Abstract
We analyzed several studies of non-verbal communication (prosody and facial expressions) completed in our lab and conducted a secondary analysis to compare performance on receptive vs. expressive tasks by adolescents with ASD and their typically developing peers. Results show a significant between-group difference for the aggregate score of expressive tasks, but not for the aggregate score of receptive tasks. There was also a significant within-group difference among individuals with ASD for expressive vs. receptive performance. Our data indicate that adolescents with ASD can achieve receptive accuracy in non-verbal communication, but show significant qualitative deficits in expressive skills across a range of tasks, which may have a significant negative impact on their success as social communicators.
Keywords: Autism, Prosody, Facial Expressions, Expressive, Receptive
One of the hallmark characteristics of individuals with autism spectrum disorders (ASD) is their difficulty understanding and producing non-verbal aspects of social communication, such as prosody and facial expressions (Kanner, 1943). More recent studies, however, have shown that the abilities of individuals with ASD in non-verbal communication are significantly more nuanced. Several studies of prosody have documented deficits in lexical stress, or affective and grammatical marking in expressive and receptive modalities (Diehl, Watson, Bennetto, McDonough, & Gunlogson, 2009; Paul, Augustyn, Klin, & Volkmar, 2005; Peppé, McCann, Gibbon, O'Hare, & Rutherford, 2007; Shriberg et al., 2001), while others have shown preserved abilities to process emotional prosody or produce lexical stress (Boucher, Lewis, & Collis, 2000; Grossman, Bemis, Plesa Skwerer, & Tager-Flusberg, 2010) Similarly, some studies of facial expressions have documented deficits in the ability to decode emotions from faces (Adolphs, Sears, & Piven, 2001; Boucher, et al., 2000; Celani, Battacchi, & Arcidiacono, 1999), particularly if the facial expressions are more complex (Golan, Baron-Cohen, & Golan, 2008). Other studies, however, have revealed facial emotion recognition skills equal to those of their typically developing (TD) peers (Gepner, Deruelle, & Grynfeltt, 2001; Grossman, Klin, Carter, & Volkmar, 2000; Rosset et al., 2008). In addition to studies showing quantifiable differences in non-verbal communication, individuals with ASD have also been shown to exhibit facial and vocal expressions that are perceived as qualitatively “odd” (Grossman et al., 2008; Macdonald et al., 1989; Yirmiya, Kasari, Sigman, & Mundy, 1989).
Most studies of nonverbal communication in ASD focus on only one or a few specific aspects of facial expressions or prosody in a single modality. Even when performance is elicited across receptive and expressive modalities, the results can only inform our understanding of the individual skill tested, such as lexical prosody, or communicative facial expressions. In order to understand whether performances of individuals with ASD on these individual tasks are related to an underlying deficit in non-verbal communication, we must look at their performance across a range of expressive and receptive tasks.
The purpose of this investigation was to conduct a cross-study analysis of several studies of non-verbal communication conducted in our lab. We wanted to determine the pattern of competence for receptive vs. expressive skills in a range of prosody, facial expression, and auditory-visual (AV) integration tasks for a small group of adolescents with high-functioning autism who had participated in several of our studies over the course of three years. Based on existing documentation of the “odd” nature of facial and vocal expressions in this population, our hypothesis was that the cross-study analysis would reveal general deficits for a group of individuals with high-functioning ASD compared to a group of their TD peers in the expressive, but not the receptive modality of non-verbal language.
Method
Participants
We selected data from participants who shared the same inclusion and exclusion criteria and had successfully completed at least three out of six studies of non-verbal communication conducted over the course of three years. These criteria were used to obtain data from as many participants as possible who had participated in a large number of the studies involved. This method allowed us to analyze data for several individuals across a range of studies, rather than attempting to interpret data of participants who had participated in only one or two of the studies included. Two groups were included in this analysis: children and adolescents with ASD (N=7 or 11, depending on task) and typically developing TD controls (N=5 or 6 depending on task) ranging from 9–18 years old. Inclusion criteria for participant with ASD were meeting criteria for ASD or autism on the ADOS, confirmed by clinical impression and inclusion for TD participants was determined by a lack of developmental delays or differences in social or communication ability based on standardized testing. Exclusion criteria for both groups were: frank neurological diseases (other than ASD), cerebral palsy, genetic disorders, significant dysmorphology without diagnosis, mental retardation, or mild to moderate hearing loss in at least one ear. Participants were originally recruited through local schools, advertisements placed in local magazines, newspapers, the internet, advocacy groups for families of children with autism, and word of mouth.
