Abstract
Mammalian apoptosis and yeast programmed cell death (PCD) share a variety of features including reactive oxygen species production, protease activity and a major role played by mitochondria. In view of this, and of the distinctive characteristics differentiating yeast and multicellular organism PCD, the mitochondrial contribution to cell death in the genetically tractable yeast Saccharomyces cerevisiae has been intensively investigated. In this mini-review we report whether and how yeast mitochondrial function and proteins belonging to oxidative phosphorylation, protein trafficking into and out of mitochondria, and mitochondrial dynamics, play a role in PCD. Since in PCD many processes take place over time, emphasis will be placed on an experimental model based on acetic acid-induced PCD (AA-PCD) which has the unique feature of having been investigated as a function of time. As will be described there are at least two AA-PCD pathways each with a multifaceted role played by mitochondrial components, in particular by cytochrome c.
Keywords: yeast, programmed cell death, mitochondria, acetic acid, cytochrome c, protein trafficking, intracellular signaling
The unicellular yeast Saccharomyces cerevisiae has been established as a good model to elucidate molecular mechanisms underlying programmed cell death (PCD) pathways. S. cerevisiae PCD shares many morphological and biochemical features with apoptosis, the major form of mammalian PCD, although there are some peculiar differences. PCD have been described to occur in yeast in different physiological scenarios (Carmona-Gutierrez et al., 2010). Indeed, chromatin condensation, nuclear DNA fragmentation and phosphatidylserine externalization onto the cell surface are general markers of both mammalian and yeast PCD cells. A characteristic feature of mammalian apoptosis is the activation of caspases, proteases that initiate and execute cell death through degradation of cell components. Yeast contains only one gene homolog of caspases, named YCA1, encoding for yeast metacaspase (Madeo et al., 2002) which has substrate specificity different from caspases (Wilkinson and Ramsdale, 2011). Glyceraldehyde-3-phosphate dehydrogenase has been identified as the first YCA1-specific substrate degraded en route to H2O2-induced PCD (Silva et al., 2011), but yeast PCD mechanisms occurring both in YCA1-dependent and -independent manner as well as the role of other proteases in yeast PCD remain to be established (Madeo et al., 2009; Wilkinson and Ramsdale, 2011).
Both in yeast and in mammalian PCD mitochondria play a major role in final pro-survival or pro-death decision. Accordingly, the mitochondria-mediated PCD pathway in yeast resembles the mammalian intrinsic pathway, and shows remarkable complexity with respect to different proteins and pathways involved (Eisenberg et al., 2007; Pereira et al., 2008). Alterations in mitochondrial structure and function during PCD depend on a variety of specific triggers, respiratory or fermentative growth conditions, and on overall cell metabolism. First evidence for a mitochondria-dependent yeast PCD pathway was obtained in acetic acid-induced PCD (AA-PCD), with cells showing cytochrome c (cyt c) release into the cytosol and production of mitochondrial reactive oxygen species (ROS). Mitochondrial dysfunction occurs as shown by mitochondrial depolarization, and a large decrease in cyt c oxidase (COX) activity together with higher resistance to AA-PCD of respiratory-deficient cells, lacking either mtDNA or unable to form active cyt c or ATP synthase (Ludovico et al., 2002). Key regulators of mitochondrial metazoan apoptosis are the Bcl-2 family proteins which include both pro-apoptotic and anti-apoptotic members harboring multiple or single Bcl-2 homology (BH) domains (BH1-4). These proteins regulate mitochondrial outer membrane permeabilization (MOMP) followed by the release of pro-apoptotic factors including cyt c (Wang and Youle, 2009; Wasilewski and Scorrano, 2009). Recent discovery of a yeast BH3-only protein (Ybh3p) mediating both AA- and H2O2-induced PCD (Büttner et al., 2011) supports the hypothesis of the origin of the eukaryotic PCD systems through acquisition of several PCD effectors as a consequence of mitochondrial endosymbiosis (Koonin and Aravind, 2002). Indeed, yeast Ybh3p translocates to mitochondria inducing PCD and mitochondrial membrane depolarization through interaction with the mitochondrial phosphate carrier (Mir1p) and a core subunit of the respiratory complex III (Cor1p; Büttner et al., 2011). Thus, Ybh3p resembles mammalian Bax that can permeabilize mitochondria, whereas mammalian BH3-only proteins require Bax and Bak to release cyt c, suggesting that the most ancestral function of the BH3-like proteins may be to trigger changes in the IMM (Oettinghaus et al., 2011).
Whether yeast PCD does resemble and/or predate apoptotic death in multicellular organisms or is a distinct form of PCD in itself is still a matter of investigation. Indeed, it remains controversial as to whether metacaspases are distant relatives of caspases or are more closely related to other classes of proteases. Moreover even if yeast encodes a BH3-only protein as recent studies suggest, yeast homologs of Bcl-2 proteins on which BH3-only proteins act are still unknown. Notwithstanding this, the central role of mitochondria in yeast PCD underlines the importance of dissecting the PCD process in this unicellular organism.
In this review we consider the mitochondrial proteins involved in yeast PCD execution and regulation (see Table 1). Most of them are involved in either electron transfer along the respiratory chain and oxidative phosphorylation, or mitochondrial dynamics, or mitochondrial permeabilization and protein trafficking from mitochondria to cytosol and vice versa. These points will be dealt with separately.
Table 1.
