Abstract
Background:
To investigate the clinical relevance of CK-19mRNA-positive circulating tumour cells (CTCs) detected before the initiation of front-line treatment in patients with metastatic breast cancer (MBC).
Methods:
The presence of CTCs was detected in 298 patients with MBC using a real-time PCR (RT-PCR assay. In 44 patients, the detection of CTCs was evaluated by both the CellSearch and the RT-PCR assay. Interaction with known prognostic factors and association of CTCs with clinical outcome were investigated.
Results:
There was a strong correlation between the detection of CTCs by both assays. CK-19mRNA-positive CTCs were detected in 201 (67%) patients and their detection was independent of various patients’ clinico-pathological characteristics. The median progression-free survival (PFS; 9.2 vs 11.9 months (mo), P=0.003) and the overall survival (OS; 29.7 vs 38.9 mo, P=0.016) were significantly shorter in patients with detectable CK-19mRNA-positive CTCs compared with patients without detectable CTCs. Multivariate analysis demonstrated that oestrogen receptor status, performance status and detection of CTCs were emerged as independent prognostic factors associated with decreased PFS and OS.
Conclusion:
The detection of CK-19mRNA-positive CTCs in patients with MBC before front-line therapy could define a subgroup of patients with dismal clinical outcome.
Keywords: breast cancer, CK-19mRNA, tumour cells
The prognosis of patients with metastatic breast cancer (MBC) mainly depends on several patient and tumour characteristics such as age, hormone receptor and HER2 status, histological grade, site of metastasis and performance status, (PS; Chung and Carlson, 2003; Ellis et al, 2004), which in the daily practice influence clinical decisions.
Recent studies have shown that the detection of cytokeratin-expressing (CK+) cancer cells in the blood (circulating tumour cells (CTCs)) or bone marrow (disseminated tumour cells) also holds significant prognostic information for patients with breast cancer. Indeed, the detection of peripheral blood CK-19mRNA-positive CTCs in patients with early disease has been proven as an independent prognostic factor for reduced disease-free survival (DFS) and overall survival (OS) (Stathopoulou et al, 2002, 2003; Xenidis et al, 2003, 2006, 2009; Ignatiadis et al, 2007). Moreover, a pooled analysis showed that bone marrow CK-positive disseminated tumour cells, detected in 31% of stage I–III breast cancer patients, have an independent prognostic impact (Braun et al, 2005). Similarly, in the metastatic setting, Cristofanilli et al (2004), using the CellSearch platform, reported that the detection of ⩾5 CTCs/7.5 ml blood at baseline and before the administration of front-line or second-line treatment was associated with a poor clinical outcome both in terms of progression-free survival (PFS) and OS. These findings were further confirmed by several other investigators using either the CellSearch (Cristofanilli et al, 2007; Dawood et al, 2008; Nole et al, 2008) or immunocytochemical assays (Bidard et al, 2008). In addition, the prognostic value of CTCs, as detected by the CellSearch system, was unrelated to hormonal receptor status, site of recurrence, previous treatments or the tumour burden (Cristofanilli et al, 2007; Dawood et al, 2008; Nole et al, 2008).
Our group, using a molecular (real-time PCR (RT-PCR)) assay, based on the detection of CK-19mRNA transcripts in peripheral blood mononuclear cells (PBMCs) of patients with early-stage breast cancer (Stathopoulou et al, 2003), demonstrated in a cohort of 444 patients that their detection was an independent factor associated with an increased risk of relapse and disease-related death (Xenidis et al, 2003, 2006; Ignatiadis et al, 2007). In addition, it was shown that adjuvant chemotherapy does not always eliminate CK-19mRNA-positive CTCs, and that patients with chemotherapy-resistant CTCs have an extremely reduced median DFS and OS (Xenidis et al, 2009). As clinical relapses are believed to be originated from these chemotherapy- and hormone-resistant CK-19mRNA-positive CTCs (Xenidis et al, 2007), we sought to determine the clinical relevance of the detection of these cells in patients with newly diagnosed MBC, before the initiation of front-line chemotherapy, using the same RT-PCR assay.
Patients and methods
Patients and clinical samples
A longitudinal trial for the study of micrometastatic disease in breast cancer is ongoing in our Department since 1997. All consenting patients are assessed for the presence of CK-19mRNA CTCs as part of their laboratory evaluation at the time of staging of their recurrent or newly diagnosed metastatic disease and before starting any systemic treatment. In this retrospective, single-centre study, a total of 298 consecutive patients with MBC and sample available for CK-19mRNA determination, who were treated in our Department with first-line chemotherapy during the period from February 1997 to June 2009 were included in the current analysis. During the same period, 393 patients were treated with first-line chemotherapy. Patients who received front-line hormonal therapy were excluded from this analysis. Patients had to have measurable or evaluable disease and a PS of 0–3 (ECOG). Prior adjuvant chemotherapy was allowed, provided that disease relapse occurred at least 12 months (mo) after its completion. Patients presenting a clinical relapse during adjuvant hormone therapy were also eligible, provided that it occurred at least 12 mo after the completion of adjuvant chemotherapy. The oestrogen receptor (ER) and the progesterone receptor (PR) status of primary tumours were determined by immunocytochemistry. The assessment of HER2 expression was evaluated using the HercepTest (Dako, Glostrup, Denmark) using the DAKO-score guidelines, and samples with score 2+ were further analysed by FISH. All patients were treated with systemic chemotherapy as front-line treatment for their metastatic disease; there was no patient who received front-line hormone treatment. Patients were treated with various standard chemotherapy regimens decided by their responsible physicians according to the disease characteristics; responsible physicians had no access to CTC results. A total of 32 (10.7%) patients received either FEC or AC, 104 (34.9%) a combination of taxane plus anthracycline, 125 (41.9%) a non-anthracycline taxane-based regimen and 37 (12.4%) other regimens; finally, 41 (13.8%) patients received trastuzumab combined with chemotherapy (a taxane-based regimen in 31 and vinorelbine in 10 patients). All clinical data were prospectively collected.
