Abstract
Background:
Vitamin D could play a protective role in multiple sclerosis.
Methods:
In an observational, uncontrolled study, vitamin D3 supplementation (3010 IU/day on average) was given to 156 consecutive patients with relapsing–remitting multiple sclerosis, under first-line immunomodulatory therapy and with initial 25-OH-D serum level lower than 100 nmol/l (40 ng/ml). Relapses were determined for 29.1 ± 8.4 months during vitamin D and 29.8 ± 10.1 months before supplementation. The 25-OH-D level was measured before supplementation and several times during supplementation. The incidence rate of relapses before and during supplementation was estimated using negative binomial regression models with follow-up durations as offset terms. The incidence rate and incidence rate ratio of relapses at various 25-OH-D levels were also calculated using negative binomial regression models.
Results:
In 76 patients, immunomodulatory therapy preceded vitamin D supplementation (by 4.2 ± 2.7 years) and in 80 patients both treatments were started simultaneously. Under supplementation, the 25-OH-D level increased from 49 ± 22 nmol/l to 110 ± 26 nmol/l on average. Pooling data collected before and during supplementation, we found a significant strong inverse relationship between the relapse incidence rate and the 25-OH-D level (p < 0.0001), suggesting that vitamin D did indeed influence the relapse rate. Results of univariate, bivariate and multivariate analyses were analogous: in the multivariate model adjusted for age, disease duration and previous use of immunomodulatory therapy, every 10 nmol increase in 25-OH-D level was associated with a reduction in the relapse incidence rate of 13.7%. Dividing iteratively the population made up of pooled periods into two subgroups according to the 25-OH-D levels, the relapse incidence rate ratio decreased as the 25-OH-D level increased up to 110 nmol/l, but a plateau effect was observed beyond this limit.
Conclusion:
Further studies are warranted for accurate quantification of the vitamin D effect.
Keywords: multiple sclerosis, relapse rate, vitamin D, vitamin D supplementation
Introduction
It has recently been suggested that hypovitaminosis D is widespread in mid- and high-latitude countries [Holick, 2007, 2011; Hagenau et al. 2009; Pierrot-Deseilligny and Souberbielle, 2011] and it is nowadays generally accepted that this insufficiency may constitute one of the risk factors for multiple sclerosis (MS) [Ascherio and Munger, 2007; Ebers, 2008; Ascherio et al. 2010; Pierrot-Deseilligny and Souberbielle, 2010; Hanwell and Banwell, 2011; Ramagopalan et al. 2011]. Furthermore, several studies in MS patients have shown that vitamin D plays an anti-inflammatory and immunomodulatory role via diverse immunological mechanisms [Mahon et al. 2003; Correale et al. 2009, 2010; Royal et al. 2009; Bartosik-Psujek et al. 2010; Smolders et al. 2008a, 2009, 2010, 2011b; Lysandropoulos et al. 2011], potentially resulting in beneficial effects after the start of the disease. It has also recently been reported in two different association studies that the spontaneous 25-OH-D serum level (DSL) of patients with relapsing–remitting MS (RRMS) was inversely correlated in a linear fashion with the relapse rate, which supports a protective role of vitamin D, at least at the beginning of the disease [Mowry et al. 2010; Simpson et al. 2010]. The results of a few small phase I/II trials using vitamin D have also suggested that a beneficial effect of this vitamin could exist in MS patients [Goldberg et al. 1986; Wingerchuk et al. 2005; Burton et al. 2010; Soilu-Hänninen et al. 2012], maybe except in very benign forms of the disease [Kampman et al. 2012]. However, a neurological therapeutic effect of vitamin D in MS can only be ascertained and accurately quantified by randomized, controlled trials (RCTs), which are now beginning in several countries, including in France [Munger and Ascherio, 2011; Smolders et al. 2011a; Dorr et al. 2012]. Since the RCT results will not be available for another 2–3 years and most MS patients are currently in a state of vitamin D insufficiency, including at the earliest stages of the disease [Pierrot-Deseilligny and Souberbielle, 2010], we recommended checking serum titration of vitamin D in MS patients and, from a general medical point of view alone, supplementing with vitamin D those who are in a state of insufficiency [Pierrot-Deseilligny, 2009]. We have been applying these practical clinical measures to our MS patients since mid-2008 and we have retrospectively studied the effect of itamin D supplementation added to first-line immunomodulatory therapies (IMTs) on the relapse rate of 156 consecutive RRMS patients over an average of 2.5 years. It should be noted that this study differs from previous association studies in MS patients, most of whom were not being supplemented with vitamin D or were receiving only low vitamin doses, since our main aim here was to determine whether a significant relationship also exists between the DSL and the relapse rate in MS patients systematically supplemented with ‘physiological’ vitamin D doses.
Patients and methods
Patients
In May 2008, we began systematically testing the vitamin D serum level in all our MS patients and supplementing those with a DSL lower than 100 nmol/l (40 ng/ml). In 2011, we retrospectively included in this study all consecutive patients fulfilling the following four criteria: (1) RRMS patients (see definition below) spontaneously coming to and subsequently followed up in our neurological department (‘Neurologie 1’, Salpêtrière Hospital, Paris); (2) who were treated with first-line IMT; (3) if they had a DSL lower than 100 nmol/l at the initial blood titration and, if so, were subsequently receiving vitamin D supplementation (see below); and (4) who were eventually followed up under this bitherapy for at least 6 months in our department. The time of prescription of vitamin D supplementation divided the whole follow-up period into two subperiods, i.e. prior to and during vitamin D supplementation (see below).
RRMS patients
We included only patients aged between 18 and 65 years. The diagnostic criteria for MS and its relapsing–remitting form were those generally used [McDonald et al. 2001; Polman et al. 2005]. Patients had to have had at least two clinical relapses at any time since the beginning of the disease and also had to have a magnetic resonance imaging scan revealing lesions consistent with the diagnosis of MS. Patients with clinically isolated syndromes or secondary progressive MS were ineligible. Relapses were defined as new or recurrent neurological symptoms not associated with fever or infection, lasting at least 24 hours and accompanied by new, objective neurological findings [McDonald et al. 2001].