Yeast mitochondrial proteins involved in PCD regulation.
| Gene (protein) | Mammalian homolog | PCD trigger | Role in PCD | Reference |
|---|---|---|---|---|
| AAC1/AAC2/AAC3 (ADP/ATP carrier isoforms) | ANT | Acetic acid, diamide, H2O2 | MOMP | Pereira et al. (2007) |
| AIF1 (apoptosis-inducing factor) | AIF | Acetic acid, bostrycin, H2O2 | Pro-apoptotic released factor translocating to the nucleus | Wissing et al. (2004), Xu et al. (2010) |
| ATP10 (ATP synthase assembly factor) | ATP synthase | Acetic acid | Pro-apoptotic factor | Ludovico et al. (2002) |
| CIT1 (citrate synthase) | CS | Aging, heat | GSH biosynthesis, antioxidant activity | Lee et al. (2007) |
| COR1 (complex III core subunit) | QCR1 | Acetic acid +Ybh3 overexpression | ETC,YBH3 interaction | Büttner et al. (2011) |
| CYC1/CYC7 (cytochrome c isoforms 1, 2) | Cytochrome c | Acetic acid, amiodarone/α-factor, ASF1/CIA1 deletion, aspirin, cdc48S565G, Bax heterologous expression, H2O2, hyperosmotic stress, salt stress | Pro-apoptotic released factor, ETC electron donor, ROS scavenger | Manon et al. (1997), Yamaki et al. (2001), Ludovico et al. (2002), Pozniakovsky et al. (2005), Silva et al. (2005), Braun et al. (2006), Pereira et al. (2007), Giannattasio et al. (2008), Sapienza et al. (2008), Gao et al. (2011) |
| CYC3 (cytochrome c heme lyase) | CCHL | Acetic acid, amiodarone, hyperosmotic stress | Cyt c holoenzyme formation | Ludovico et al. (2002), Pozniakovsky et al. (2005), Silva et al. (2005) |
| FIS1 (mitochondrial fission protein) | hFIS | Acetic acid, BAR0329, ethanol, heat shock, H2O2 | Mitochondrial dynamics | Fannjiang et al. (2004), Kitagaki et al. (2007), Bink et al. (2010) |
| L14-A (mitochondrial 60S ribosomal protein) | – | Grapefruit seed extract | Unknown | Cao et al. (2012) |
| MIR1 (mitochondrial phosphate carrier) | PHC | Acetic acid +Ybh3 overexpression | Energetic metabolism,YBH3 interaction | Büttner et al. (2011) |
| NDI1 (internal NADH dehydrogenase) | AMID | NDI1 overexpression | ROS production | Li et al. (2006) |
| NUC1 (mitochondrial nuclease) | Endo G | Acetic acid, amiodarone, ethanol, H2O2 | Pro-apoptotic released factor translocating to the nucleus | Büttner et al. (2007), Kitagaki et al. (2007) |
| POR1 (porin) | VDAC | Acetic acid, H2O2,diamide | Anti-apoptotic factor | Pereira et al. (2007) |
| RSM23 (mitochondrial 40S ribosomal protein) | hDAP-3 | YCA1 overexpression | Pro-apoptotic factor | Madeo et al. (2002) |
| TIM18 (translocase of the inner mitochondrial membrane) | – | Arsenite | MOMP | Du et al. (2007) |
| YME1 (catalytic subunit of i-AAA protease complex) | – | Heterologous expression of Bax | Complex IV degradation | Manon et al. (2001) |
| YSP1 (yeast suicide protein 1) | – | α-Factor, amiodarone | Mitochondrial dynamics | Pozniakovsky et al. (2005) |
| YSP2 (yeast suicide protein 2) | – | Acetic acid, amiodarone | Mitochondrial dynamics | Sokolov et al. (2006) |
The S. cerevisiae mitochondrial proteins reported in this table have been implicated in PCD induced by different triggers through biochemical and/or genetic studies.ANT, adenine nucleotide translocator; MOMP, mitochondrial outer membrane permeabilization; CS, citrate synthase; GSH, glutathione; QCR1, ubiquinol–cytochrome c reductase core protein;YBH3, yeast BH3-only; ETC, electron transport chain; CCHL, cyt c heme lyase; hFIS, human homolog of Fis1p; BAR0329, 4-{[3-(4-chlorobenzyl)- 2-methoxyquinolin-6-yl]methyl}piperazine-1-carboximidamide; PHC, phosphate carrier; AMID, apoptosis-inducing factor-homologous mitochondrion-associated inducer of death; Endo G, endonuclease G; VDAC, voltage-dependent anion channel; hDAP-3, human death associated protein.
ELECTRON TRANSFER ALONG THE RESPIRATORY CHAIN AND OXIDATIVE PHOSPHORYLATION
Yeast internal NADH dehydrogenase (NDI1) is the homolog of metazoan AMID, the apoptosis-inducing factor (AIF)-homologous mitochondrion-associated inducer of death. Ndi1p overexpression can cause PCD, probably due to ROS production in mitochondria, only when cells are grown in glucose-rich media. However this occurs in yeast cells lacking mitochondrial superoxide dismutase both during fermentative and respiring growth (Li et al., 2006). Yme1p is a mitochondrial AAA-type protease involved in the coordinated assembly of COX. Yme1p activation results in a decrease of COX level en route to Bax-induced cell death; however since under fermentative conditions, when COX activity is strongly repressed, YME1 deletion slightly delays Bax-induced cell death, some other unidentified Yme1p substrate could also play a role in this process (Manon et al., 2001). Analysis of the effect of oxidative phosphorylation inhibitors on yeast PCD has shown conflicting results depending on the PCD trigger. Although AA-PCD is insensitive to antimycin or oligomycin, myxothiazol and cyanide prevented amiodarone/α-factor-induced PCD (Ludovico et al., 2002; Pozniakovsky et al., 2005; Guaragnella et al., 2011b). Yeast cells grown in the presence of both antimycin and oligomycin and subsequently treated with acetic acid in the presence of both these compounds displayed a higher sensitivity to AA-PCD (Pereira et al., 2007). Yet, fully assembled and functional F0F1-ATPase and cyt c are required for Bax-induced PCD and AA-PCD to occur (Matsuyama et al., 1998; Ludovico et al., 2002; Guaragnella et al., 2011a).
