience that suggests capsule passage will occur with routine constipation management.
Clinical applications
Evidence continues to mount that a significant portion of GI dysmotility is multiregional, as seen in patients with slow transit constipation who also have delayed transit by gastric or small bowel scintigraphy [Hasler, 2007]. While the clinical role of WMC pressure data requires more clinical investigation, transit time data from WMC offers a unique opportunity to assess the whole GI tract with one noninvasive ambulatory standardized test. The American and European Neurogastroenterology and Motility Societies reiterate this concept in their position paper on GI transit time stating that the WMC is recommended for regional and whole gut transit time evaluation in individuals with upper GI symptoms, gastroparesis, small bowel dysfunction, constipation or colonic disorders, or in those with alterations of GI motility in multiple regions [Rao et al. 2011].
Several WMC studies have shown that many patients with symptoms of dysmotility in one GI region often have abnormalities in other regions. Sarosiek and colleagues reported that both CTT (p = 0.005) and WGTT (p <0.001) were significantly longer in patients with gastroparesis than in healthy controls [Sarosiek et al. 2010]. Similarly, a study by Rao and colleagues showed that in constipated patients not only was CTT (p < 0.0001) delayed compared with healthy controls, but GET (p = 0.0123) was also significantly delayed [Rao et al. 2009]. A recent study by Kuo and colleagues examining WMC measurements in relation to clinical suspicion revealed that in patients with a clinical suspicion of gastroparesis, many also had delayed SBTT (20.4%) or CTT (53.2%). Conversely, patients with suspected delayed CTT also had delayed GET (41.7%) or delayed SBTT (14.3%) (Table 5) [Kuo et al. 2011].
Table 5.
Conventional test and wireless motility capsule findings in relation to clinical suspicion.
| Clinical suspicion | Conventional test sensitivity | Wireless motility capsule findings |
||
|---|---|---|---|---|
| Capsule sensitivity in target region | Capsule specificity in target region | Abnormal transit in other regions | ||
| Suspected gastric emptying delay | 17/44 (38.6%) | 24/52 (46.2%) | 19/28 (67.9%) | Small intestine: 10/49 (20.4%) Colon: 25/47 (53.2%) |
| Suspected small intestinal transit delay | 4/6 (66.7%) | 1/11 (9.1%) | 54/64 (84.4%) | Stomach: 5/13 (38.5%) Colon: 4/10 (40%) |
| Suspected colonic transit delay | 9/16 (56.2%) | 32/55 (58.2%) | 11/18 (61.1%) | Stomach: 25/60 (41.7%) Small intestine: 8/56 (14.3%) |
Adapted from Kuo et al. [2011].
In the above-mentioned retrospective analysis by Kuo and colleagues of 83 clinical patients referred to tertiary centers with suspected gastric, small intestinal, or colonic transit dysmotility, abnormal transit was found in 68% of the cases with 35.1% showing generalized transit abnormalities in two or more regions (Figure 2). The authors noted that WMC testing eliminated the need for gastric scintigraphy (17%), small bowel barium transit (54%), and colon ROMs (68%). The WMC findings led to new diagnoses in 53% of patients and significantly influenced management decisions in 67% of cases through modified nutritional regimens (14%), surgical referrals (6%), and new medications (60%). Examples of the latter include prescribing prokinetics, such as metoclopramide, to patients with suspected slow transit constipation who were found to have delayed gastric emptying by WMC and using laxatives like PEG 3350 in patients with gastroparesis who were noted to have slow colonic transit [Kuo et al. 2011].
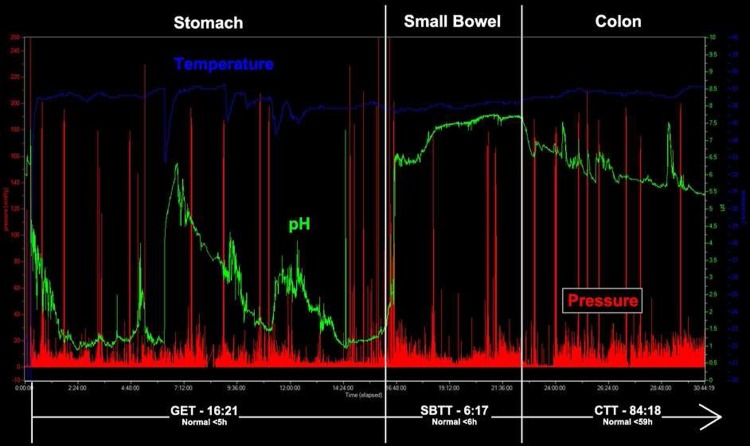
Figure 2.