Standardized testing
The Kaufman Brief Intelligence Test, Second Edition (K-BIT 2; Kaufman & Kaufman, 2004) was used to assess IQ, receptive vocabulary ability was measured by the Peabody Picture Vocabulary Test (PPVT-III; Dunn & Dunn, 1997), and reading ability by the Woodcock-Johnson III Diagnostic Reading Battery (WJ III DRB, Woodcock, Mather, & Schrank, 2004). All participants had IQ and receptive vocabulary scores within the normal range (Table 1). Using a multivariate ANOVA with group as the independent variable we verified that the ASD and TD groups did not differ significantly in age, (F (1,16) = .17, p = .68), verbal IQ (F (1,16) = .72, p = .41), nonverbal IQ (F (1,16) = .21, p = .65), receptive vocabulary (F (1,16) = .16, p = .7), and reading skills (F (1,13) = 1.23, p = .29). A chi-squared analysis showed that the groups did not differ in the distribution of gender (χ2 (1, N = 17) = 2, p = .52).
Table 1.
Descriptive Characteristics of Participant groups
| ASD (n=11) M(SD) |
TD (n=6) M(SD) |
|
|---|---|---|
| Age | 13:7 (2:11) Range: 9:5 – 18:10 |
14:2 (2:5) Range: 9:5 – 15:8 |
| Sex | 8 male, 3 female | 6 male, 0 female |
| Verbal IQ | 104.18 (19.57) | 112.33 (17.5) |
| Nonverbal IQ | 113.73 (10.11) | 116.17 (11.3) |
| PPVT-III | 108.03 (31.56) | 113.33 (9.5) |
| WJ III DRB | 104 (11.59) | 112.33 (16.66) |
Diagnosis of ASD
Participants in the ASD group met DSM-IV criteria for autistic disorder, based on expert clinical impression and confirmed by the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994) and the Autism Diagnostic Observation Schedule (ADOS) Module 3 (Lord, Rutter, DiLavore, & Risi, 1999), which were administered by trained examiners. Participants with known genetic disorders were excluded. Based on their ADOS scores, nine participants met criteria for autism and two met criteria for ASD.
Measures included
The data shown here represent a synthesis of nine measures taken from six studies conducted at our lab. We selected the measures that represented the central data focus of each study. The methods and results for each measure and study are described here and summarized in Table 2.
Table 2.
Summary of measures
| Measure | Expressive/ Receptive |
Study # | Main Finding |
|---|---|---|---|
| Facial expression awkwardness (4-point code) | Expressive | 1 | ASD more awkward than TD (but accurate) |
| Prosody awkwardness (4 point code) | Expressive | 1 | ASD more awkward than TD (but accurate) |
| Utterance length for noun phrases (milliseconds) | Expressive | 2 | ASD longer than TD (but accurate) |
| Utterance length for compound nouns (milliseconds) | Expressive | 2 | ASD longer than TD (but accurate) |
| Onset asynchrony detection, 10 frames (accuracy) | Receptive | 3 | ASD as accurate as TD |
| Onset asynchrony detection, 12 frames (accuracy) | Receptive | 3 | ASD as accurate as TD |
| Face-Voice matching, low-intensity & within-valence (accuracy) | Receptive | 4 | ASD less accurate than TD |
| Emotional facial expression sequencing (accuracy) | Receptive | 5 | ASD less accurate than TD |
| Emotional facial expression sequencing without eyes (accuracy) | Receptive | 6 | ASD as accurate as TD (accuracy low for both groups) |
1. Production of emotional facial and vocal expressions
We analyzed emotional communicative facial and vocal (prosody) expressions of adolescents with ASD elicited during a story-retelling task of four brief stories. Each story contained at least one sentence with happy, fearful, angry, and positive surprise emotion. Fifteen adolescents with ASD and 12 TD controls watched each story and retold it to a camera, using the printed text to assist in retrieval. We edited the resulting videos to obtain separate audio and video clips containing a single emotion each and coded each clip for awkwardness of the expressed emotion. The purpose of this measure was to capture the qualitative differences in production of non-verbal communication by individuals with ASD, who were rated as significantly more awkward than their TD peers in facial and vocal expressions (Grossman, et al., 2008).