Thus, complexes participating in oxidative phosphorylation have key roles in yeast PCD different from electron transport and ATP synthesis, likely ROS production. Interestingly, deletion of mitochondrial citrate synthase (CIT1) results in higher sensitivity to oxidative stress and PCD induction, due to impairment of reduced glutathione (GSH) biosynthesis (Lee et al., 2007), suggesting that other metabolic pathways are also involved in oxidative stress.
MITOCHONDRIAL DYNAMICS
Extensive mitochondrial fragmentation is recognized as a general feature in yeast PCD. Fis1p, Dnm1p, and Mdv1p/Net2p, which constitutes the machinery responsible for mitochondrial fission in healthy cells (Fannjiang et al., 2004), are involved in mitochondrial fragmentation/degradation and cell death induced by different stimuli (Fannjiang et al., 2004; Kitagaki et al., 2007; Bink et al., 2010). Indeed, DNM1 gene deletion extends life span by increasing cellular resistance to PCD induction (Scheckhuber et al., 2007). In distinction from its pro-apoptotic function in mammals, yeast Fis1p is a mitochondrial protein which inhibits DNM1-mediated cell death by inhibiting the fission function of Dnm1p, differently from its role in mitochondrial fission during normal growth (Fannjiang et al., 2004). This inhibitory function of Fis1p can be functionally replaced by human Bcl-2 and Bcl-xL, supporting the idea that Fis1p is a functional homolog of anti-apoptotic Bcl-2 family proteins (Cheng et al., 2008a) and, together with Ybh3p (Büttner et al., 2011), is a component of an ancestral mitochondrial PCD pathway. The pro-survival role of FIS1 was confirmed in studies using different apoptotic triggers, such as virus-encoded toxin, ethanol, and fungicidal derivative BAR0329 (Ivanovska and Hardwick, 2005; Kitagaki et al., 2007; Bink et al., 2010). However, FIS1 may have an additional long-term survival function which appears to be independent of DNM1 and MDV1. Indeed, FIS1 deletion results in acquisition of a secondary mutation in the stress-response gene WHI2 that confers sensitivity to cell death (Cheng et al., 2008b).
Genetic screens have revealed the existence of two novel genes, named yeast suicide protein 1 (YSP1) and yeast suicide protein 2 (YSP2), required for mitochondrial fragmentation en route to amiodarone-induced PCD (Pozniakovsky et al., 2005; Sokolov et al., 2006). It has been proposed that Ysp2p acts downstream of ROS production due to intracellular acidification, following AA-PCD induction (Sokolov et al., 2006). No homologous genes have been found in higher organisms.
MITOCHONDRIAL PERMEABILITY AND PROTEIN TRAFFICKING FROM MITOCHONDRIA TO CYTOSOL AND VICE VERSA
As in mammals, the release of pro-apoptotic mitochondrial proteins occurs en route to yeast PCD. Cyt c was the first mitochondrial protein shown to have an apoptotic function different from its role as an electron carrier in the respiratory chain. Cyt c release from mitochondria occurs commonly in yeast PCD both in response to a variety of stimuli, including acetic acid (Ludovico et al., 2002; Giannattasio et al., 2008), amiodarone/α-factor (Pozniakovsky et al., 2005), H2O2 (Pereira et al., 2007), aspirin (Sapienza et al., 2008), salt stress (Gao et al., 2011), and as a result of heterologous expression of the mammalian BAX (Manon et al., 1997). Cyt c release was also observed in yeast strains lacking the histone chaperone ASF1/CIA1 (Yamaki et al., 2001) and with a mutation in CDC48 (cdc48S565G; Braun et al., 2006). Deletion of cyt c isoforms or heme lyase, necessary for cyt c maturation, inhibits yeast PCD triggered by different stimuli (Ludovico et al., 2002; Severin and Hyman, 2002; Pozniakovsky et al., 2005; Silva et al., 2005; Yang et al., 2008; Gao et al., 2011), except ethanol (Kitagaki et al., 2007).
Mammalian AIF is a FAD-containing oxidoreductase localized in the mitochondrial intermembrane space whose specific enzymatic activity remains unknown (Sevrioukova, 2011). AIF is a caspase-independent death effector and also plays a vital mitochondrial role in healthy cells (Hangen et al., 2010). Similarly to AIF, the yeast homolog Aif1p translocates to the nucleus in response to apoptotic stimuli (Wissing et al., 2004). AIF1 disruption rescues yeast cells from oxygen stress and delays age-induced PCD. Conversely, overexpression of AIF1 strongly stimulates H2O2-induced PCD; this effect is attenuated by disruption of YCA1. Contrarily, AIF1-dependent bostrycin-induced cell death was shown to be independent of YCA1 (Xu et al., 2010).
Nuc1p is the yeast homolog of metazoan endonuclease G (EndoG), a mitochondrial protein with DNase/RNase activity involved in apoptotic DNA degradation (Li et al., 2001). Overexpression of Nuc1p promotes yeast PCD. Nuc1p-mediated PCD is shown to be AIF1- and YCA1-independent, which favors the existence of multiple, redundant pathways regulating cell death. Nuc1p translocates from mitochondria to the nucleus upon death induction. Nuc1p-dependent death depends on its interaction with AAC2, as well as with histone H2B and KAP123, coding for karyopherin involved in nuclear import, indicating that the pro-death role of Nuc1p requires nuclear import and chromatin association (Büttner et al., 2007). When mitochondrial respiration is increased NUC1 deletion inhibits apoptotic death, whereas under respiration repressing conditions, NUC1 deletion sensitizes yeast cells to non-apoptotic death, this showing a dual, pro-life and pro-death role for NUC1 (Büttner et al., 2007; Kitagaki et al., 2007).