IMT treatment
Moreover, to be enrolled in the study, RRMS patients were required either to be already under a first-line IMT before vitamin D supplementation (Group 1), regardless of the time this treatment was introduced, or to have started this IMT simultaneously with vitamin D supplementation (Group 2). The first-line IMT was glatiramer acetate (GA, i.e. Copaxone®, SC once daily), interferon beta 1b (Betaseron®, SC every other day) or interferon beta 1a (Rebif® 44 or 22, SC 3 times weekly, or Avonex®, IM once a week). It is generally accepted that GA and the three types of interferon betas (IFBs) have largely analogous effects on the relapse rate of RRMS patients [Haas and Firzlaff, 2005; Gajofatto et al. 2009]. Furthermore, since it was possible to switch from one IMT to one of the other three types during the study period, the IMT considered was that taken together with vitamin D. Patients under natalizumab for ‘aggressive’ MS were not included since this treatment is clearly more active on the relapse rate than first-line IMTs [Polman et al. 2006; Castillo-Trivino et al. 2011; Kaufman et al. 2011].
25-OH-D serum level
Calcaemia, assessed by a standard chemistry method, and the 25-OH-D serum level, measured by radio-immunoassay (DiaSorin, Saluggia, Italy), were determined prior to vitamin D supplementation. The 25-OH-D serum level (DSL) is usually measured to evaluate vitamin D status since it is representative of the overall vitamin D store in the body [Heaney, 2000; Zerwekh, 2008]. No patients had previously been supplemented with vitamin D and none had hypercalcaemia (i.e. over 104 mg/l) at the initial blood test. Patients with an initial DSL lower than 100 nmol/l were prescribed a vitamin D supplementation to take in addition to their IMT. The 100 nmol/l threshold was chosen (1) since it was close to the minimum DSL above which the odds ratio for MS had been shown to be reduced in a prospective nested-case control study [Munger et al. 2006]; and (2) since it could be considered significantly higher than the 75 nmol/l value, often regarded nowadays as the lower limit for optimal bone and extra-bone effects of vitamin D [Holick, 2004; Hollis, 2005; Bischoff-Ferrari et al. 2006, 2009; Vieth et al. 2007], when the measurement uncertainty of the 25-OH-D assay is taken into account [Binkley and Krueger, 2008; Cavalier et al. 2010]. Once started, vitamin D supplementation had to have been maintained whatever the season. Calcaemia and DSL were tested 6 months after the beginning of supplementation and, subsequently, once or twice a year, to detect possible hypercalcaemia and to adjust the vitamin D dose, if necessary. The successive DSLs during vitamin D supplementation were recorded and averaged (DSL2).
Vitamin D supplementation
Vitamin D supplementation was in the form of a drinkable ampoule of 100 000 IU/l of vitamin D3 (cholecalciferol, Uvédose ®) to be taken once a month if the initial DSL was lower than 75 nmol/l or every 6 weeks if it was between 75 and 100 nmol/l. No calcium supplementation was prescribed. If, at the 6-month control, the DSL was still low or if it was above 200 nmol/l, supplementation was adjusted to 100,000 IU every 3 weeks or every 2 months, as appropriate, and further readjusted later, if necessary. The mean daily vitamin D supplementation was calculated over the entire follow-up period. Thus, all patients were in a state of relative vitamin D insufficiency prior to supplementation and the aim was for their DSL to be kept within the 75-200 nmol/l range during supplementation. This target range is nowadays often considered the ‘normal’ range [Souberbielle et al. 2010] in medical laboratories. Accordingly, such vitamin D supplementation, using an authorized medication, administered at moderate doses, to reach and remain within a physiological DSL, in patients initially in a state of vitamin D insufficiency, was considered routine care. Nevertheless, all patients were informed of possible (minor) side effects due to vitamin D supplementation. Two patients complained of brief slight nausea after taking their monthly drinkable ampoule of 100,000 IU of vitamin D: they were subsequently given a daily vitamin D dose of 1600 IU in tablet form, with no recurrence of this symptom. Two other patients had a benign skin eruption after taking their ampoules: they were also switched to the tablet form without recurrence of this sign.
Clinical visits
Clinical visits were scheduled every 6 months to check patients’ clinical state, assess (using a structured interview) possible side effects of the IMT and/or vitamin D supplementation, check the results of the most recent blood tests (DSL and calcaemia), performed just before an intake of vitamin D, and renew medication prescriptions (IMT and vitamin D). Patients were also seen for unscheduled visits within 72 hours after the development of new symptoms so that they could be assessed for possible relapses and treated with intravenous methylprednisolone (3–5 g), if necessary.
Clinical data
The primary clinical outcome measure was the incidence rate of MS relapses: relapses were recorded over the 36 months preceding vitamin D supplementation or since the date of the first relapse if disease duration was less than 36 months; relapses were also recorded during the period of vitamin D supplementation, which had to have lasted for at least 6 months. The same methods were therefore used for defining and recording relapses before and during vitamin D supplementation.
Gender, age, disease duration (from the first clinical relapse) and Expanded Disability Status Scale (EDSS) score were recorded at the start of vitamin D supplementation. The length of the period preceding vitamin D supplementation and the length of the period during supplementation were also recorded.