2. Production of lexical stress
We elicited lexical stress prosody productions of homophone compound nouns and noun phrases (HOTdog vs. hot DOG) from 16 adolescents with ASD and 15 TD controls. Audio recordings of participants productions were analyzed for whole word length, which is expected to be longer for noun phrases than compound nouns. The purpose of this measure was to determine whether individuals with ASD could accurately differentiate two types of lexical stress, as well as to capture the acoustic differences underlying that differentiation. Participants with ASD accurately expressed the two versions of each stimulus, but had significantly longer productions than their TD peers (Grossman, et al., 2010).
3. Perception of auditory-visual speech synchrony
We determined whether 25 adolescents with ASD and 25 TD controls could detect onset asynchrony of speech through auditory-visual (AV) integration. We used 12 video clips and digitally separated the audio from the video track to slip them out of synch by 4, 6, 8, 10, 12, and 14 frames. Our data showed that at least 10 frames were required to achieve greater than chance level accuracy for both groups. The purpose of this measure was to determine whether individuals with ASD were able to detect AV asynchrony in conditions that allowed for reliable detection by their TD peers (10 and 12 frames). There were no significant group differences for any slip rate (Grossman, Schneps, & Tager-Flusberg, 2009).
4. Receptive face-voice matching of emotional expressions
We investigated whether individuals with ASD could match emotional voices to emotional facial expressions when emotional intensity was low. We recorded semantically neutral sentences in two positive (happiness, surprise) and two negative (anger, sadness) emotions with high and low emotional intensity. Twenty-two adolescents with ASD and 22 TD controls matched each sentence to one of two facial expressions, which were either differentiated across-valence (e.g. happy and sad) or within-valence (e.g. sad and angry). The purpose of this measure was to assess whether individuals with ASD were vulnerable to manipulations of difficulty in both the auditory and visual components of an emotional face-voice matching task. Participants with ASD were significantly less accurate than TD peers for trials with low emotional intensity and within-valence face contrasts (Grossman, Kennedy, & Tager-Flusberg, 2009).
5. Sequencing of emotional facial expressions
We examined whether individuals with ASD could recreate the dynamic sequence of emotional facial expressions. We recorded a female actor portraying five basic emotions and extracted six still images from each video. We provided 25 adolescents with ASD and 15 TD participants with the first and last frame of each sequence and asked them to place the remaining four images into the correct sequence. The purpose of this measure was to ascertain the familiarity of individuals with ASD with the dynamic sequences of emotional facial expressions. The ASD group was significantly less accurate at sequencing emotional facial expressions than the TD group (Grossman & Tager-Flusberg, 2008).
6. Sequencing of emotional facial expressions without eyes
We replicated study #5, but masked the eyes on the photographs. The purpose of this measure was to determine whether the performance of individuals with ASD in this task changed when eye information was eliminated. We found no significant group differences for sequencing of emotional facial expressions without eyes in 22 participants with ASD and 22 TD controls (Grossman & Tager-Flusberg, 2008).
Data analysis
We created z-scores for each measure to compare across tasks. Each participants data were normalized by subtracting the TD groups mean and dividing the result by the TD groups standard deviation (individual_result – TD_group_mean/TD_group_StDev). We used the TD groups means and standard deviations to establish z-scores since the TD group represents the normative behavior on each task. The purpose of calculating z- scores was to establish whether the ASD group deviates significantly from the normative performance across tasks, which is why the TD mean was used to establish both groups z-scores. We then created aggregate values by calculating the mean z-score of the four expressive measures and the mean z-score of the five receptive tasks for each participant.