The yeast genome also harbors a gene, called NMA111, homologous to vertebrate HtrA2/Omi mitochondrial serine protease, which mediates apoptosis once released to the cytosol where it can antagonize the inhibitor of apoptosis protein XIAP (Vande Walle et al., 2008). Differently from HtrA2/Omi, yeast Nma111p is a nuclear protein that, under cellular stress conditions such as H2O2-induced PCD, tends to aggregate inside the nucleus without its expression level being upregulated, suggesting that aggregation of Nma111p is correlated to its death-mediating character (Fahrenkrog et al., 2004).
Mitochondrial protein release and MOMP are crucial events in yeast PCD. Certain mitochondrial proteins possibly involved in MOMP en route to yeast PCD have been identified. Yeast possesses the homologous genes of the putative core components of mammalian permeability transition pore, ADP/ATP carrier proteins (AAC1,2,3), yeast voltage-dependent anion channel (POR1), and a mitochondrial cyclophilin (CPR3). While Por1p was proposed to have a pro-survival role and Cpr3p had no effect on yeast PCD, only deletion of AAC proteins was shown to protect cells from AA- but not H2O2-induced PCD, and to inhibit cyt c release (Pereira et al., 2007). In addition, the AAC proteins and the vacuolar protease Pep4p have been shown to have a role in mitochondrial degradation en route to AA-PCD; Pep4p is released from the vacuole upon AA-PCD induction, suggesting a vacuole-mitochondrial cross-talk during yeast PCD (Pereira et al., 2010).
The mitochondrial inner membrane translocase, Tim18, was shown to be involved in arsenic-induced yeast cell death (Du et al., 2007), this raising a question about the possible involvement of this translocase in MOMP. Tim18 is part of the Tim54–Tim22 complex, Tim22 being a mitochondrial receptor for the pro-apoptotic protein Bax (Kovermann et al., 2002).
Two other proteins, Mmi1p and Mcd1p, have been shown to translocate to mitochondria en route to yeast PCD. The former functionally links microtubules and mitochondria (Rinnerthaler et al., 2006). The latter causes the decrease of mitochondrial membrane potential amplifying PCD in a cyt c-dependent manner (Yang et al., 2008).
A CASE STUDY: THE ROLE OF CYTOCHROME c IN YEAST PCD
Although cyt c release occurs en route to yeast PCD, so far in S. cerevisiae there is no evidence of the existence of a functional homolog of the apoptosome (Huttemann et al., 2011). Accordingly, yeast cyt c is unable to activate caspases in cytosolic extracts from metazoan cells (Kluck et al., 2000; Bender et al., 2012). Thus, some questions need to be answered: which event/s triggers cyt c release? Is cyt c released from damaged mitochondria? What is the role of the released cyt c en route to PCD, and is it strictly required for PCD to occur? In this regard, the definition of the sequence of events leading to the death cascade turns out to be useful.
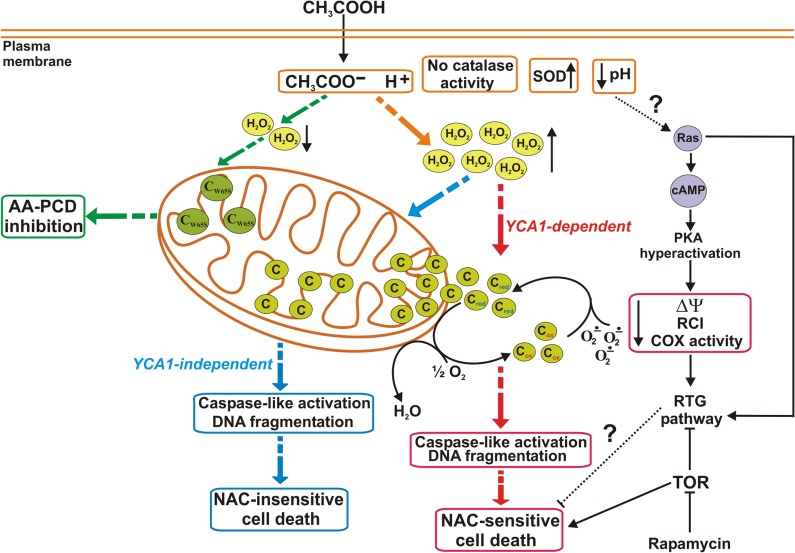
After the discovery of the occurrence of AA-PCD in yeast (Ludovico et al., 2001, 2002), in a series of papers a detailed time course of certain events was investigated (Giannattasio et al., 2005, 2008; Guaragnella et al., 2006, 2007, 2008, 2010a; Ribeiro et al., 2006; Pereira et al., 2007). These events can be classified as pre- and post-cyt c release (Figure 1). Loss of cell viability is complete after 200 min of acetic acid treatment with accumulation of cells with fragmented nuclear DNA. The earliest event (15 min) following acetic acid challenge is ROS production, with a different role for H2O2 and superoxide anion, whose levels are modulated by catalase and superoxide dismutase. En route to death cyt c starts to be released at 60 min from coupled and intact mitochondria; maximum release is reached at 150 min. Later on cyt c is degraded, possibly by yet unidentified proteases. The latest event of AA-PCD is caspase-like activation occurring at 200 min from death induction. Mitochondria are functionally implicated in this death scenario. In fact, up to 150 min released cyt c can act both as an electron donor as well as a ROS scavenger. However, en route to death a progressive impairment of mitochondrial functions, evidenced by a decrease of the respiratory control index, a collapse of the mitochondrial membrane potential, a decrease in COX activity and in cytochromes a + a3 levels, have been observed.
FIGURE 1.