Before the initiation of front-line treatment, patients had complete clinical, biochemical and imaging staging (including computed tomography scans of chest and abdomen, as well as a whole-body bone scan) and serum levels of tumour markers (CEA and/or CA 15.3). Response to treatment was assessed according to the World Health Organization criteria (Miller et al, 1981) with the same techniques that were used at baseline, without knowledge of the CTCs’ results.
Peripheral blood (20 ml in EDTA) was obtained at the middle of vein puncture before the initiation of chemotherapy for the detection of CTCs using the molecular assay, whereas 7.5 ml of blood in CellSave_tubes were used for detection of CTCs using the CellSearch system (Veridex Corporation, Warren, NJ, USA). All patients provided a written informed consent to participate in the study, which had been approved by the Ethics and Scientific Committees of our Institution.
Detection of CTCs by CellSearch
Samples were maintained at room temperature and processed within 24 h. All evaluations were carried out with no knowledge of the patient’s clinical status. The standardised CellSearch technique for the detection of CTCs in whole blood has been reported previously (Cristofanilli et al, 2004). Briefly, CTCs expressing the epithelial cell adhesion molecule were immunomagnetically enriched and stained with 4,2-diamidino-2-phenylindole dihydrochloride, cytokeratins 8,18,19 and CD45. Circulating tumour cells morphology was confirmed in all cases. Quantitative results were expressed as number of CTCs per 7.5 ml blood.
RNA extraction and real-time RT-PCR assay for CK-19mRNA-positive cells
Peripheral blood mononuclear cells were obtained by Ficoll-Hypaque gradient-density centrifugation within 2–4 h from vein puncture, and total RNA isolation was carried out in a laminar flow hood under RNAse-free conditions by using Trizol (Invitrogen, Grand Island, NY, USA; Stathopoulou et al, 2002). The isolated RNA was dissolved in RNA storage buffer (Ambion, Grand Island, NY, USA) and stored at −80 oC until used. RNA concentration was determined by absorbance reading at 260 nm with the NanoDrop spectophotometer (Wilmington, DE, USA) at the time of CK-19mRNA analysis. RNA integrity was tested by PCR amplification of the β-actin housekeeping gene. RNA samples prepared from the MCF-7 breast cancer and ARH-77 leukaemic cell lines were used as positive and negative controls, respectively (Stathopoulou et al, 2002).
Reverse transcription of RNA was carried out by synthesising cDNA using the Superscript III Platinum Two-Step qRT-PCR Kit (Invitrogen) according to the manufacturer’s instructions; the real-time RT-PCR assay, performed in a total volume of 10 μl in the LightCycler glass capillaries, for the detection of CK-19mRNA-positive cells and its analytical methodological details (specificity, sensitivity, cut-off for positivity), the used primers and probes, as well as the cycling protocol have been previously described (Stathopoulou et al, 2003). The quality of cDNAs was always evaluated by real-time PCR using the β-actin housekeeping gene. The lower detection limit for positivity of the assay was determined to be ⩾0.6 MCF-7 cell equivalents/5 μg RNA for the patients’ PBMCs (Stathopoulou et al, 2003). Using this cut-off value, only 2 out of 89 healthy individuals tested were found positive (Stathopoulou et al, 2003).