Results
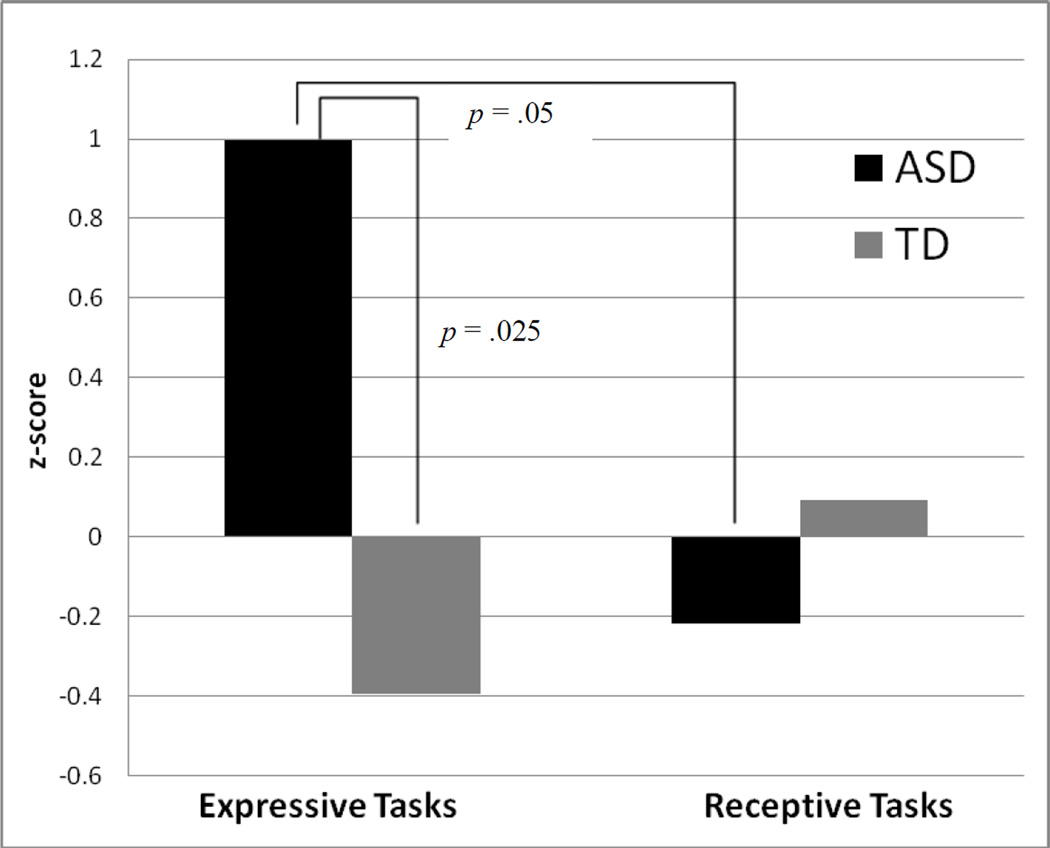
We conducted an across-group comparison of the aggregate expressive and receptive scores (Figure 1). A Wilcoxon Rank Sums test revealed that the ASD group had significantly larger z-scores than the TD group for the expressive aggregate (W (nASD = 7, nTD = 5) = 16, p = .025), but not the receptive aggregate (W (nASD = 11, nTD = 6) = 66, p = .25) indicating a greater deviation from the expected norm for expressive tasks by individuals with ASD than their TD peers. We also conducted within-group analyses to establish whether either group showed differences in z-scores for expressive vs. receptive tasks. A Wilcoxon Signed Rank Test showed that the ASD group had significantly larger z-scores for the expressive than the receptive aggregate (S = 12, p = .05), indicating that participants with ASD had significantly greater deviation from the typical mean for expressive tasks than receptive tasks. The TD group showed no such difference (S = −5.5, p = .19).
Figure 1.
Aggregate z-scores for expressive vs. receptive tasks
Discussion
The purpose of our study was to determine differences for receptive vs. expressive non-verbal communication skills between adolescents with ASD and their TD peers across a range of studies. Our hypothesis was that adolescents with ASD would show greater deviation from expected performance in expressive than receptive tasks. The data presented here clearly support that hypothesis.
Receptive ability is usually described in form of accuracy, as was the case for the five measures included in the receptive aggregate. Expressive ability, however, is more difficult to capture, particularly for non-verbal language, and is often described using qualitative codes. Two of our measures constituting the expressive aggregate were based on perceptual coding using a four-point scale (“natural,” “slightly awkward,” “moderately awkward,” “unnatural”) and two captured utterance length in milliseconds. These measures could be perceived as more open-ended than measures of accuracy and the argument could be made that they allow for greater individual variation, thereby explaining the differences in z-scores between expressive and receptive aggregates in the ASD group. However, the TD individuals in our analysis did not show this significant difference between expressive and receptive aggregates. TD adolescents, despite a certain level of individual expressive variation, and despite the open-ended nature of the measures used, still produce facial and vocal expressions within a fairly narrow measurable range, while their peers with ASD demonstrate significantly larger deviations from that expected range.