Cytochrome c and mitochondrial dysfunction in AA-PCD pathways. At extracellular acidic pH values acetic acid enters yeast cells and dissociates into acetate and protons causing intracellular acidification. In NAC-sensitive AA-PCD (red dashed arrows) hydrogen peroxide (H2O2) accumulates early, superoxide dismutase (SOD) activity increases, while catalase activity is undetectable; cyt c is released to the cytosol in a YCA1-dependent manner as a functional protein, acting as an electron donor (cred) to the electron transport chain and as a superoxide anion (O2-.) scavenger; in a late phase, mitochondrial functions progressively decline, as revealed by a decrease in mitochondrial membrane potential (ΔΨ), respiratory control index (RCI), and cyt c oxidase (COX) activity. Caspase-like activity increases and DNA fragmentation occurs. The NAC-insensitive (blue dashed arrows) AA-PCD takes place in a YCA1-independent manner without cyt c release, yet caspase-like activation and DNA fragmentation occur in a late phase. In cells expressing a catalytically inactive form of iso-1-cyt c (CW65S; green dashed arrows), no release of mutant cyt c occurs with inhibition of AA-PCD, and there is a decrease in H2O2 production. Possible involvement of certain signaling pathways in the interplay between PCD and cell adaptation is also shown: intracellular acidification caused by AA-PCD induction may stimulate RAS–cAMP–PKA signaling pathway, causing mitochondrial dysfunction, which can activate retrograde (RTG) pathway. The RTG pathway is positively and negatively regulated by Ras and TOR pathways, respectively. The TOR pathway is found at the crossroad of AA-PCD and RTG signaling, which may play a role in AA-PCD resistance.
The AA-PCD time course clearly shows that ROS accumulation and caspase-like activation occur upstream and downstream of cyt c release, respectively. Functional genomics and biochemical studies on knock-out cells lacking YCA1 and/or the genes encoding the two yeast cyt c isoforms allowed the elucidation of causal relationships among ROS levels, cyt c release and caspase-like activation and two separate pathways activated by acetic acid have been identified. Particularly, it has been found that ROS and YCA1 are required for cyt c release, since both prevention of ROS production by the antioxidant N-acetyl cysteine (NAC) and YCA1 disruption result in the inhibition of cyt c release (Guaragnella et al., 2010a,b). How YCA1 is related to cyt c release remains to be elucidated. Nevertheless, a recent report suggests that YCA1 has a role in mitochondrial respiratory functions (Lefevre et al., 2012). Interestingly, AA-PCD still occurs, although with a lower death rate compared to wild type cells, without cyt c release in ADP/ATP carrier as well as YCA1 and/or cyt c knock-out cells (Pereira et al., 2007; Guaragnella et al., 2010b). This confirms on one hand that YCA1 and cyt c act as pro-apoptotic proteins in yeast AA-PCD, but on the other hand that they are dispensable for PCD occurrence, showing the existence of YCA1/cyt c-independent AA-PCD pathway (Figure 1). In this pathway ROS accumulate early, caspase-like activity increase, and DNA fragmentation occurs. Importantly, YCA1/cyt c-independent AA-PCD is insensitive to NAC. This evidence suggests that cyt c still present in mitochondria might play a role in AA-PCD. Recent studies performed on yeast cells expressing a stable but catalytically inactive iso-1-cyt c (W65Scyc1) unable to reduce COX have shown inhibition of AA-PCD, with a decrease of ROS production, no cyt c release, this being independent of electron flow impairment, and an increase in caspase-like activation (Figure 1). Thus, cyt c release does not depend on cyt c function as an electron carrier and when still associated to the mitochondrial membrane, cyt c in its reduced form has a role in AA-PCD by regulating ROS production and caspase-like activity (Guaragnella et al., 2010a,b, 2011b). Regulation of ROS production by mitochondrial cyt c during AA-PCD may be exerted either directly by the cyt c peroxidase system able to scavenge both superoxide anion and H2O2 (Korshunov et al., 1999) or by a change in cyt c–cardiolipin interaction or inefficient cardiolipin peroxidation by ROS (Kagan et al., 2005; Bayir et al., 2006; Sinibaldi et al., 2010; Huttemann et al., 2011). These issues require further investigations.
CONCLUSIONS AND PERSPECTIVES
In the light of results emerging from research into yeast PCD we feel that there is consensus that the response to any stimulus leading to PCD depends on the intrinsic status of the cells, for instance the growth phase or the metabolic and environmental conditions. Paradigmatic of this is that in response to acetic acid in cells with increased mitochondrial respiration yeast activates a Nuc1p-dependent PCD pathway (Büttner et al., 2007), whereas in stationary growth phase yeast is less sensitive to acetic acid (Ludovico et al., 2002), and after acid stress adaptation it is highly resistant to AA-PCD induction (Giannattasio et al., 2005; Ždralević et al., 2012).
Although a number of mitochondrial proteins participating in yeast PCD have been identified, how they work en route to PCD remains to be fully established. Further aspects also need to be investigated, including the fact that mitochondria are important organelles in the cross-talk between death- and life-promoting signaling pathways. Indeed, RAS–cAMP–PKA (Longo, 2003; Roosen et al., 2005; Gourlay et al., 2006; Leadsham and Gourlay, 2010), target of rapamycin (TOR) kinase (Almeida et al., 2009) and retrograde (Jazwinsky, 2003; Liu and Butow, 2006) signaling pathways have been shown to control yeast cell PCD and aging through mitochondrial function regulation (Figure 1).
Mitochondrial dysfunction and the mode of cell response to it underlie different pathological conditions such as neurodegeneration. Alterations in mitochondrial functions have long been observed also in cancer cells and targeting mitochondria as an anti-cancer therapeutic strategy has gained momentum recently (Gogvadze et al., 2009). Since yeast shares with cancer cells the metabolic features identified as the underlying causes of the Warburg effect (Ruckenstuhl et al., 2009; Diaz-Ruiz et al., 2010), it is a suitable model organism to identify cell compounds responsible for tumorigenesis for development of targeted cancer drugs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financed by Fondazione Cassa di Risparmio di Puglia to Nicoletta Guaragnella, CNR project MERIT to Ersilia Marra, and a grant from the Italian Ministry of Economy and Finance to the CNR for the Project FaReBio di Qualità to Sergio Giannattasio. Maša Ždralević is a recipient of a CNR PhD fellowship in “Biology and Biotechnologies,” Università del Salento, Italy. Lucia Antonacci is a recipient of a CNR contract granted by Fondazione Cassa di Risparmio di Puglia. We thank Professor Shawn Doonan for critically reading of the manuscript.