Statistical analysis
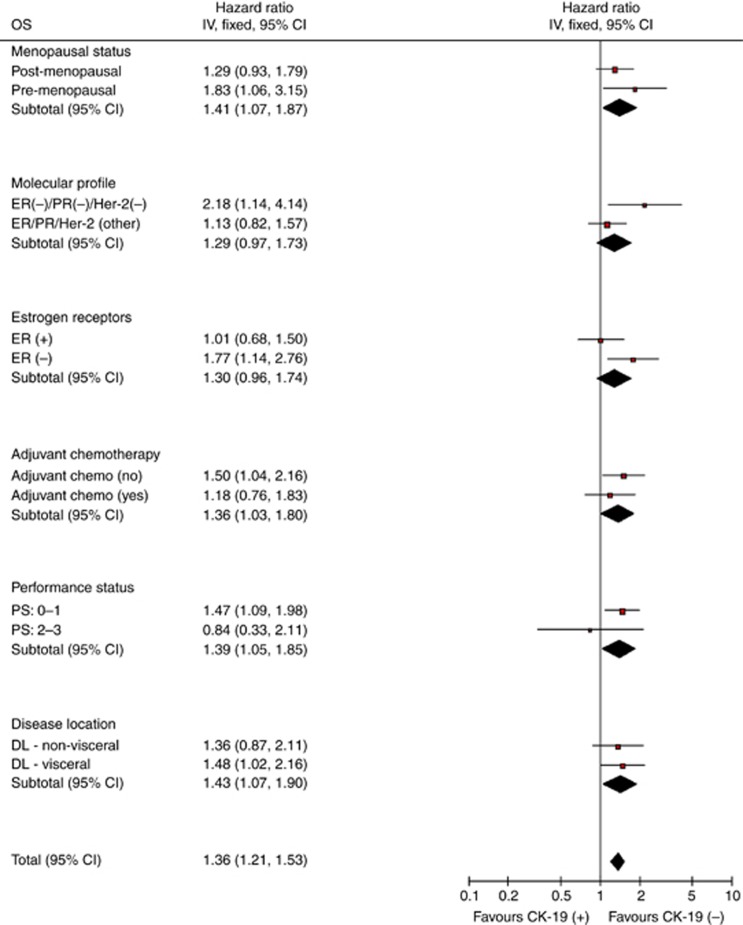
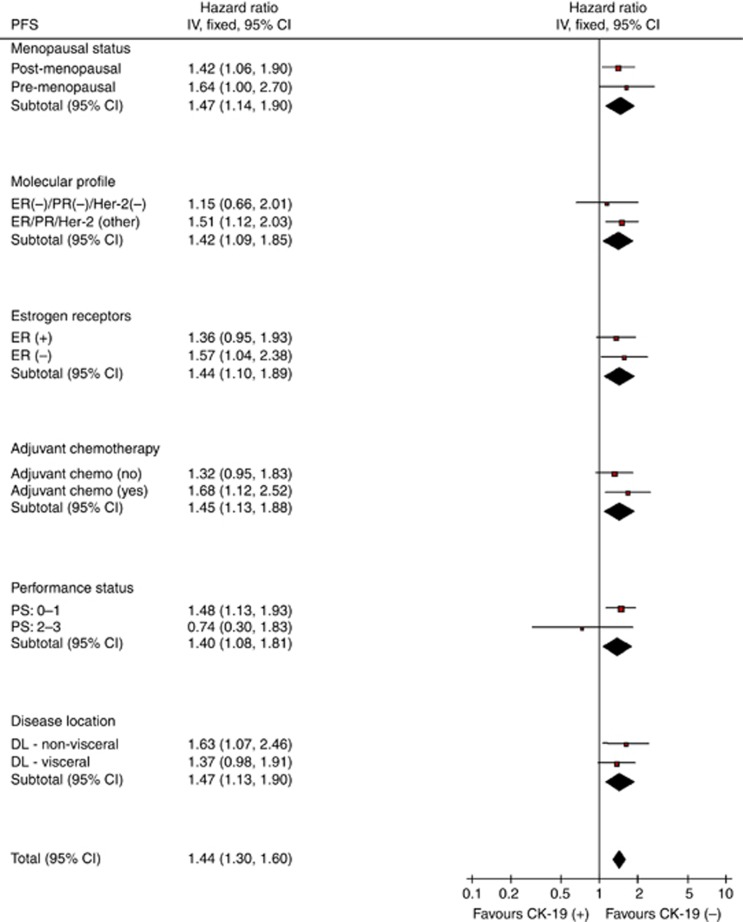
This is a retrospective analysis aiming to investigate the clinical relevance of the detection of CK-19mRNA-positive CTCs, evaluated prospectively and blindly, at the time of diagnosis of metastatic disease before the initiation of any systemic treatment. Therefore, there was no predetermined sample size estimation in this study because of its observational nature. Progression-free survival was defined as the time elapsed between the date of the administration of the first chemotherapy cycle, and either the date of clinical or radiological progression, or death or the last follow-up. Overall survival was measured from the date of the administration of the first chemotherapy cycle until the date of death or last follow-up. To detect significant differences between survival curves, Log-rank or Breslow tests were used. Cox proportional hazards model for time to event data was used to evaluate the hazard of every important variable, as well as for the construction of a multivariate model, explaining the relative significant influence of the covariates on outcomes (unconditional backwards procedure). The interaction between the prognostic value of the detection of CK-19mRNA and known clinico-pathological prognostic factors was tested using the heterogeneity tests for OS and PFS (Figures 1 and 2). Analysis included contingency tables and χ2-tests for categorical variables used to assess differences between groups. All statistical tests were carried out at the 5% significance level using the statistical software SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Figure 1.
Forest plot: overall survival for CK-19mRNA pre-chemotherapy.
Figure 2.
Forest plot: Progression-free survival for CK-19 pre-chemotherapy.
Results
Patients’ demographics
During the study period, 298 consecutive patients with MBC at first presentation were evaluated for the presence of circulating CK-19mRNA-positive CTCs before the administration of any systemic treatment. The patients’ median age was 59 years (range, 27–83), 218 (73.2%) were post-menopausal and 166 (55.7%) had visceral disease. A total of 136 (45.6%) patients had hormone receptor (either ER or PR)-positive/HER2-negative tumours, 61 (20.5%) HER2-positive tumours (3+ or FISH+) and 66 (22.1%) triple-negative tumours. Another 129 (43.3%) patients had received prior adjuvant treatment, most of them in the context of research protocols of the Hellenic Oncology Research Group, whereas 169 (56.7%) patients were diagnosed with metastatic disease from the beginning. There was no difference in terms of the main clinico-pathological characteristics in patients with detectable and undetectable CK-19mRNA-positive CTCs (Table 1). At the time of analysis, 16 (5.4%) patients are still in first remission and 58 (19.5%) are alive.
Table 1. Patients’ demographics and clinico-pathological characteristics.