The expressive tasks from which the measures for this analysis were taken also included measures of accuracy, which were not included in this analysis. These measures showed that individuals with ASD were at least as capable as their TD peers at producing accurate facial and vocal emotional expressions, as well as lexical stress. The significant differences in the expressive aggregate scores presented here are therefore based on productions that were categorically accurate, but qualitatively very different from those of their TD peers. Qualitative differences, in these expressive stimuli, refer to productions that were either perceived as awkward in perceptual coding or significantly longer in timing measures, despite being categorically accurate in all cases. Furthermore, these qualitative differences were evident in an analysis based on the combined scores of tasks involving lexical stress, emotional prosody, and emotional facial expressions, indicating that qualitative expressive differences are found in a range of expressive non-verbal tasks and not just related to one aspect of non-verbal communication, such as facial vs. vocal expression, or emotional vs. grammatical marking. This fundamental and cross-skill qualitative expressive difference is what appears to drive the significant between-group differences for the expressive aggregate, as well as the within-group difference for participants with ASD for the expressive vs. receptive aggregates.
Limitations and clinical implications
The data presented here are based on a small group of participants and only a few studies, making it difficult to generalize our results to the general population of adolescents with ASD or all aspects of non-verbal language competence. It must also be pointed out that the studies presented here were not specifically designed for a receptive-expressive comparison. Future studies should include more participants across a wider range of matched expressive and receptive tasks to determine whether these within- and across-group qualitative differences for expressive measures remain consistent. It has been suggested that expressive prosody that is qualitatively poor may have a significant negative impact on the social success of a speaker, despite preserved intelligibility (Peppé, 2009). Our data show that this concept can be expanded across a range of non-verbal communication skills. Individuals with ASD can produce facial expressions and prosody that accurately express their intent, but are nevertheless atypical or awkward. Future research in this area should focus on qualitative measures of non-verbal communication and determine better ways to describe the complex features that create this perceived awkwardness across all aspects of non-verbal communication so we can ultimately devise intervention programs to improve the social appropriateness of facial and vocal expressions in this population.
Highlights.
We compared performance across receptive and expressive non-verbal tasks
Participants were adolescents with high-functioning autism and typical peers
Adolescents with ASD may have preserved receptive nonverbal skills
Adolescents with ASD have significant qualitative deficits in nonverbal expression
Qualitative expressive differences may create a barrier to social communication
Acknowledgements
The authors wish to thank Yavni Bar-Yam, Rhyannon Bemis, Steven Borawksi, Karen Condouris, Chris Connolly, Sarah Delahanty, Danielle Delosh, Curtis Deutsch, Bob Duggan, Alex B. Fine, Kerri Green, Alex Griswold, Ann Hunt, Robert M. Joseph, Meaghan Kennedy, Margaret Kjelgaard, Maria Kobrina, Janice Lomibao, Toby McElheny, Geraldine Owen, Loren Rubinstein, and Matthew Schneps for their assistance in stimulus creation, task administration, and data analysis. We also thank the children and families who gave their time to participate in this study. Funding was provided by NAAR, NIDCD (U19 DC03610; H. Tager-Flusberg, PI) which is part of the NICHD/NIDCD Collaborative Programs of Excellence in Autism, and by grant M01-RR00533 from the General Clinical Research Ctr. program of the National Center for Research Resources, National Institutes of Health. The corresponding author is currently supported by NIDCD (R21 DC010867-01; R Grossman, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A version of this paper was presented as a poster at the International Meeting for Autism Research in May 2011.
References
- 1.Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism [doi: 10.1162/089892901564289] Journal of Cognitive Neuroscience. 2001;13(2):232–240. doi: 10.1162/089892901564289. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- 2.Boucher J, Lewis V, Collis GM. Voice processing abilities in children with autism, children with specific language impairments, and young typically developing children. Journal of Child Psychology and Psychiatry. 2000;41(7):847–857. [PubMed] [Google Scholar]