REFERENCES
- Almeida B., Ohlmeier S., Almeida A. J., Madeo F., Leao C., Rodrigues F., Ludovico P. (2009). Yeast protein expression profile during acetic acid-induced apoptosis indicates causal involvement of the TOR pathway. Proteomics 9 720–732 [DOI] [PubMed] [Google Scholar]
- Bayir H., Fadeel B., Palladino M. J., Witasp E., Kurnikov I. V., Tyurina Y. Y., Tyurin V. A., Amoscato A. A., Jiang J., Kochanek P. M., Dekosky S. T., Greenberger J. S., Shvedova A. A., Kagan V. E. (2006). Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochim. Biophys. Acta 1757 648–659 [DOI] [PubMed] [Google Scholar]
- Bender C. E., Fitzgerald P., Tait S. W., Llambi F., Mcstay G. P., Tupper D. O., Pellettieri J., Sanchez Alvarado A., Salvesen G. S., Green D. R. (2012). Mitochondrial pathway of apoptosis is ancestral in metazoans. Proc. Natl. Acad. Sci. U.S.A. 109 4904–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bink A., Govaert G., Francois I. E., Pellens K., Meerpoel L., Borgers M., Van Minnebruggen G., Vroome V., Cammue B. P., Thevissen K. (2010). A fungicidal piperazine-1-carboxamidine induces mitochondrial fission-dependent apoptosis in yeast. FEMS Yeast Res. 10 812–818 [DOI] [PubMed] [Google Scholar]
- Braun R. J., Zischka H., Madeo F., Eisenberg T., Wissing S., Büttner S., Engelhardt S. M., Buringer D., Ueffing M. (2006). Crucial mitochondrial impairment upon CDC48 mutation in apoptotic yeast. J. Biol. Chem. 281 25757–25767 [DOI] [PubMed] [Google Scholar]
- Büttner S., Eisenberg T., Carmona-Gutierrez D., Ruli D., Knauer H., Ruckenstuhl C., Sigrist C., Wissing S., Kollroser M., Frohlich K. U., Sigrist S., Madeo F. (2007). Endonuclease G regulates budding yeast life and death. Mol. Cell 25 233–246 [DOI] [PubMed] [Google Scholar]
- Büttner S., Ruli D., Vogtle F. N., Galluzzi L., Moitzi B., Eisenberg T., Kepp O., Habernig L., Carmona-Gutierrez D., Rockenfeller P., Laun P., Breitenbach M., Khoury C., Frohlich K. U., Rechberger G., Meisinger C., Kroemer G., Madeo F. (2011). A yeast BH3-only protein mediates the mitochondrial pathway of apoptosis. EMBO J. 30 2779–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Xu W., Zhang N., Wang Y., Luo Y., He X., Huang K. (2012). A mitochondria-dependent pathway mediates the apoptosis of GSE-induced yeast. PLoS ONE 7 e32943 10.1371/journal.pone.0032943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Gutierrez D., Eisenberg T., Büttner S., Meisinger C., Kroemer G., Madeo F. (2010). Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 17 763–773 [DOI] [PubMed] [Google Scholar]
- Cheng W. C., Leach K. M., Hardwick J. M. (2008a). Mitochondrial death pathways in yeast and mammalian cells. Biochim. Biophys. Acta 1783 1272–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W. C., Teng X., Park H. K., Tucker C. M., Dunham M. J., Hardwick J. M. (2008b). Fis1 deficiency selects for compensatory mutations responsible for cell death and growth control defects. Cell Death Differ. 15 1838–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz R., Rigoulet M., Devin A. (2010). The Warburg and Crabtree effects: on the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim. Biophys. Acta 1807 568–576 [DOI] [PubMed] [Google Scholar]
- Du L., Yu Y., Li Z., Chen J., Liu Y., Xia Y., Liu X. (2007). Tim18, a component of the mitochondrial translocator, mediates yeast cell death induced by arsenic. Biochemistry (Mosc) 72 843–847 [DOI] [PubMed] [Google Scholar]
- Eisenberg T., Büttner S., Kroemer G., Madeo F. (2007). The mitochondrial pathway in yeast apoptosis. Apoptosis 12 1011–1023 [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B., Sauder U., Aebi U. (2004). The S.cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J. Cell Sci. 117 115–126 [DOI] [PubMed] [Google Scholar]
- Fannjiang Y., Cheng W. C., Lee S. J., Qi B., Pevsner J., Mccaffery J. M., Hill R. B., Basanez G., Hardwick J. M. (2004). Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 18 2785–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Ren Q., Liou L. C., Bao X., Zhang Z. (2011). Mitochondrial DNA protects against salt stress-induced cytochrome c-mediated apoptosis in yeast. FEBS Lett. 585 2507–2512 [DOI] [PubMed] [Google Scholar]
- Giannattasio S., Atlante A., Antonacci L., Guaragnella N., Lattanzio P., Passarella S., Marra E. (2008). Cytochrome c is released from coupled mitochondria of yeast en route to acetic acid-induced programmed cell death and can work as an electron donor and a ROS scavenger. FEBS Lett. 582 1519–1525 [DOI] [PubMed] [Google Scholar]
- Giannattasio S., Guaragnella N., Corte-Real M., Passarella S., Marra E. (2005). Acid stress adaptation protects Saccharomyces cerevisiae from acetic acid-induced programmed cell death. Gene 354 93–98 [DOI] [PubMed] [Google Scholar]
- Gogvadze V., Orrenius S., Zhivotovsky B. (2009). Mitochondria as targets for cancer chemotherapy. Semin. Cancer Biol. 19 57–66 [DOI] [PubMed] [Google Scholar]