Diffuse gastrointestinal dysmotility tracing using the wireless motility capsule, with prolonged GET, SBTT, and CTT. Transit times are in hh:mm. CTT, colonic transit time; GET, gastric emptying time; SBTT, small bowel transit time.
Rao and colleagues examined the diagnostic utility of the WMC in comparison to conventional motility tests in patients with suspected upper and lower GI dysmotility who also had normal endoscopic evaluations. New diagnostic information was obtained with the WMC test in 53% of the lower GI (p = 0.006) and 47% of the upper GI group (p = 0.001), with 43% of the patients receiving new additional diagnoses after the WMC test. Often, the WMC test revealed GI transit anomalies in regions other than the suspected region. The WMC test led to changes in management plans in 30% of subjects in the lower GI group and 50% of subjects in the upper GI group. Treatment modifications included prescriptions for laxatives, prokinetics, antiemetics, antidepressants, or nutritional and behavioral modifications. The WMC testing also guided further workup: a finding of normal CTT in a patient with constipation and difficulty with defecation led to recommendations for anorectal manometry to assess for pelvic floor dysfunction [Rao et al. 2011].
The complete GI tract profile offered by the WMC may provide a useful tool in the management of a patient with severe constipation who is considering colectomy as a treatment option. Over 18% of patients thought to be suffering solely from prolonged colon transit were found on evaluation to have gastroparesis as their dominant motility disorder [Camilleri et al. 2010]. In constipated patients with diffuse GI dysmotility, colectomy has a higher incidence of poor outcomes. Out of 21 patients with colonic inertia who underwent total abdominal colectomy (TAC), 90% had a successful outcome in long-term follow up, while only 13% of the 16 patients with generalized intestinal dysmotility had prolonged relief after TAC [Redmond et al. 1995]. The WMC is well suited for a full regional and whole gut transit time evaluation and may change management decisions confirming or avoiding surgery and reduce additional testing in this population.
Limitations
While the WMC provides a full GI tract-transit profile in a standardized protocol, the pressure profiles are limited by nonstationary, single point pressure measurements throughout the GI tract. As a new method of measuring GI Ct, new standards need to be developed and validated before the relevance of this information is clear. Encouraging results have been published regarding the utility of these measurements: detecting decreased contraction frequency in the stomach in gastroparetics [Kloetzer et al. 2010]; observing a blunting of the small bowel fed response in gastroparetics [Brun et al. 2010]; measuring abnormal small bowel pressure parameters when both GES and GET were normal [Lee et al. 2009]; or distinguishing differences in regional Ct activity in constipated patients [Hasler et al. 2009]. The WMC, with only one pressure sensor, is unable to detect a pressure wave front, which limits its utility in comparison to traditional manometric testing. However, with the invasive nature and limited availability of manometry, the WMC may have significant potential as further investigation continues to evolve the clinical utility of WMC pressure data.
The WMC cannot distinguish the absolute time of emptying of a meal or distinguish between liquid and solid emptying; rather it measures the total meal emptying. Furthermore, the WMC measures gastric emptying indirectly through the use of a physiologic meal. Scintigraphy testing leads to a more physiologic assessment of transit time.
As a nondigestible capsule that needs to be ingested, the WMC should not be administered to those patients with suspected strictures, fistulas, or GI obstructive symptoms. In addition, it should be used with caution for anyone with a history of gastric bezoars, dysphagia, or disorders of swallowing, recent GI surgery, Crohn’s disease, or diverticulitis.
Summary
The WMC is a novel technology offering a noninvasive, nonradioactive, standardized method to assess intraluminal pH, temperature, and pressure, allowing for the measurement of gastric, small bowel, colon, and whole GI transit times. As a single ambulatory test, it allows for an assessment of isolated and diffuse motility transit abnormalities. Interpretation of Ct measurements obtained by the WMC is limited compared with manometry testing, but continues to evolve. The WMC should be considered by gastroenterologists to evaluate regional and whole gut transit time for patients with motility and functional GI disorders.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Braden Kuo serves as a consultant for the SmartPill Corporation and has conducted clinical trials on their behalf.