- 3.Celani G, Battacchi MW, Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. J Autism Dev Disord. 1999;29(1):57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- 4.Diehl JJ, Watson D, Bennetto L, McDonough J, Gunlogson C. An acoustic analysis of prosody in high-functioning autism. Applied Psycholinguistics, Published Online by Cambridge University Press 21 May. 2009 [Google Scholar]
- 5.Dunn LM, Dunn LM. Peabody picture vocabulary test. 3 ed. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 6.Gepner B, Deruelle C, Grynfeltt S. Motion and emotion: A novel approach to the study of face processing by young autistic children. Journal of Autism & Developmental Disorders. 2001;31(1):37–45. doi: 10.1023/a:1005609629218. [DOI] [PubMed] [Google Scholar]
- 7.Golan O, Baron-Cohen S, Golan Y. The ‘reading the mind in films’ task [child version]: Complex emotion and mental state recognition in children with and without autism spectrum conditions. Journal of Autism and Developmental Disorders. 2008;38(8):1534–1541. doi: 10.1007/s10803-007-0533-7. doi: 10.1007/s10803-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 8.Grossman JB, Klin A, Carter AS, Volkmar FR. Verbal bias in recognition of facial emotions in children with asperger syndrome. Journal of Child Psychology and Psychiatry. 2000;41(3):369–379. [PubMed] [Google Scholar]
- 9.Grossman RB, Bemis RH, Plesa Skwerer D, Tager-Flusberg H. Lexical and affective prosody in children with high-functioning autism. Journal of Speech, Language and Hearing Research. 2010;53(3):778. doi: 10.1044/1092-4388(2009/08-0127). (716). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman RB, Edelson L, Rubinstein L, Lomibao J, Borawski S, Tager-Flusberg H. Production of emotional prosody and facial expressions in adolescents with autism; Paper presented at the International Meeting for Autism Research; May 2008; London, England. 2008. [Google Scholar]
- 11.Grossman RB, Kennedy M, Tager-Flusberg H. "Who said that?" affective face and voice matching in adolescents with autism; Paper presented at the 2009 International Meeting for Autism Research; Chicago, IL. 2009. [Google Scholar]
- 12.Grossman RB, Schneps MH, Tager-Flusberg H. Slipped lips: Onset asynchrony detection of auditory-visual language in autism. Journal of Child Psychology and Psychiatry. 2009;50(4):491–497. doi: 10.1111/j.1469-7610.2008.02002.x. doi: JCPP2002 [pii] 10.1111/j.1469-7610.2008.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman RB, Tager-Flusberg H. Reading faces for information about words and emotions in adolescents with autism. Research in Autism Spectrum Disorders. 2008;2(4):681–695. doi: 10.1016/j.rasd.2008.02.004. doi: 10.1016/j.rasd.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 15.Kaufman A, Kaufman N. Manual for the kaufman brief intelligence test. second edition. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 16.Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule - wps (ados-wps) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- 17.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald H, Rutter M, Howlin P, Rios P, Conteur AL, Evered C, et al. Recognition and expression of emotional cues by autistic and normal adults. Journal of Child Psychology and Psychiatry. 1989;30(6):865–877. doi: 10.1111/j.1469-7610.1989.tb00288.x. doi: 10.1111/j.1469-7610-989.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 19.Paul R, Augustyn A, Klin A, Volkmar FR. Perception and production of prosody by speakers with autism spectrum disorders. J Autism Dev Disord. 2005;35(2):205–220. doi: 10.1007/s10803-004-1999-1. [DOI] [PubMed] [Google Scholar]
- 20.Peppé S, McCann J, Gibbon F, O'Hare A, Rutherford M. Receptive and expressive prosodic ability in children with high-functioning autism. Journal of Speech Language & Hearing Research. 2007;50(4):1015–1028. doi: 10.1044/1092-4388(2007/071). [DOI] [PubMed] [Google Scholar]
- 21.Peppé SJE. Why is prosody in speech-language pathology so difficult? International Journal of Speech-Language Pathology. 2009;11(4):258–271. [Google Scholar]
- 22.Rosset D, Rondan C, Da Fonseca D, Santos A, Assouline B, Deruelle C. Typical emotion processing for cartoon but not for real faces in children with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(5):919–925. doi: 10.1007/s10803-007-0465-2. doi: 10.1007/s10803-007-0465-2. [DOI] [PubMed] [Google Scholar]
- 23.Shriberg LD, Paul R, McSweeny JL, Klin AM, Cohen DJ, Volkmar FR. Speech and prosody characteristics of adolescents and adults with high-functioning autism and asperger syndrome. J Speech Lang Hear Res. 2001;44(5):1097–1115. doi: 10.1044/1092-4388(2001/087). [DOI] [PubMed] [Google Scholar]
- 24.Woodcock RW, Mather N, Schrank FA. Woodcock-johnson iii diagnostic reading battery. Rolling Meadows, IL: Riverside Publishing; 2004. [Google Scholar]
- 25.Yirmiya N, Kasari C, Sigman M, Mundy P. Facial expressions of affect in autistic, mentally retarded and normal children. Journal of Child Psychology and Psychiatry. 1989;30(5):725–735. doi: 10.1111/j.1469-7610.1989.tb00785.x. doi: 10.1111/j.1469-7610.1989.tb00785.x. [DOI] [PubMed] [Google Scholar]