Statistics
One of the primary predictors of the relapse incidence rate was the DSL. DSL1 was determined just before vitamin D supplementation and was considered to be representative of the vitamin D status in the period preceding supplementation (see the discussion). DSL2 was the mean of all DSLs ascertained over the period of vitamin D supplementation. However, the DSL could noticeably vary in some patients over this period (see the discussion) and this could be a concern for estimating the relationship between the DSL and the relapse rate during this period. Therefore, the period under vitamin D supplementation was divided into a variable number of subperiods defined by DSL determinations, each period starting and ending with a DSL determination. For each subperiod, the DSL was the mean of the starting and ending determinations. The number of relapses that occurred between two vitamin D determinations was attributed to the considered subperiod. As the global follow-up time and the duration of each subperiod were variable for each patient and because the assumptions of the Poisson model were violated (due to an overdispersion), a negative binomial regression model, using the logarithms of the duration of the period before vitamin D supplementation and of the duration of each subperiod under vitamin D supplementation as offset terms, was used to assess the impact of the DSL on the number of relapses. Incidence rates (IRs) of relapses and the corresponding 95% confidence intervals (CIs) were calculated before and during vitamin D supplementation along with the incidence rate ratio (IRR) of relapses (univariate analyses). Potential confounding variables, namely gender, age, disease duration, EDSS score at the start of vitamin D supplementation, previous use of an IMT prior to vitamin D supplementation (Group 1 versus Group 2) and the type of IMT (GA versus IFB) used concomitantly with vitamin D supplementation were introduced separately in the model (bivariate analyses). Furthermore, factors associated with IRR (p < 0.20) were introduced in a multivariate model. In addition, the relationship between the DSL and the IR was estimated after having pooled data collected before and during vitamin D supplementation. Finally, IRs and IRRs were estimated according to various DSL thresholds after iterative binary division of the pooled data according to DSLs. The dataset was divided into quintiles according to the DSL value, and the IR for each quintile was calculated and displayed graphically for the whole population, Group 1 and Group 2. Moreover, the evolution of the IRR was displayed graphically for the 3 groups of patients. Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA).
Results
Study population
Of the 171 RRMS patients who began vitamin D supplementation between May 2008 and October 2010, 156 met the criteria to be included in this cohort. Among the 15 patients who were not eligible, eight were lost to follow up within less than 6 months after the start of vitamin D supplementation, and the other seven interrupted either the IMT (five for pregnancy and two for skin problems) or vitamin D (non compliance) for more than 3 months during the first 6 months of vitamin D supplementation. The number of patients who started vitamin D supplementation during each of the four seasons was analogous. This cohort was followed up for 29.1 ± 8.4 months (median = 31 months; range: 6–42 months) during vitamin D supplementation and for an analogous period of 29.8 ± 10.1 months (range: 4–36 months) before vitamin D supplementation. In 76 patients, an IMT had already been present for a variable period (4.2 ± 2.7 years; median = 4.0 years; range: 0.5–12 years) when vitamin D supplementation was started (Group 1) and in 80 patients the first IMT was started simultaneously with vitamin D supplementation (Group 2) (Table 1). Clinical characteristics were consistent with a population of RRMS patients, including for EDSS score (Table 1).
Table 1.
Characteristics of patients.
| All patients | Group 1 | Group 2 | |
|---|---|---|---|
| n = 156 | n = 76 | n = 80 | |
| Age* (years) | 39±10 | 41±10 | 36±10 |
| Mean±SD [range] | [18–65] | [20–61] | [18–65] |
| Males: No. (%) | 41 (26%) | 13 (24%) | 23 (29%) |
| Females: No. (%) | 115 (74%) | 58 (76%) | 57 (71%) |
| Disease duration* (years) | 7.2±6.4 | 9.3±6.3 | 5.3±5.9 |
| Mean±SD [range] | [0.3–30] | [1–30] | [0.3–30] |
| EDSS* Mean [range] | 2.2 [0–6] | 2.3 [0–6] | 2.1 [0–6] |
| IMT: No. (%) | |||
| - Glatiramer acetate | 84 (53.8%) | 34 (44.7%) | 50 (62.5%) |
| - Interferons beta | 72 (46.2%) | 42 (55.3%) | 30 (37.5%) |
| DSL1 (nmol/l) | 49.5±22.1 | 53.6±21.5 | 45.5±21.9 |
| Mean±SD [range] | [5–98] | [13–98] | [5–95] |
| DSL2 (nmol/l) | 110±26 | 113± 26 | 106± 25 |
| Mean±SD [range] | [52–200] | [65–200] | [52–165] |
| Period before supplementation (months) | 29.8±10 | 34.2±4.9 | 25.6±12 |
| Mean±SD [range] | [4–36] | [12–36] | [4–36] |
| Period during supplementation (months) | 29.1±10.4 | 32.6±6.6 | 26.6±8.5 |
| Mean±SD [range] | [6–42] | [12–42] | [6–42] |
DSL1, 25-OH-D serum level just prior to vitamin D supplementation; DSL2, averaged 25-OH-D serum level during the period under vitamin D supplementation; EDSS, Expanded Disability Status Scale; Group 1, IMT started prior to vitamin D supplementation; Group 2, IMT started concomitantly with vitamin D supplementation; IMT, first-line immunomodulatory treatment; SD, standard deviation; *at the beginning of vitamin D supplementation.
All patients were treated with a first-line IMT simultaneously with vitamin D: GA in 84 cases (54%) and IFB in 72 cases (46%) (Table 1), subdivided into Betaseron® in 37 cases (24%), Rebif® in 26 cases (17%) and Avonex® in 9 cases (6%). Patients under the different types of IFB were considered as one therapeutic subgroup for statistical subanalyses.
The dose of vitamin D supplementation was 3010 ± 993 IU/day (median 3200; range: 800–5800 IU/day) in the whole cohort and was analogous in Groups 1 and 2. It should be noted that 53% of our patients received a vitamin D dose of 3288 IU/day (i.e. corresponding to 100,000 IU/month) whereas 20% needed less supplementation (i.e. between 800 and 3288 IU/day) and 27% needed more supplementation (i.e. between 3289 and 5800 UI/day) to arrive at the targeted DSL range. There was no correlation between the vitamin D dose and the DSL2 (Pearson’s test, r = 0.12; p = 0.13).
Safety and tolerance
Calcaemia did not significantly change after vitamin D supplementation, passing from 93.4 ± 3.9 mg (median: 93 mg; range: 85–104 mg) at the first titration to 93.4 ± 4.0 mg (median: 93 mg; range 83–103 mg) at the last titration (Student’s t-test, p = 0.86) (Table 1). No cases of hypercalcaemia (over 104 mg/ml) or urinary lithiasis were observed during the study period. No serious side effects related to vitamin D were observed.