Contributor Information
Khoa Tran, Pediatric GI, Massachusetts General Hospital for Children, Boston, MA, USA.
Rita Brun, Gastrointestinal Unit, Massachusetts General Hospital, Boston, MA, USA.
Braden Kuo, Gastrointestinal Unit, Massachusetts General Hospital, 55 Fruit St, GRJ 724, Boston, MA 02114, USA.
References
- Abell T.L., Camilleri M., Donohoe K., Hasler W.L., Lin H.C., Maurer A.H., et al. (2008) Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol 36: 44–54 [DOI] [PubMed] [Google Scholar]
- Agrawal A., Houghton l.A., Reilly B., Morris J., Whorwell P.J. (2009) Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol 104: 1998–2004 [DOI] [PubMed] [Google Scholar]
- Brun M., Michalek W., McCallum R., Kock K.L., Sitrin M.D., Wo J.M., et al. (2010) Comparison of small bowel postprandial response in healthy, gastroparetic and constipated subjects as measured by wireless motility capsule (WMC). Gastroenterology 138: S–459 [Google Scholar]
- Brun M., Michalek W., Surjanhata B., Kuo B. (2011a) Small bowel transit time (SBTT) by wireless motility capsule (WMC): normal values and analysis of pressure profiles in different subgroups of patients with slow SBTT. Gastroenterology 140: S–865 [Google Scholar]
- Camilleri M., Thorne N.K., Ringel Y., Hasler W.L., Kuo B., Esfandyari T., et al. (2010) Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil 22: 874–882; e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilly D., Kantor S., Knight L.C., Maurer A.H., Fisher R.S., Semler J., et al. (2008) Gastric emptying of a non-digestible solid: assessment with simultaneous Smartpill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 20: 311–319 [DOI] [PubMed] [Google Scholar]
- De Schryver A., Samsom M., Smout A. (2002) In search of objective manometric criteria for colonic high-amplitude propagated pressure waves. Neurogastroenterol Motil 14: 375–381 [DOI] [PubMed] [Google Scholar]
- Evans D.F., Pye G., Bramley R., Clark A.G., Dyson T.J., Hardcastle J.D. (1988) Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 29: 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2009) Smartpill GI Monitoring System, version 2.0. Market approval notification 30 October 2009 Silver Spring, MD: Food and Drug Administration, Department of Health and Human Sevices [Google Scholar]
- Guyton A.C., Hall J. (2011) Textbook of Medical Physiology. 12th edition Philadelphia, PA: Saunders [Google Scholar]
- Hasler W.L. (2007) Gastroparesis: symptoms, evaluation, and treatment. Gastroenterol Clin North Am 36: 619–647 [DOI] [PubMed] [Google Scholar]
- Hasler W.L., Coleski R., Chey W.D., Koch K.L., McCallum R.W., Wo J.M., et al. (2008) Differences in intragastric pH in diabetic vs. idiopathic gastroparesis: relation to degree of gastric retention. Am J Physiol Gastrointest Liver Physiol 294: G1384–G1391 [DOI] [PubMed] [Google Scholar]
- Hasler W.L., Saad R.J., Rao S.S., Wilding G.E., Parkman H.P., Koch K.L., et al. (2009) Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol Gastrointest Liver Physiol 297: G1107–G1114 [DOI] [PubMed] [Google Scholar]
- Kloetzer L., Chey W.D., McCallum R.W., Koch K.L., Wo J.M., Sitrin M., et al. (2010) Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil 22: 527–533; e117 [DOI] [PubMed] [Google Scholar]
- Kuo B., Maneerattanaporn M., Lee A.A., Baker J.R., Wiener S.M., Chey W.D., et al. (2011) Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci 56: 2928–2938 [DOI] [PubMed] [Google Scholar]
- Kuo B., McCallum R.W., Koch K.L., Sitrin M.D., Wo J.M., Chey W.D., et al. (2008) Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 27: 186–196 [DOI] [PubMed] [Google Scholar]