| All patients n=300 | CK-19 (+) n=199 (66.8%) | CK-19 (–) n=99 (33.2%) | χ 2 -test | |
|---|---|---|---|---|
| Age | Mann–Whitney (P-values) | |||
| Median (min-max) | 59.0 (27–83) | 59.0 (27–83) | 60.0 (29–81) | 0.426 |
| Menopausal status | ||||
| Pre (%) | 80 (26.8) | 55 (27.6) | 25 (25.3) | |
| Post (%) | 218 (73.2) | 144 (72.4) | 74 (74.7) | 0.680 |
| Performance status | ||||
| 0–1 (%) | 265 (88.9) | 176 (88.4) | 89 (89.9) | |
| 2–3 (%) | 25 (8.4) | 18 (9.0) | 7 (7.1) | |
| Missing (%) | 8 (2.7) | 5 (2.5) | 3 (3.0) | 0.661 |
| Molecular profile | ||||
| ?R- and/or PR-positive/??R2-negative (%) | 136 (45.6) | 84 (42.2) | 52 (52.5) | |
| ?R- and/or PR-positive/??R2-positive (%) | 25 (8.4) | 13 (6.5) | 12 (12.1) | |
| ?R-/PR-negative/??R2-positive (%) | 36 (12.1) | 29 (14.6) | 7 (7.1) | |
| Triple-negative (%) | 66 (22.1) | 49 (24.6) | 17 (17.2) | |
| Unknown (%) | 35 (11.7) | 24 (12.1) | 11 (11.1) | 0.034 |
| Disease location | ||||
| (a) Visceral (%) | 166 (55.7) | 107 (53.8) | 59 (59.6) | |
| Non-visceral (%) | 114 (38.3) | 79 (39.7) | 35 (35.4) | |
| Missing (%) | 18 (6.0) | 13 (6.5) | 5 (5.1) | 0.441 |
| (b) Bones (%) | 108 (36.2) | 72 (36.2) | 36 (36.4) | |
| No bones (%) | 182 (57.7) | 114 (57.3) | 58 (58.6) | |
| Missing (%) | 18 (6.0) | 13 (6.5) | 5 (5.1) | 0.999 |
| Adjuvant chemotherapy | ||||
| No (%) | 169 (56.7) | 105 (52.8) | 64 (64.6) | |
| Yes (%) | 129 (43.3) | 94 (47.2) | 35 (35.4) | 0.063 |
| Front-line chemotherapy | ||||
| Taxane-based (%) | 125 (41.9) | 78 (39.2) | 47 (47.5) | |
| Anthracycline-based (%) | 32 (10.7) | 20 (10.1) | 12 (12.1) | |
| Taxane/anthracycline –based (%) | 104 (34.9) | 72 (36.2) | 32 (32.3) | |
| Other (%) | 37 (12.4) | 29 (14.6) | 8 (8.1) | 0.276 |
| Survival status | ||||
| Alive (%) | 58 (19.5) | 29 (14.6) | 29 (29.3) | |
| Dead (%) | 240 (80.5) | 170 (85.4) | 70 (70.7) | 0.003 |
Abbreviations: ER=oestrogen receptor; PR=progesterone receptor.
Detection of CTCs by CellSearch and RT-PCR
In 44 patients treated from January 2007 to May 2009, the detection of CTCs was performed in parallel using the two different assays. Circulating tumour cells were detected in 17 (38.6%) patients using the CellSearch assay (cut-off, ⩾5 CTCs/7.5 ml), and in 24 (54.6%) using the RT-PCR assay (P=0.001). The RT-PCR assay failed to detect CTCs in two (4.5%) patients in whom CellSearch could detect CTCs; conversely, CellSearch failed to detect CTCs in nine (20.5%) patients in whom RT-PCR could detect CTCs (Table 2). However, there was a statistically significant correlation of the detection of CTCs between the two assays (r=0.373, P=0.0001). The correlation remained significant when the cut-offs for CTC detection with the CellSearch were set at ⩾1 and ⩾2 CTCs (Table 2).
Table 2. Comparison of detection of CTCs by both CellSearch and RT-PCR assays.
|
CellSearch
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| ⩾1 CTC/7.5 ml |
⩾2CTCs/7.5 ml |
⩾5CTCs/7.5 ml |
|||||||
| Negative ( n =17) | Positive ( n =27) | P -value | Negative ( n =20) | Positive ( n =24) | P -value | Negative ( n =27) | Positive ( n =17) | P -value | |
| RT-PCR-negative | 12 (27.3%) | 8 (18.8%) | 0.008 | 14 (31.8%) | 6 (13.6%) | 0.003 | 18 (40.9%) | 2 (4.5%) | 0.001 |
| RT-PCR-positive | 5 (11.4%) | 19 (43.2%) | 6 (13.6%) | 18 (40.9%) | 9 (20.5%) | 15 (34.1%) | |||
| Spearman’s test: r=0.385, P=0.01 | r=0.373, P=0.0001 | r=0.373, P=0.0001 | |||||||
Abbreviations: CTC=circulating tumour cell; RT=real-time PCR.
Detection of CTCs
CK-19mRNA-positive cells were detected in 199 (66.8%) patients. There was no association between the detection of CK-19mRNA-positive CTCs and the menopausal status, the PS, the hormone receptor or HER2/neu status, the presence of visceral or bone metastases and the administered front-line treatment (Table 1). The median number of CK-19mRNA-positive CTCs was 1.0 MCF-7 cell equivalents/5 μg RNA (range, 0.0–1221.0). Front-line chemotherapy resulted in an objective response (complete plus partial response) in 95 (48.2%) out of 199 patients with detectable CK-19mRNA-positive CTCs and in 58 (58.6%) out of 99 patients without detectable CK-19mRNA-positive CTCs (P=0.109). There was no significant correlation between the detection of CTCs and the presence of visceral (53.8% vs 59.6% in CK-19mRNA-positive and CK-19mRNA-negative patients, respectively; P=0.441) or bone (36.2% and 36.4%, respectively; P=0.999) disease.