Relapses
The incidence rate of relapses before (IR1) and during (IR2) vitamin D supplementation and the IRR are given in Table 2. The IRR was 0.25 (95% CI 0.20–0.32) for the whole cohort. This means that the IR was reduced by 75% under IMT and vitamin D supplementation in this cohort. The IRR was significantly lower in patients of Group 2 than in patients of Group 1 (p < 0.0001). Age (p = 0.0005) and disease duration (p < 0.0001), but not gender (p = 0.14), EDSS score at inclusion (p = 0.69) or type of IMT (GA versus IFB, p = 0.06), were significantly associated with the IR in adjusted analyses. However, a similar IRR was found in multiple regression models (IRR for the whole cohort = 0.26 [95% CI 0.20–033]).
Table 2.
Relapse incidence rate and incidence rate ratio.
| IR1 | IR2 | IRR | |
|---|---|---|---|
| Whole population | 0.70 [0.62–0.79] | 0.18 [0.14–0.22] | 0.25 [0.20–0.32] |
| Group 1 | 0.50 [0.41–0.61] | 0.16 [0.11–0.22] | 0.32 [0.24–0.43] |
| Group 2 | 0.89 [0.78–1.02] | 0.20 [0.14–0.28] | 0.22 [0.15–0.32] |
IR, relapse incidence rate; IR1, before vitamin D supplementation; IR2, under vitamin D supplementation; IRR, incidence rate ratio, results are expressed with two-sided 95% confidence interval; Group 1, IMT started prior to vitamin D supplementation; Group 2, IMT started concomitantly with vitamin D supplementation; IMT, immunomodulatory treatment.
25-OH-D serum level
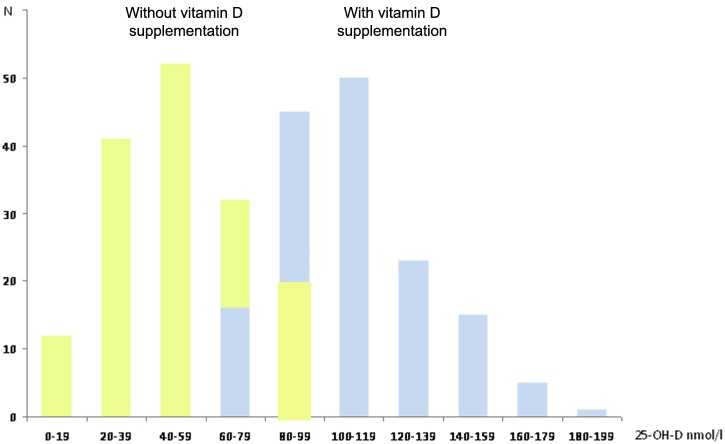
The DSL increased from 49 ± 22 nmol/l (median: 50 nmol/l; range: 5–98 nmol/l) before vitamin D supplementation (DSL1) to 110 ± 26 nmol/l (median: 107 nmol/l; range: 70–185 nmol/l) on average under vitamin D supplementation (DSL2), which represents an increase of 60 ± 29 nmol/l (median 59 nmol/l; range: 19–146 nmol/l) in the DSL level (Table 1 and Figure 1). The results were analogous in Groups 1 and 2 (Table 1), with no difference in the effect of vitamin D supplementation on DSL between groups (p = 0.18, analysis of covariance [ANCOVA] adjusted for DSL1).
Figure 1.
Effect of vitamin D supplementation on 25-OH-D serum level in all patients.
In yellow, before vitamin D supplementation; in blue, during vitamin D supplementation (averaged serum level); X-axis: 25-OH-D serum level; Y-axis: number of patients.
Relationship between relapses and 25-OH-D serum level
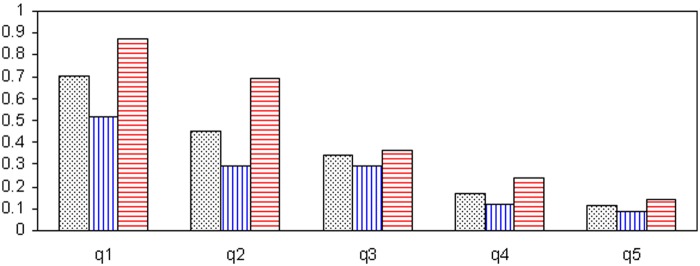
DSL1s were often low whereas DSL2s were almost consistently higher (Figure 1). The data collected before and during vitamin D supplementation were pooled to evaluate the relationship between relapses and the DSL. The population was first divided into quintiles according to the DSL: the IR was clearly lower when the DSL was higher, as illustrated in Figure 2. This effect was analogous in the whole population, Group 1 and Group 2. Moreover, in univariate analysis, there was a log-linear relationship between the DSL and the IR (p < 0.0001): for every 10 nmol/l increase in DSL there was a 14.90% decrease in IR (95% CI 11.70–18.00%). This effect remained virtually unchanged when the model was adjusted in bivariate analyses for the patient’s age (11.3% decrease in IR for every 10 nmol/l increase in DSL), disease duration (11.5% decrease in IR), EDSS score at inclusion (11.7% decrease in IR) and previous use of an IMT prior to inclusion (10.8% decrease in IR). In addition, in univariate analyses conducted separately in Groups 1 and 2, results were analogous: IRs were reduced by 13.2% in Group 1 and 14.2% in Group 2 for every 10 nmol/l increase in DSL (p < 0.0001). Gender and the type of IMT were not associated with the IR. Finally, in a multivariate model adjusted for the patient’s age, disease duration and previous use of an IMT prior to inclusion, results were similar since there was a 13.7% decrease in IR (95% CI 10.64–16.64%) for every 10 nmol/l increase in DSL (p < 0.0001).
Figure 2.
Relapse incidence rate according to 25-OH-D serum level.
X-axis: quintile of 25-OH-D serum levels; Y-axis: incidence rate. Q1 to q5 are quintiles of 25-OH-D serum levels, numbers are relapse incidence rate: q1 : ≤ 55.5 nmol/l; q2: > 55.5 to ≤ 78.5 nmol/l; q3: > 78.5 to ≤ 97.25 nmol/l; q4: > 97.25 to ≤ 121.5 nmol/l; q5: >121.5 nmol/l. In black, whole population; in blue, Group 1 (IMT started prior to vitamin D supplementation); in red, Group 2 (IMT started concomitantly with vitamin D supplementation).