- Gourlay C. W., Du W., Ayscough K. R. (2006). Apoptosis in yeast – mechanisms and benefits to a unicellular organism. Mol. Microbiol. 62 1515–1521 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Antonacci L., Giannattasio S., Marra E., Passarella S. (2008). Catalase T and Cu,Zn-superoxide dismutase in the acetic acid-induced programmed cell death in Saccharomyces cerevisiae. FEBS Lett. 582 210–214 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Antonacci L., Passarella S., Marra E., Giannattasio S. (2007). Hydrogen peroxide and superoxide anion production during acetic acid-induced yeast programmed cell death. Folia Microbiol. 7 237–240 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Antonacci L., Passarella S., Marra E., Giannattasio S. (2011a). Achievements and perspectives in yeast acetic acid-induced programmed cell death pathways. Biochem. Soc. Trans. 39 1538–1543 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Passarella S., Marra E., Giannattasio S. (2011b). Cytochrome c Trp65Ser substitution results in inhibition of acetic acid-induced programmed cell death in Saccharomyces cerevisiae. Mitochondrion 11 987–991 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Bobba A., Passarella S., Marra E., Giannattasio S. (2010a). Yeast acetic acid-induced programmed cell death can occur without cytochrome c release which requires metacaspase YCA1. FEBS Lett. 584 224–228 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Passarella S., Marra E., Giannattasio S. (2010b). Knock-out of metacaspase and/or cytochrome c results in the activation of a ROS-independent acetic acid-induced programmed cell death pathway in yeast. FEBS Lett. 584 3655–3660 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Pereira C., Sousa M. J., Antonacci L., Passarella S., Corte-Real M., Marra E., Giannattasio S. (2006). YCA1 participates in the acetic acid induced yeast programmed cell death also in a manner unrelated to its caspase-like activity. FEBS Lett. 580 6880–6884 [DOI] [PubMed] [Google Scholar]
- Hangen E., Blomgren K., Benit P., Kroemer G., Modjtahedi N. (2010). Life with or without AIF. Trends Biochem. Sci. 35 278–287 [DOI] [PubMed] [Google Scholar]
- Huttemann M., Pecina P., Rainbolt M., Sanderson T. H., Kagan V. E., Samavati L., Doan J. W., Lee I. (2011). The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion 11 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska I., Hardwick J. M. (2005). Viruses activate a genetically conserved cell death pathway in a unicellular organism. J. Cell Biol. 170 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinsky M. S. (2003). “Mitochondria, metabolism and aging in yeast,” in Model Systems in Aging, eds Nystrom T., Osiewacz H. D. (Heidelberg: Springer; ) 39–59 [Google Scholar]
- Kagan V. E., Tyurin V. A., Jiang J., Tyurina Y. Y., Ritov V. B., Amoscato A. A., Osipov A. N., Belikova N. A., Kapralov A. A., Kini V., Vlasova Ii, Zhao Q., Zou M., Di P., Svistunenko D. A., Kurnikov I. V., Borisenko G. G. (2005). Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1 223–232 [DOI] [PubMed] [Google Scholar]
- Kitagaki H., Araki Y., Funato K., Shimoi H. (2007). Ethanol-induced death in yeast exhibits features of apoptosis mediated by mitochondrial fission pathway. FEBS Lett. 581 2935–2942 [DOI] [PubMed] [Google Scholar]
- Kluck R. M., Ellerby L. M., Ellerby H. M., Naiem S., Yaffe M. P., Margoliash E., Bredesen D., Mauk A. G., Sherman F., Newmeyer D. D. (2000). Determinants of cytochrome c pro-apoptotic activity. The role of lysine 72 trimethylation. J. Biol. Chem. 275 16127–16133 [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Aravind L. (2002). Origin and evolution of eukaryotic apoptosis: the bacterial connection. Cell Death Differ. 9 394–404 [DOI] [PubMed] [Google Scholar]
- Korshunov S. S., Krasnikov B. F., Pereverzev M. O., Skulachev V. P. (1999). The antioxidant functions of cytochrome c. FEBS Lett. 462 192–198 [DOI] [PubMed] [Google Scholar]
- Kovermann P., Truscott K. N., Guiard B., Rehling P., Sepuri N. B., Muller H., Jensen R. E., Wagner R., Pfanner N. (2002). Tim22, the essential core of the mitochondrial protein insertion complex, forms a voltage-activated and signal-gated channel. Mol. Cell 9 363–373 [DOI] [PubMed] [Google Scholar]
- Leadsham J. E., Gourlay C. W. (2010). cAMP/PKA signaling balances respiratory activity with mitochondria dependent apoptosis via transcriptional regulation. BMC Cell Biol. 11 92 10.1186/1471-2121-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., Hoe K. L., Maeng P. J. (2007). Yeast cells lacking the CIT1-encoded mitochondrial citrate synthase are hypersusceptible to heat- or aging-induced apoptosis. Mol. Biol. Cell 18 3556–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre S., Sliwa D., Auchere F., Brossas C., Ruckenstuhl C., Boggetto N., Lesuisse E., Madeo F., Camadro J. M., Santos R. (2012). The yeast metacaspase is implicated in oxidative stress response in frataxin-deficient cells. FEBS Lett. 586 143–148 [DOI] [PubMed] [Google Scholar]
- Li L. Y., Luo X., Wang X. (2001). Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412 95–99 [DOI] [PubMed] [Google Scholar]
- Li W., Sun L., Liang Q., Wang J., Mo W., Zhou B. (2006). Yeast AMID homologue Ndi1p displays respiration-restricted apoptotic activity and is involved in chronological aging. Mol. Biol. Cell 17 1802–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Butow R. A. (2006). Mitochondrial retrograde signaling. Annu. Rev. Genet. 40 159–185 [DOI] [PubMed] [Google Scholar]
- Longo V. D. (2003). The Ras and Sch9 pathways regulate stress resistance and longevity. Exp. Gerontol. 38 807–811 [DOI] [PubMed] [Google Scholar]