Clinical outcome according to the detection of CK-19mRNA-positive cells
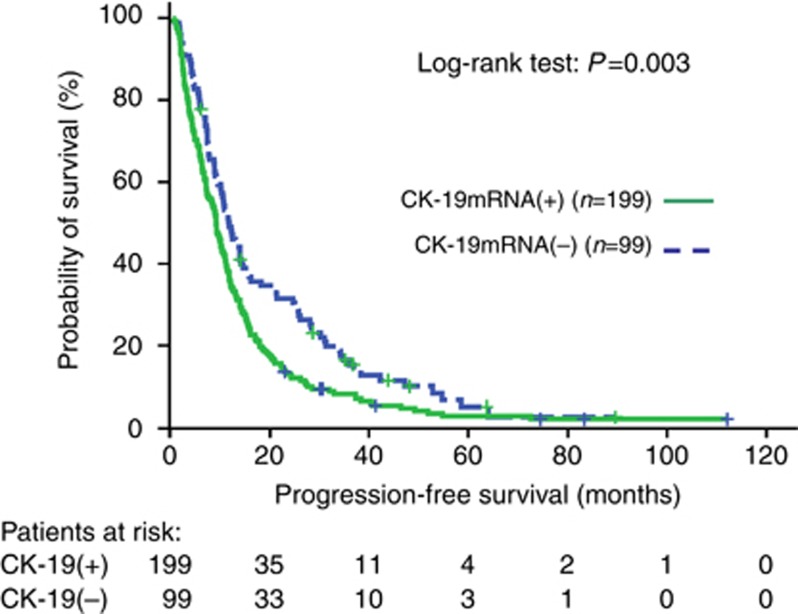
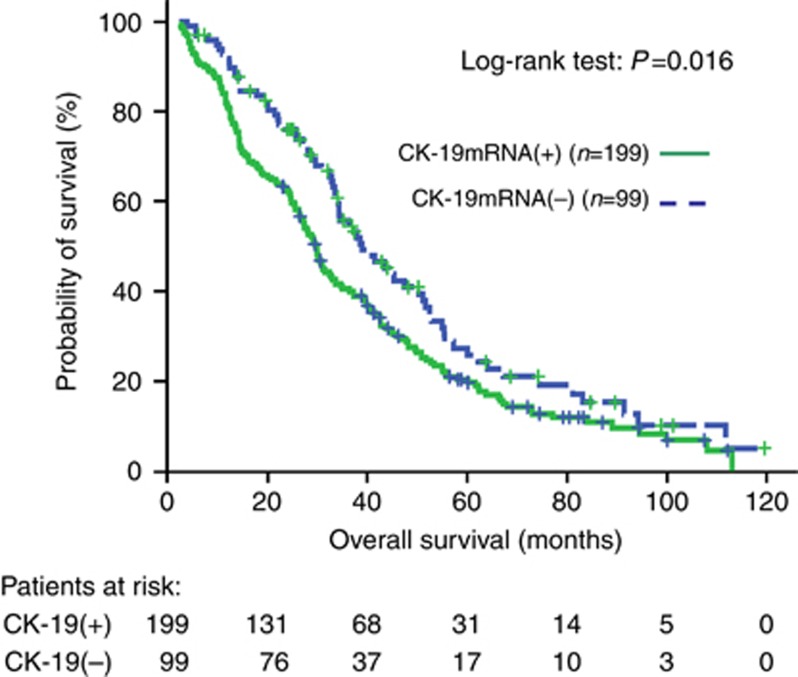
At the time of analysis, 240 (80.5%) patients had died. The median follow-up was 83.3 mo (range, 2.2–119.6). The median PFS was significantly lower in patients with detectable CK-19mRNA-positive CTCs compared with patients without detectable CTCs (9.2 vs 11.9 mo; P=0.003, Supplementary Table 1, Figure 3). The median OS was significantly higher in patients without detectable CK-19mRNA-positive CTCs compared to patients with detectable CTCs (38.9 vs 29.7 mo; P=0.016, Supplementary Table 1, Figure 4).
Figure 3.
Progression-free survival Kaplan–Meier curves for patients with or without CK-19mRNA-positive CTCs before the initiation of front-line chemotherapy.
Figure 4.
Overall survival Kaplan–Meier curves for patients with or without CK-19mRNA-positive CTCs before the initiation of front-line chemotherapy.
Univariate and multivariate analysis
The univariate analysis using the menopausal status, the hormone receptor status (ER and PR), the HER2/neu status, the PS, the disease localisation (bone or visceral), the adjuvant chemotherapy and the detection of CK-19mRNA-positive CTCs at baseline revealed that PS, ER, PR, HER2/neu, adjuvant chemotherapy and CTC status at baseline were significantly associated with PFS (Table 3). Multivariate analysis demonstrated that only the ER negativity (hazard ratio (HR) 1.627; 95% CI: 1.267–2.090) and the detection of CK-19mRNA-positive CTCs (HR 1.424; 95% CI: 1.092–1.857) could be emerged as independent factors associated with a decreased PFS (Table 4). Similarly, the univariate analysis for the OS using the same parameters as for PFS, demonstrated that the same factors (PS, ER, PR and CTC status) were significantly associated with OS (Table 3). Interestingly, although triple-negative histological subtype was not associated with worse outcome in the univariate analysis, when we analysed the prognostic significance of triple-negative histology according to the presence of CTCs, it was shown that triple-negative/CK-19mRNA-positive patients had worse prognosis compared with CK-19mRNA-positive patients with other histological subtypes (median OS 26.6 mo vs 32.9 mo, respectively). Multivariate analysis revealed that the ER negativity (HR 1.480; 95% CI: 1.129–1.940), worse PS (HR 1.957; 95% CI: 1.199–3.193) and the detection of CTCs at baseline (HR 1.371; 95% CI: 1.019–1.845) emerged as independent factors associated with a decreased OS (Table 4).
Table 3. Univariate analysis for PFS and OS.
|
PFS
|
OS
|
|||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P -values | HR | 95% CI | P -values |
| Menopausal status (pre vs post) | 1.110 | 0.853–1.446 | 0.437 | 1.197 | 0.903–1.587 | 0.210 |
| ER status (− vs +) | 1.677 | 1.308–2.150 | <0.001 | 1.433 | 1.098–1.869 | 0.008 |
| PR status (− vs+) | 1.442 | 1.118–1.859 | 0.005 | 1.334 | 1.019–1.747 | 0.036 |
| HR status (ER(−)/PR(−) vs other) | 1.641 | 1.272–2.117 | <0.001 | 1.385 | 1.058–1.815 | 0.018 |
| HER/2 status (+ vs −) | 1.420 | 1.061–1.900 | 0.019 | 1.140 | 0.831–1.563 | 0.418 |
| HR/HER-2 (triple-negative vs other) | 1.302 | 0.983–1.724 | 0.065 | 1.345 | 0.996–1.816 | 0.053 |
| Adjuvant chemotherapy (yes vs no) | 1.293 | 1.019–1.639 | 0.034 | 1.254 | 0.972–1.619 | 0.082 |
| Bone disease (yes vs no) | 0.985 | 0.769–1.262 | 0.908 | 1.210 | 0.927–1.579 | 0.161 |
| Visceral disease (yes vs no) | 1.093 | 0.855–1.396 | 0.478 | 1.049 | 0.806–1.365 | 0.724 |
| CK-19 pre chemo (+ vs −) | 1.456 | 1.132–1.875 | 0.003 | 1.406 | 1.064–1.858 | 0.016 |
| Performance status (2–3 vs 0–1) | 1.667 | 1.100–2.525 | 0.016 | 2.184 | 1.425–3.347 | <0.001 |
Abbreviations: CI=confidence interval; ER=oestrogen receptor; HR=hazard ratio; OS=overall survival; PFS=progression-free survival; PR=progesterone receptor.