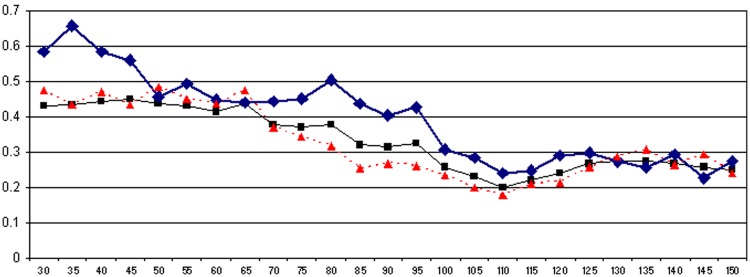
To estimate what a target DSL might be, we performed iterative analyses with the same model, introducing various DSL threshold values as covariate. For each analysis, the whole data set combining data before and during vitamin D supplementation was divided into two subgroups: patients with a DSL less than the tested threshold and patients with a DSL greater or equal to the chosen threshold. Threshold values varied from 30 to 150 nmol/l in steps of 5 nmol/l. IRs and IRRs were calculated at each threshold. IRR values according to the DSL are graphically displayed in Figure 3 for the whole population, Group 1 and Group 2. There was a nadir in IRR for DSLs between 100 and 120 nmol/l. The IRR decreased for DSLs between 30 and 110 nmol/l, with a plateau effect for DSLs higher than 120 nmol/l in the three groups of patients. The IRR was higher in Group 1 than in Group 2 for low DSLs but analogous in both subgroups above 120 nmol/l.
Figure 3.
Evolution of relapse incidence rate ratio according to 25-OH-D serum level.
X-axis: 25-OH-D serum level (nmol/l); Y-axis: relapse incidence rate ratio. In black, whole population; in blue, Group 1 (IMT started prior to vitamin D supplementation); in red, Group 2 (IMT started concomitantly with vitamin D supplementation).
Discussion
Study design
In the present study, we systematically supplemented with vitamin D our RRMS patients who were in a state of relative insufficiency since, according to the recent literature, vitamin D supplementation could be important for the maintenance of health in general [Pierrot-Deseilligny and Souberbielle, 2010]. Moreover, it has now been widely reported that, even as early as the first stages of the disease, most MS patients are in a state of vitamin D insufficiency, whatever DSL is chosen to define it, i.e. between 50 and 100 nmol/l [Pierrot-Deseilligny and Souberbielle, 2010]. The spontaneous DSL (before supplementation) was 49 ± 22 nmol/l in our cohort. Therefore, the first aim of our intervention was to bring the DSL of our MS patients to slightly above the 75–100 nmol/l zone, which is often nowadays considered as the critical physiological lower limit for extra-bone beneficial effects of vitamin D [Hollis, 2005; Vieth et al. 2007; Souberbielle et al. 2010], maybe including the prevention of MS [Munger et al. 2006]. After adjustments to the vitamin D supplementation dose in some patients, when the DSL either remained below the 75–100 nmol/l zone or transitorily exceeded 200 nmol/l, an averaged serum level of 110 ± 26 nmol/l was reached in this cohort during the follow up (with, therefore, an increase of 60 nmol/l, on average), corresponding to an average daily supplementation of 3010 IU of vitamin D. It should be noted that this supplementation was within the range of 1000–4000 IU/day of vitamin D often nowadays considered as the daily physiological requirement [Heaney et al. 2003, 2009; Grant and Holick, 2005; Vieth, 2006; Bischoff-Ferrari et al. 2006; Hall et al. 2010; Schwalfenberg et al. 2010], even if this point is not yet consensual [Ross et al. 2011; Heaney and Holick, 2011]. Furthermore, the doses of vitamin D supplementation used here were found to be safe and well tolerated, with no cases of hypercalcaemia or serious side effects after a mean follow up of 2.5 years.
We paid particular attention to the relapse rate since the anti-inflammatory and immunomodulatory effects of vitamin D (see the introduction) could particularly influence this variable. All patients of this cohort were under a first-line IMT and vitamin D supplementation. In terms of its main clinical characteristics (Table 1), our cohort was analogous to the cohorts of RRMS patients usually encountered. Although vitamin D supplementation appears to have been somewhat beneficial in this cohort, only the statistical relationship existing between the relapse rate and the vitamin D status will be discussed here.
Relationship between the relapse rate and vitamin D status
Main results
The main finding of this study was a strongly significant inverse relationship between vitamin D status, expressed by the DSL, and the IR, in univariate as well as multivariate analyses (p < 0.0001 for all). In bivariate analyses, this vitamin D effect was similar when adjusted for gender, age, disease duration, degree of disability (EDSS) and the type of IMT (GA versus IFB) used simultaneously with vitamin D supplementation. Importantly, this effect was also independent of the presence or absence of an IMT prior to inclusion (Group 1 versus Group 2). The results of these diverse univariate analyses and of multivariate analysis (adjusted for age, disease duration and the previous use of an IMT prior to inclusion) strongly suggest that the reduction of IR could not be explained by the sole action of the IMT.
Furthermore, an important quantitative point found here using univariate analyses was that every 10 nmol/l increase in DSL was associated with a 14.9% reduction of IR in the whole cohort and with a 13.2% and a 14.4% reduction of IR in Groups 1 and 2, respectively. In the multivariate analysis, IR was reduced by 13.7% for every 10 nmol/l increase in DSL. Therefore, the results were analogous whatever the analyses and groups, which further suggests a significant vitamin D effect added to the IMT action.
However, we cannot accurately differentiate between the respective actions of these two treatments, even after focusing analyses on Group 1 (under IMT prior to vitamin D supplementation), since the duration of IMT was not homogeneous in this group (4.2 ± 2.7 years; range: 0.5–12 years): this means that the period under IMT was shorter in many patients than the whole period taken into account to determine the IR before supplementation, with, consequently, also a part of IMT action in the relapse reduction which cannot be differentiated from the vitamin D effect. Thus, the accurate quantification of the vitamin D effect added to the IMT action requires the use of RCTs.