- Lee A., Michalek W., Kuo B.; The SmartPill Trial Group (2009) Variable upper gastrointestinal tract pathophysiological motility in gastroparesis. Neurogastroenterol Motil 21: 73 [Google Scholar]
- Lundin E., Graf W., Garske U., Nilsson S., Maripuu E., Karlbom U. (2007) Segmental colonic transit studies: comparison of a radiological and a scintigraphic method. Colorectal Dis 9: 344–351 [DOI] [PubMed] [Google Scholar]
- Maqbool S., Parkman H.P., Friedenberg F.K. (2009) Wireless capsule motility: comparison of the Smartpill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 54: 2167–2174 [DOI] [PubMed] [Google Scholar]
- Metcalf A., Phillips S., Zinsmeister A., MacCarty R., Beart R., Wolff B. (1987) Simplified assessment of segmental colonic transit. Gastroenterology 92: 40–47 [DOI] [PubMed] [Google Scholar]
- Michalek W., Semler J.R., Kuo B. (2011) Impact of acid suppression on upper gastrointestinal pH and motility. Dig Dis Sci 56: 1735–1742 [DOI] [PubMed] [Google Scholar]
- Miner P., Jr, Katz P.O., Chen Y., Sostek M. (2003) Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol 98: 2616–2620 [DOI] [PubMed] [Google Scholar]
- Mojaverian P., Chan K., Desai A., John V. (1989) Gastrointestinal transit of a solid indigestible capsule as measured by radiotelemetry and dual gamma scintigraphy. Pharm Res 6: 719–724 [DOI] [PubMed] [Google Scholar]
- Mojaverian P., Ferguson R.K., Vlasses P.H., Rocci M.L., Jr, Oren A., Fix J.A., et al. (1985) Estimation of gastric residence time of the Heidelberg capsule in humans: effect of varying food composition. Gastroenterology 89: 392–397 [DOI] [PubMed] [Google Scholar]
- Rao S.S. (2005) Clinical utility of diagnostic test for constipation in adults: a systematic review. Am J Gastroenterol 100: 1605–1615 [DOI] [PubMed] [Google Scholar]
- Rao S.S. (2009) Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastrointest Endosc Clin N Am 19: 117–139 [DOI] [PubMed] [Google Scholar]
- Rao S.S., Camilleri M., Hasler W.L., Maurer A.H., Parkman H.P., Saad R., et al. (2011) Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil 23: 8–23 [DOI] [PubMed] [Google Scholar]
- Rao S.S., Kuo B., McCallum R.W., Chey W.D., Dibaise J.K., Hasler W.L., et al. (2009) Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol 7: 537–544 [DOI] [PubMed] [Google Scholar]
- Rao S.S., Mysore K., Attaluri A., Valestin J. (2011) Diagnostic utility of wireless motility capsule in gastrointestinal dysmotility. J Clin Gastroenterol 45: 684–690 [DOI] [PubMed] [Google Scholar]
- Redmond J., Smith G., Barofsky I., Ratych R.E., Goldborough D.C., Schuster M.M. (1995) Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol 90: 748–753 [PubMed] [Google Scholar]
- Sarosiek I., Selover K.H., Katz L.A., Semler J.R., Wilding G.E., Lackner J.M., et al. (2010) The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther 31: 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. (2003) Manometric techniques for the evaluation of colonic motor activity: current status. Neurogastroenterol Motil 15: 483–513 [DOI] [PubMed] [Google Scholar]
- SmartPill Corporation (2009) pH.p Capsule Operational Specifications. www.smartpillcorp.com
- Tougas G., Eaker E.Y., Abell T.L., Abrahamsson H., Boivin M., Chen J., et al. (2000) Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 95: 1456–1462 [DOI] [PubMed] [Google Scholar]
- Urma D., Parkman H., Koch K., Sitrin M.D., Bahadur S., Lackner J.M., et al. (2005) Postprandial small intestinal motility response recording with a wireless capsule technology. Neurogastroenterol Motil 17: 623 [Google Scholar]
- Zarate N., Mohammed S.D., O’Shaughnessy E., Newell M., Yazaki E., Williams N.S., et al. (2010) Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol 299: G1276–G1286 [DOI] [PubMed] [Google Scholar]