Table 4. Multivariate analysis for PFS and OS.
|
PFS
|
OS
|
|||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P -values | HR | 95% CI | P -values |
| ER status (− vs +) | 1.627 | 1.267–2.090 | <0.001 | 1.480 | 1.129–1.940 | 0.005 |
| Performance status (2–3 vs 0–1) | 1.957 | 1.199–3.193 | 0.007 | |||
| CK-19 pre chemo (+ vs –) | 1.424 | 1.092–1.857 | 0.009 | 1.371 | 1.019–1.845 | 0.037 |
Abbreviations: CI=confidence interval; ER=oestrogen receptor; HR=hazard ratio; OS=overall survival; PFS=progression-free survival.
Discussion
The aim of the current study was to evaluate the prognostic value of CK-19mRNA-positive CTCs in women with newly diagnosed recurrent or de novo MBC, using our previously described RT-PCR assay, which has been validated in patients with early breast cancer (Stathopoulou et al, 2003; Xenidis et al, 2006, 2009; Ignatiadis et al, 2007). The results of this retrospective study demonstrate that the detection of CK-19mRNA-positive CTCs at baseline and before the initiation of any systemic treatment is a poor prognostic factor, as it was independently associated with a significantly reduced median PFS and OS.
Recent studies demonstrated the prognostic value of baseline CTCs measurement in MBC. Indeed, the study by Cristofanilli et al (2004) demonstrated that the detection of ⩾5 CTCs per 7.5 ml of blood from MBC patients was associated with a shorter PFS and OS, and has superior and independent prognostic value than the tumour burden and disease phenotype. This finding was subsequently confirmed by the same investigators in untreated patients with MBC eliminating factors, which may influence the results (i.e., differences between prior therapies, stage of the disease, line of treatment, etc); in this particular study, CTC measurement performed at baseline and before initial therapy was found to be highly predictive for worse PFS and OS (Cristofanilli et al, 2005). In a similar study, Dawood et al (2008) confirmed that the detection of <5 CTCs/7.5 ml of blood at baseline in patients with newly diagnosed MBC was associated with a significantly better OS compared with those with ⩾5 CTCs/7.5 ml of blood, irrespectively of the hormone receptor and HER-2/neu status, site of first metastasis or whether the patient had recurrent or de novo metastatic disease. Similar results have been reported by other investigators who evaluated the presence of CTCs using either the CellSearch platform (Bidard et al, 2010; Pierga et al, 2012) or other techniques (Wong et al, 2006; Bidard et al, 2008; Nakamura et al, 2010). In a recent report, the MD Anderson group reported that the risk of death was linearly increased with increasing CTC count in all molecular tumour subtypes; however, it was higher in ER+ and triple-negative than in HER2+ tumours (Giordano et al, 2011).
The detection rate of CK-19mRNA transcripts in peripheral blood was 67% in this group of patients with metastatic disease. Using the same RT-PCR assay, CK-19mRNA-positive CTCs were detected in 181 (40.8%) of 444 patients with early breast cancer (Ignatiadis et al, 2007). Van der Auwera et al (2010), using a RT-PCR for CK-19mRNA showed that 26% of patients with MBC were CTC-positive, whereas Reinholz et al (2011) were able to detect CK19+mRNA cells in 56–75% of 86 patients with metastatic disease. Using a multi-marker RT-PCR assay for four marker genes, including CK-19 (Bosma et al, 2002), tumour cells were detected in 31% of patients with advanced breast cancer (Weigelt et al, 2003), and using the AdnaTest BreastCancer, 52% of metastatic patients were tested positive for CTCs (Tewes et al, 2009). It should be mentioned here, however, that the use of various enrichment procedures, the variability in the millilitres of blood analysed, and the different methods used for the evaluation of the sensitivity and specificity of the assays, preclude firm conclusions to be drawn regarding the actual incidence of CTC detection. To further complicate the issue, in the above-mentioned reports, different numbers of patients have been evaluated in different stages of metastatic disease. In the present study, 20 ml of peripheral blood were used to detect CTCs in previously untreated patients with advanced breast cancer. Usually, smaller volumes of blood are used for gene expression studies in metastatic patients, which could explain, at least in part, the relatively high incidence of CTC detection reported here.
Nevertheless, in the present study, the detection of CK-19mRNA-positive CTCs in metastatic patients was significantly and independently associated with worse outcome. The independent prognostic value of CTC detection using this method was observed irrespectively of the quantitative burden of CTCs, as patients were classified according to the presence or the absence of the CK-19mRNA transcripts. The cut-off of ⩾0.6 MCF-7 cell equivalents/5 μg RNA of the patients’ PBMCs, which has been determined in a previous analytical study (Stathopoulou et al, 2003) and has been shown to be able to define patients with early breast cancer at high risk for relapse and disease-related death (Xenidis et al, 2006, 2009; Ignatiadis et al, 2007), was also revealed appropriate for the selection of poor prognosis patients with newly diagnosed MBC. Despite the fact that no quantitative data have been reported, preliminary results seem to indicate that quantitative classification of patients according to the numerical burden of CTCs is not superior than the qualitative one (data not shown). This might be a difference with the results obtained with the CellSearch platform (Giordano et al, 2011). In a small cohort of patients, the detection of CTCs was performed, in parallel, using both the CellSearch platform and the RT-PCR assay; the findings indicate that there was a close correlation concerning the detection of CTCs between the two assays, suggesting that they, probably, recognise similar sub-populations of CTCs. Obviously, the small number of patients limits the statistical power of these comparisons necessitating a comparison of the two assays in a larger number of patients. In any case, this correlation could, at least partly, explain the fact that our findings are in line with those of the literature concerning the independent prognostic value of CTC detection before the initiation of any systemic treatment.