Methodological points
It should first be noted that vitamin D supplementation was delivered once a month in our study and that DSLs once vitamin D supplementation had been started were measured a few days before a monthly intake. However, we know from pharmacokinetic studies that after a single dose of 100,000 IU of cholecalciferol [Ilahi et al. 2008] (i.e. the same dose as in our study), the DSL decreases only slightly for the second half of the month following the intake, and that, with regular monthly intakes of vitamin D when compared with analogous weekly or daily intakes [Ish-Shalom et al. 2009], the results of DSLs are almost identical.
Furthermore, the DSL1 was an approximation of the vitamin D status existing over a period of up to 36 months before vitamin D supplementation, but, in the absence of any vitamin D supplementation during this period, the vitamin D status was likely mainly influenced by the patients’ lifestyle and their habits regarding sunshine exposure, which are unlikely to change radically from one year to another. Furthermore, the DSL2 could vary somewhat since the vitamin D dose had to be adjusted in about half of the patients, namely those not yet within the 75–200 nmol/l range after 6 or 12 months of follow up during vitamin D supplementation; however, the analysis of successive subperiods (i.e. the periods between two consecutive determinations of DSL) has largely taken into account these variations, resulting in a more accurate determination of the DSL for each subperiod. The wide range of DSL2s was likely due to imponderable variations (1) in vitamin D metabolism, possibly linked to genetic variations, as described recently [Wang et al. 2010], (2) in compliance with vitamin D intake or even (3) in changes in the lifestyle habits of some patients, once they were aware of a potential role of the environment in the course of their disease. In contrast, the seasons were equally represented for the DSL1 (i.e. just prior to vitamin D supplementation) and the subsequent successive dosages during vitamin D supplementation also balanced a possible seasonal effect on the DSL2. Therefore, in spite of the aforementioned points, the fact remains that we found a strong significant effect of vitamin D status on the IR.
Reverse causality, i.e. the disease itself worsening the vitamin D insufficiency by limiting sunlight exposure, has been invoked to try to account for the correlations found between the spontaneous DSL and the relapse rate of RRMS patients in simple association studies. However, whatever the reason for vitamin D insufficiency, vitamin D supplementation in our study led to a dramatic increase in DSL, and the DSL itself was strongly associated with a decreased risk of relapses. Furthermore, the DSL level reached under supplementation was considerably higher than would be expected if the reduction in relapse rate had modified patients’ lifestyle habits regarding sunlight exposure. Therefore, reverse causality cannot reasonably be invoked to explain our results.
Comparison with previous studies
Our study shows a clear relationship between vitamin D status and the relapse rate of RRMS patients, as previously reported in several association studies [Van der Mei et al. 2007; Smolders et al. 2008b; Mowry et al. 2010; Simpson et al. 2010], but our study is the first so far to observe this finding after systematic vitamin D supplementation. Furthermore, the global quantitative effect of the DSL increase on the relapse reduction found here (13.7% decrease in IR for every 10 nmol/l increase in DSL in the multivariate analysis) confirms the quantitative predictions made in two recent association studies [Mowry et al. 2010; Simpson et al. 2010], in which broadly similar figures were reported in RRMS patients, most of whom were not being supplemented with vitamin D. It should be noted that in one of these studies [Simpson et al. 2010], most of the patients were also under first-line IMT, as in our cohort. However, the fundamental difference between our study and these previous studies is that almost all of our patients have to a greater or lesser extent eventually benefited from the vitamin D effect thanks to active, systematic supplementation. The overall result was a relatively low final IR in absolute terms, whatever the group (Table 2). However, this point will not be further commented on since it can only be ascertained through the use of RCTs. Furthermore, it remains to be determined whether vitamin D provides a significant immunomodulatory therapeutic effect by itself or potentiates the action of first-line IMT, as shown experimentally with interferon β [Van Etten et al. 2007].
Plateau effect
One of the most interesting and original quantitative points raised by our study is the plateau effect on the IRR observed for DSLs higher than 110–120 nmol/l, in the whole cohort as well as in Groups 1 and 2 (Figure 3). Interestingly, concerning the beginning of the plateau effect close to 110–120 nmol/l, it appears to be just over the proposed 75–100 nmol/l lower limit of the physiological extra-bone actions of vitamin D [Holick, 2004; Hollis, 2005; Vieth et al. 2007; Souberbielle et al. 2010]. Our study is the first to find a plateau effect of the action of vitamin D on the relapse rate of MS patients. This finding suggests that a much higher dose supplementation, resulting in very high DSLs (close to 400 nmol/l) [Burton et al. 2010], i.e. far beyond the physiological range, might not be useful from a therapeutic point of view. However, further studies are required to determine accurately the optimal zone within which the DSL should be if it is to have a favourable effect on the relapse rate in RRMS patients.
Conclusions
Taken together, these results suggest that vitamin D supplementation, likely via the anti-inflammatory and immunomodulatory properties of this vitamin, exerts a protective effect for relapses in RRMS patients concomitantly receiving IMTs. However, we were unable to differentiate between the role of the IMT and that of vitamin D supplementation; further RCTs are required to quantify accurately the specific vitamin D add-on effect. While awaiting the results of such clinical trials, which will not be available for several years, it appears wise to supplement all MS patients currently in a state of vitamin D insufficiency in order to bring their DSLs to just over the 100 nmol/l level, since such supplementation already seems unavoidable from a general medical point of view, is safe and might also be neurologically beneficial for the course of the disease.
Footnotes
Funding: This work received support from Bayer and Sanofi for the statistical analysis used in this study.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Contributor Information
Charles Pierrot-Deseilligny, Service de Neurologie 1, Hôpital de la Salpêtrière, Assistance Publique-Hôpitaux de Paris, Université Pierre et Marie Curie (Paris VI), Paris, France.
Sophie Rivaud-Péchoux, INSERM URMS 975, CNRS 7225, Université Pierre et Marie Curie (Paris VI), Paris, France.
Pierre Clerson, Orgamétrie biostatistiques, Roubaix, France.
Raphaël de Paz, Service de Neurologie 1, Hôpital de la Salpêtrière, Assistance Publique-Hôpitaux de Paris, Université Pierre et Marie Curie (Paris VI), Paris, France.
Jean-Claude Souberbielle, Service d’explorations fonctionnelles, Hôpital Necker-Enfants-Malades, Assistance Publique-Hôpitaux de Paris, Université René Descartes (Paris V), Paris, France.
References
- Ascherio A., Munger K.L. (2007) Environmental risk factors for multiple sclerosis. Part II: non-infectious factors. Ann Neurol 61: 504–513 [DOI] [PubMed] [Google Scholar]
- Ascherio A., Munger K.L., Simon K.C. (2010) Vitamin D and multiple sclerosis. Lancet Neurol 9: 599–612 [DOI] [PubMed] [Google Scholar]
- Bartosik-Psujek H., Tabarkiewicz J., Pocisnska K., Stelmasiak Z., Rolinski J. (2010) Immunomodulatory effects of vitamin D on monocyte-derived dendritic cells in multiple sclerosis. Mult Scler 16: 1513–1516 [DOI] [PubMed] [Google Scholar]
- Binkley N., Krueger D. (2008) Evaluation and correction of low vitamin D status. Curr Osteoporos Rep 6: 95-99 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H.A., Giovannucci E., Willett W.C., Dietrich T., Dawson-Hughes B. (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84: 18–28 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H.A., Willett W.C., Wong J.B., Stuck A.E., Staehelin B., Orav E.J., et al. (2009) Prevention of non-vertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 169: 551–561 [DOI] [PubMed] [Google Scholar]
- Burton J.M., Kimball S., Vieth R., Bar-Or A., Dosch H.M., Cheug R., et al. (2010) A phase I/II dose escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 74: 1852–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Trivino T., Mowry E.M., Gajofatto A., Chabas D., Crabtree-Hartman E., Cree B.A., et al. (2011) Switching multiple sclerosis patients with breakthrough disease to second-line therapy. PLoS One 6(2): e16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier E., Rozet E., Gadisseur R., Carlisi A., Monge M., Chapelle J.P., et al. (2010) Measurement uncertainty of 25-OH vitamin D determination with different commercially available kits: impact on the clinical cut offs. Osteoporos Int 21: 1047–1051 [DOI] [PubMed] [Google Scholar]
- Correale J., Ysrraelit M.C., Gaitan M.I. (2009) Immunomodulatory aspects of vitamin D in multiple sclerosis. Brain 132: 1146–1160 [DOI] [PubMed] [Google Scholar]
- Correale J., Ysrraelit M.C., Gaitan M.I. (2010) Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol 185: 4948-4958 [DOI] [PubMed] [Google Scholar]
- Dorr J., Ohlraun S., Skarabis H., Paul F. (2012) Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS Trial): study protocol for a randomized trial. Trials 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebers G.C. (2008) Environmental factors and multiple sclerosis. Lancet Neurol 7: 268–277 [DOI] [PubMed] [Google Scholar]
- Gajofatto A., Baccheti P., Grimes B., High A., Waubant E. (2009) Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler 15: 50–58 [DOI] [PubMed] [Google Scholar]
- Goldberg P., Fleming M.C., Picard E.H. (1986) Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses 21: 193–200 [DOI] [PubMed] [Google Scholar]
- Grant W.B., Holick M.F. (2005) Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev 10: 94–111 [PubMed] [Google Scholar]
- Haas J., Firzlaff M. (2005) Twenty-four-month comparison of immunomodulatory treatments – a retrospective open label study in 308 RRMS patients treated with beta interferons or glatiramer acetate (Copaxone®). Eur J Neurol 12: 425–431 [DOI] [PubMed] [Google Scholar]
- Hagenau T., Vest R., Gissel T.N., Poulsen C.S., Erlandsem M., Mosekilde L., et al. (2009) Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int 20: 133–140 [DOI] [PubMed] [Google Scholar]
- Hall L.M., Kimlin M.G., Aronov P.A., Hammock B.D., Slusser J.R., Woodhouse L.R. (2010) Vitamin D intake needed to maintain target serum 25-hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. J Nutr 140: 542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanwell H.E.C., Banwell B. (2011) Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta 1812: 202–212 [DOI] [PubMed] [Google Scholar]
- Heaney R. (2000) Vitamin D: how much do we need and how much is too much? Osteoporos Int 11: 553–555 [DOI] [PubMed] [Google Scholar]
- Heaney R.P., Davies K.M., Chen T.C., Holick M.F., Bager-Lux J. (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77: 204–210 [DOI] [PubMed] [Google Scholar]
- Heaney R.P., Holick M.F. (2011) Why the IOM recommendations for vitamin D are deficient. J Bone Min Res 26: 455–457 [DOI] [PubMed] [Google Scholar]
- Heaney R.P., Horst R.L., Cullen D.M., Armas L.A. (2009) Vitamin D3 distribution and status in the body. J Am Coll Nutr 28: 252–256 [DOI] [PubMed] [Google Scholar]
- Holick M.F. (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80(Suppl.): 1678S–1688S [DOI] [PubMed] [Google Scholar]
- Holick M.F. (2007) Vitamin D deficiency. N Engl J Med 357: 266–281 [DOI] [PubMed] [Google Scholar]
- Holick M.F. (2011) Evolutionary, physiological and health perspectives. Curr Drug Targets 12: 4-18 [DOI] [PubMed] [Google Scholar]
- Hollis B.W. (2005) Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 135: 317–322 [DOI] [PubMed] [Google Scholar]
- Ilahi M., Armas L.A.G., Heaney R.P. (2008) Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr 87: 688–691 [DOI] [PubMed] [Google Scholar]
- Ish-Shalom S., Segal E., Salganik T., Raz B., Bromberg I.L., Vieth R. (2009) Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab 93: 3430–3435 [DOI] [PubMed] [Google Scholar]
- Kampman M.T., Steffensen L.H., Mellgren S.I., Jørgensen L. (2012) Effect of vitamin D3 supplementation on relapses, disease progression and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kaufman M.D., Lee R., Norton H. (2011) Course of relapsing-remitting multiple sclerosis before, during and after natalizumab. Mult Scler 7: 90–94 [DOI] [PubMed] [Google Scholar]