The current study represents the cumulative experience of >10-year involvement of our group in the study of CK-19mRNA-positive CTCs in patients with early or/and MBC. Our study demonstrates, for the first time, that the detection of CK-19mRNA-positive CTCs in a large group of previously untreated patients with metastatic disease, using a well-validated RT-PCR assay, is clinically relevant, as it was significantly associated with an increased incidence of relapse and disease-related death. The prognostic value of the detection of CK-19mRNA-positive CTCs was independent of whether the patients presented with recurrent or de novo MBC or the different well-known clinico-pathological characteristics. Similarly, Reinholz et al (2011), showed in a smaller group of metastatic patients that the presence of CK19+mRNA CTCs was associated with poor prognosis.
Our findings, taken together with those of the literature, strongly suggest that the detection of CTCs represent an interesting marker characterising the biology of the disease, and thus, might identify subgroups of patients with different prognosis. On the basis of these findings, we strongly support the Dawood et al’s (2008) proposition that CTC’s status could be used as a new stratification factor for women with newly diagnosed MBC. However, despite these data, CTCs measurement at baseline has not yet been incorporated into cancer-staging protocols or even considered as a ‘standard’ clinical tool; the American Society of Clinical Oncology (ASCO) 2007 guidelines (Harris et al, 2007) do not recommend its routine use in clinical practice, owing to insufficient clinical evidence. Nevertheless, Dawood et al (2008) proposed that the distinction of the two different prognostic groups of patients with MBC based on the detection of CTCs could provide a biological (and not merely anatomic) distinction of prognoses upfront, on the basis of a reliable and reproducible test (e.g., stage IVA vs IVB); in addition, the upfront use of CTCs would allow patients with more aggressive or stage IVB disease to be actively enrolled into clinical trials designed to address specific questions related to the selection of more specific and targeted therapies aimed at the elimination of CTCs, which might have an impact on patients’ survival (Cristofanilli et al, 2004; Bidard et al, 2010, 2012). However, limiting factors such as the variable expression of different epithelial antigens in poorly differentiated tumours, the different sensitivity and specificity of various detection assays, and the lack of their direct comparison support the need for cautious consideration of the use of CTC measurement in the daily clinical practice. We have to wait for the data of the large validation trial, which has been undertaken by the Southwestern Oncology Group to definitively decide on the integration of CTC measurement into clinical practice and to provide the rational for an individualised therapeutic approach. Additionally, the better biological analysis of CTCs may help us to better understand the mechanism of resistance of tumour cells to the different anti-tumour agents, as well as to define new molecular targets for their elimination (Bozionellou et al, 2004). This is most important, as experimental data seem to indicate that the population of CTCs is heterogeneous and an important number of them are apoptotic (Mehes et al, 2001), whereas another subpopulation may express stem cell or/and epithelial–mesenchymal transition markers (Fehm et al, 2008; Aktas et al, 2009; Theodoropoulos et al, 2010) facilitating the disease dissemination.
In conclusion, the results of the present study demonstrate that the detection of CK-19mRNA-positive CTCs in patients with MBC at baseline and before the initiation of first-line treatment is associated with significant prognostic information. Further prospective studies are needed to investigate whether the genotypic or/and phenotypic characterisation of CTCs may allow a more effective and rational treatment of MBC, which could confer a survival benefit in these patients.
Acknowledgments
This work was partly supported by grants from the Cretan Association for Biomedical Research (CABR).
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S (2009) Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 11(4): R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard FC, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, Cottu P, Beuzeboc P, Rolland E, Mathiot C, Pierga JY (2012) Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res 14(1): R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard FC, Mathiot C, Degeorges A, Etienne-Grimaldi MC, Delva R, Pivot X, Veyret C, Bergougnoux L, de CP, Milano G, Pierga JY (2010) Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol 21(9): 1765–1771 [DOI] [PubMed] [Google Scholar]
- Bidard FC, Vincent-Salomon A, Sigal-Zafrani B, Dieras V, Mathiot C, Mignot L, Thiery JP, Sastre-Garau X, Pierga JY (2008) Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann Oncol 19(3): 496–500 [DOI] [PubMed] [Google Scholar]
- Bosma AJ, Weigelt B, Lambrechts AC, Verhagen OJ, Pruntel R, Hart AA, Rodenhuis S, van 't Veer LJ (2002) Detection of circulating breast tumor cells by differential expression of marker genes. Clin Cancer Res 8(6): 1871–1877 [PubMed] [Google Scholar]
- Bozionellou V, Mavroudis D, Perraki M, Papadopoulos S, Apostolaki S, Stathopoulos E, Stathopoulou A, Lianidou E, Georgoulias V (2004) Trastuzumab administration can effectively target chemotherapy-resistant cytokeratin-19 messenger RNA-positive tumor cells in the peripheral blood and bone marrow of patients with breast cancer. Clin Cancer Res 10(24): 8185–8194 [DOI] [PubMed] [Google Scholar]
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353(8): 793–802 [DOI] [PubMed] [Google Scholar]