- Lysandropoulos A.P., Jaquiéry E., Jilek S., Pantealo G., Schluep M., Du Pasquier R.A. (2011) Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and in healthy controls. J Neuroimmunol 233: 240–244 [DOI] [PubMed] [Google Scholar]
- Mahon B.D., Gordon S.A., Cruz J., Cosman F., Cantorna M.T. (2003) Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol 134: 128–132 [DOI] [PubMed] [Google Scholar]
- McDonald W.I., Compston A., Edan G., Goodkin D., Hartung H.P., Lublin F.D., et al. (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 50: 121–7 [DOI] [PubMed] [Google Scholar]
- Mowry E.M., Krupp L.B., Milazzo M., Chabas D., Strober J.B., Belman A.L., et al. (2010) Vitamin D status is associated with relapse rate in pediatric-onset MS. Ann Neurol 67: 618–624 [DOI] [PubMed] [Google Scholar]
- Munger K.L., Ascherio A. (2011) Prevention and treatment of MS: studying the effects of vitamin D. Mult Scler 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K.L., Levin L.I., Hollis B.W., Howard N.S., Ascherio A. (2006) Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296: 2832–2838 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C. (2009) Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol 256: 1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Souberbielle J.C. (2010) Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain 133: 1869–1888 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Souberbielle J.C. (2011) Widespread vitamin D insufficiency: a new challenge for primary prevention with particular reference to multiple sclerosis. Presse Médicale 40: 349–356 [DOI] [PubMed] [Google Scholar]
- Polman C.H., O’Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H., et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. New Engl J Med 354: 899–910 [DOI] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Edan G., Philippi M., Hartung H.P., Kappos L., et al. (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol 58: 840–846 [DOI] [PubMed] [Google Scholar]
- Ramagopalan S.V., Dyment D.A., Cader M.Z., Morrison K.M., Disanto G., et al. (2011) Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann Neurol 70: 881–886 [DOI] [PubMed] [Google Scholar]
- Ross A.C., Manson E.J., Abrams S.A., Aloia J.F., Brannon P.M., Steven K., et al. (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W., III, Mia Y., Li H., Nauton K. (2009) Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol 213: 135–141 [DOI] [PubMed] [Google Scholar]
- Schwalfenberg G.K., Genuis S.J., Hiltz M.N. (2010) Addressing vitamin D deficiency in Canada: a public health innovation whose time has come. Public Health 124: 350–359 [DOI] [PubMed] [Google Scholar]
- Simpson S., Taylor B., Blizzard L., Ponsonby A.L., Pittas F., Tremlett H., et al. (2010) Higher 25-hydroxyvitamin D is associated with lower relapse risk in MS. Ann Neurol 68: 193–203 [DOI] [PubMed] [Google Scholar]
- Smolders J., Damoiseaux J., Menheree P., Hupperts R. (2008a) Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 194: 7–17 [DOI] [PubMed] [Google Scholar]
- Smolders J., Hupperts R., Barkhof R., Grimaldi L.M., Holmoy T., Killestein J., et al. (2011a) Efficacy of vitamin D(3) as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta 1-a: a phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci 311: 44–49 [DOI] [PubMed] [Google Scholar]
- Smolders J., Menheere P., Kessels A., Damoiseaux J., Hupperts R. (2008b) Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 14: 1–5 [DOI] [PubMed] [Google Scholar]
- Smolders J., Moen S.M., Damoiseaux J., Huitinga I., Holmoy T. (2011b) Vitamin D in the healthy and inflamed central nervous system: access and function. J Neurol Sci 311: 37–43 [DOI] [PubMed] [Google Scholar]
- Smolders J., Peelen E., Thewissen M., Cohen Tervaet J.W., Menheere P., et al. (2010) Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One 5(12): e15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J., Thewissen M., Peelen E., Menheere P., Tervaert J.W., Damoiseaux J., et al. (2009) Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One 4: e6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilu-Hänninen M., Aivo J., Lindström B.M., Elovaara I., Sumelhati M.L., Färkkilä M., et al. (2012) A randomised, double blind, placebo controlled trial with vitamin D3 as an add-on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 83: 565–571 [DOI] [PubMed] [Google Scholar]
- Souberbielle J.C., Body J.J., Lappe J.M., Plebani M., Shoenfeld Y., Wang T.J., et al. (2010) Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 9: 709–715 [DOI] [PubMed] [Google Scholar]
- Van der Mei I.A., Ponsonby A.L., Dwyer T., Blizzard L., Taylor B.V., Kilpatrick T., et al. (2007) Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol 254: 581–590 [DOI] [PubMed] [Google Scholar]
- Van Etten E., Gysemans C., Branisteanu D., Verstuyf A., Bouillon R., Overbergh L., et al. (2007) Novel insights in the immune function of the vitamin D system: synergism with interferon-beta. J Steroid Biochem Mol Biol 103: 546–551 [DOI] [PubMed] [Google Scholar]
- Vieth R. (2006) What is the optimal vitamin D status for health? Prog Biophys Mol Biol 92: 26–32 [DOI] [PubMed] [Google Scholar]
- Vieth R., Bischoff-Ferrari H., Boucher B.J., Dawson-Hughes B., Garland C.F., Heaney R.P., et al. (2007) The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85: 649–650 [DOI] [PubMed] [Google Scholar]
- Wang T.J., Zhang F., Richards B., Kestenbaum B., van Meurs J.B., Berry D., et al. (2010) Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuck D.M., Lesaux J., Rice J.P., Kremenchtzky M., Ebers G.C. (2005) A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 76: 1294–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwekh J.E. (2008) Blood biomarkers on vitamin D status. Am J Clin Nutr 87: 1087S–1091S [DOI] [PubMed] [Google Scholar]