- Chung CT, Carlson RW (2003) Goals and objectives in the management of metastatic breast cancer. Oncologist 8(6): 514–520 [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Broglio KR, Guarneri V, Jackson S, Fritsche HA, Islam R, Dawood S, Reuben JM, Kau SW, Lara JM, Krishnamurthy S, Ueno NT, Hortobagyi GN, Valero V (2007) Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer 7(6): 471–479 [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8): 781–791 [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN, Terstappen LW (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23(7): 1420–1430 [DOI] [PubMed] [Google Scholar]
- Dawood S, Broglio K, Valero V, Reuben J, Handy B, Islam R, Jackson S, Hortobagyi GN, Fritsche H, Cristofanilli M (2008) Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer 113(9): 2422–2430 [DOI] [PubMed] [Google Scholar]
- Ellis M, Hayes DF, Lippman ME et al. Treatment of metastatic disease. In: Harris J, Lippman M, Morrow M et al. (eds). Diseases of the Breast. 3rd edn. Lippincott-Raven: Philadelphia, 2004, pp 1101–1159 [Google Scholar]
- Fehm T, Muller V, Alix-Panabieres C, Pantel K (2008) Micrometastatic spread in breast cancer: detection, molecular characterization and clinical relevance. Breast Cancer Res 10(Suppl 1): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Giuliano M, De LM, Eleuteri A, Iorio F, Tagliaferri R, Hortobagyi GN, Pusztai L, De PS, Hess K, Cristofanilli M, Reuben JM (2011) Artificial neural network analysis of circulating tumor cells in metastatic breast cancer patients. Breast Cancer Res Treat 129(2): 451–458 [DOI] [PubMed] [Google Scholar]
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33): 5287–5312 [DOI] [PubMed] [Google Scholar]
- Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M, Stathopoulos EN, Stathopoulou A, Lianidou E, Chlouverakis G, Sotiriou C, Georgoulias V, Mavroudis D (2007) Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol 25(33): 5194–5202 [DOI] [PubMed] [Google Scholar]
- Mehes G, Witt A, Kubista E, Ambros PF (2001) Circulating breast cancer cells are frequently apoptotic. Am J Pathol 159(1): 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, Hoogstraten G, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47(1): 207–214 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K, Suzuki E (2010) Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer 17(3): 199–204 [DOI] [PubMed] [Google Scholar]
- Nole F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, Medici M, Verri E, Adamoli L, Rotmensz N, Goldhirsch A, Sandri MT (2008) Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol 19(5): 891–897 [DOI] [PubMed] [Google Scholar]
- Pierga JY, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, Dieras V, Rolland E, Mignot L, Mathiot C, Bidard FC (2012) High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol 23(3): 618–624 [DOI] [PubMed] [Google Scholar]
- Reinholz MM, Kitzmann KA, Tenner K, Hillman D, Dueck AC, Hobday TJ, Northfelt DW, Moreno-Aspitia A, Roy V, LaPlant B, Allred JB, Stella PJ, Lingle WL, Perez EA (2011) Cytokeratin-19 and mammaglobin gene expression in circulating tumor cells from metastatic breast cancer patients enrolled in North Central Cancer Treatment Group trials, N0234/336/436/437. Clin Cancer Res 17(22): 7183–7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou A, Gizi A, Perraki M, Apostolaki S, Malamos N, Mavroudis D, Georgoulias V, Lianidou ES (2003) Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res 9(14): 5145–5151 [PubMed] [Google Scholar]
- Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis C, Apostolaki S, Malamos N, Kakolyris S, Kotsakis A, Xenidis N, Reppa D, Georgoulias V (2002) Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol 20(16): 3404–3412 [DOI] [PubMed] [Google Scholar]
- Tewes M, Aktas B, Welt A, Mueller S, Hauch S, Kimmig R, Kasimir-Bauer S (2009) Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat 115(3): 581–590 [DOI] [PubMed] [Google Scholar]
- Theodoropoulos PA, Polioudaki H, Agelaki S, Kallergi G, Saridaki Z, Mavroudis D, Georgoulias V (2010) Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett 288(1): 99–106 [DOI] [PubMed] [Google Scholar]
- Van der Auwera I, Peeters D, Benoy IH, Elst HJ, Van Laere SJ, Prove A, Maes H, Huget P, van DP, Vermeulen PB, Dirix LY (2010) Circulating tumour cell detection: a direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br J Cancer 102(2): 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Bosma AJ, Hart AA, Rodenhuis S, van 't Veer LJ (2003) Marker genes for circulating tumour cells predict survival in metastasized breast cancer patients. Br J Cancer 88(7): 1091–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NS, Kahn HJ, Zhang L, Oldfield S, Yang LY, Marks A, Trudeau ME (2006) Prognostic significance of circulating tumour cells enumerated after filtration enrichment in early and metastatic breast cancer patients. Breast Cancer Res Treat 99(1): 63–69 [DOI] [PubMed] [Google Scholar]
- Xenidis N, Ignatiadis M, Apostolaki S, Perraki M, Kalbakis K, Agelaki S, Stathopoulos EN, Chlouverakis G, Lianidou E, Kakolyris S, Georgoulias V, Mavroudis D (2009) Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol 27(13): 2177–2184 [DOI] [PubMed] [Google Scholar]
- Xenidis N, Markos V, Apostolaki S, Perraki M, Pallis A, Sfakiotaki G, Papadatos-Pastos D, Kalmanti L, Kafousi M, Stathopoulos E, Kakolyris S, Mavroudis D, Georgoulias V (2007) Clinical relevance of circulating CK-19 mRNA-positive cells detected during the adjuvant tamoxifen treatment in patients with early breast cancer. Ann Oncol 18(10): 1623–1631 [DOI] [PubMed] [Google Scholar]
- Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A, Kalbakis K, Androulakis N, Kouroussis C, Pallis T, Christophylakis C, Argyraki K, Lianidou ES, Stathopoulos S, Georgoulias V, Mavroudis D (2006) Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol 24(23): 3756–3762 [DOI] [PubMed] [Google Scholar]
- Xenidis N, Vlachonikolis I, Mavroudis D, Perraki M, Stathopoulou A, Malamos N, Kouroussis C, Kakolyris S, Apostolaki S, Vardakis N, Lianidou E, Georgoulias V (2003) Peripheral blood circulating cytokeratin-19 mRNA-positive cells after the completion of adjuvant chemotherapy in patients with operable breast cancer. Ann Oncol 14(6): 849–855 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.