- Ludovico P., Rodrigues F., Almeida A., Silva M. T., Barrientos A., Corte-Real M. (2002). Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell 13 2598–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludovico P., Sousa M. J., Silva M. T., Leao C., Corte-Real M. (2001). Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 147 2409–2415 [DOI] [PubMed] [Google Scholar]
- Madeo F., Carmona-Gutierrez D., Ring J., Büttner S., Eisenberg T., Kroemer G. (2009). Caspase-dependent and caspase-independent cell death pathways in yeast. Biochem. Biophys. Res. Commun. 382 227–231 [DOI] [PubMed] [Google Scholar]
- Madeo F., Herker E., Maldener C., Wissing S., Lachelt S., Herlan M., Fehr M., Lauber K., Sigrist S. J., Wesselborg S., Frohlich K. U. (2002). A caspase-related protease regulates apoptosis in yeast. Mol. Cell 9 911–917 [DOI] [PubMed] [Google Scholar]
- Manon S., Chaudhuri B., Guerin M. (1997). Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett. 415 29–32 [DOI] [PubMed] [Google Scholar]
- Manon S., Priault M., Camougrand N. (2001). Mitochondrial AAA-type protease Yme1p is involved in Bax effects on cytochrome c oxidase. Biochem. Biophys. Res. Commun. 289 1314–1319 [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Xu Q., Velours J., Reed J. C. (1998). The mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell 1 327–336 [DOI] [PubMed] [Google Scholar]
- Oettinghaus B., Frank S., Scorrano L. (2011). Tonight, the same old, deadly programme: BH3-only proteins, mitochondria and yeast. EMBO J. 30 2754–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C., Camougrand N., Manon S., Sousa M. J., Corte-Real M. (2007). ADP/ATP carrier is required for mitochondrial outer membrane permeabilization and cytochrome c release in yeast apoptosis. Mol. Microbiol. 66 571–582 [DOI] [PubMed] [Google Scholar]
- Pereira C., Chaves S., Alves S., Salin B., Camougrand N., Manon S., Sousa M. J., Corte-Real M. (2010). Mitochondrial degradation in acetic acid-induced yeast apoptosis: the role of Pep4 and the ADP/ATP carrier. Mol. Microbiol. 76 1398–1410 [DOI] [PubMed] [Google Scholar]
- Pereira C., Silva R. D., Saraiva L., Johansson B., Sousa M. J., Corte-Real M. (2008). Mitochondria-dependent apoptosis in yeast. Biochim. Biophys. Acta 1783 1286–1302 [DOI] [PubMed] [Google Scholar]
- Pozniakovsky A. I., Knorre D. A., Markova O. V., Hyman A. A., Skulachev V. P., Severin F. F. (2005). Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J. Cell Biol. 168 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro G. F., Corte-Real M., Johansson B. (2006). Characterization of DNA damage in yeast apoptosis induced by hydrogen peroxide, acetic acid, and hyperosmotic shock. Mol. Biol. Cell 17 4584–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler M., Jarolim S., Heeren G., Palle E., Perju S., Klinger H., Bogengruber E., Madeo F., Braun R. J., Breitenbach-Koller L., Breitenbach M., Laun P. (2006). MMI1 (YKL056c, TMA19), the yeast orthologue of the translationally controlled tumor protein (TCTP) has apoptotic functions and interacts with both microtubules and mitochondria. Biochim. Biophys. Acta 1757 631–638 [DOI] [PubMed] [Google Scholar]
- Roosen J., Engelen K., Marchal K., Mathys J., Griffioen G., Cameroni E., Thevelein J. M., De Virgilio C., De Moor B., Winderickx J. (2005). PKA and Sch9 control a molecular switch important for the proper adaptation to nutrient availability. Mol. Microbiol. 55 862–880 [DOI] [PubMed] [Google Scholar]
- Ruckenstuhl C., Büttner S., Carmona-Gutierrez D., Eisenberg T., Kroemer G., Sigrist S. J., Frohlich K. U., Madeo F. (2009). The Warburg effect suppresses oxidative stress induced apoptosis in a yeast model for cancer. PLoS ONE 4 e4592 10.1371/journal.pone.0004592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza K., Bannister W., Balzan R. (2008). Mitochondrial involvement in aspirin-induced apoptosis in yeast. Microbiology 154 2740–2747 [DOI] [PubMed] [Google Scholar]
- Scheckhuber C. Q., Erjavec N., Tinazli A., Hamann A., Nystrom T., Osiewacz H. D. (2007). Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nat. Cell Biol. 9 99–105 [DOI] [PubMed] [Google Scholar]
- Severin F. F., Hyman A. A. (2002). Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 12 R233–R235 [DOI] [PubMed] [Google Scholar]
- Sevrioukova I. F. (2011). Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid. Redox Signal. 14 2545–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A., Almeida B., Sampaio-Marques B., Reis M. I., Ohlmeier S., Rodrigues F., Vale A., Ludovico P. (2011). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a specific substrate of yeast metacaspase. Biochim. Biophys. Acta 1813 2044–2049 [DOI] [PubMed] [Google Scholar]
- Silva R. D., Sotoca R., Johansson B., Ludovico P., Sansonetty F., Silva M. T., Peinado J. M., Corte-Real M. (2005). Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Mol. Microbiol. 58 824–834 [DOI] [PubMed] [Google Scholar]
- Sinibaldi F., Howes B. D., Piro M. C., Polticelli F., Bombelli C., Ferri T., Coletta M., Smulevich G., Santucci R. (2010). Extended cardiolipin anchorage to cytochrome c: a model for protein-mitochondrial membrane binding. J. Biol. Inorg. Chem. 15 689–700 [DOI] [PubMed] [Google Scholar]
- Sokolov S., Knorre D., Smirnova E., Markova O., Pozniakovsky A., Skulachev V., Severin F. (2006). Ysp2 mediates death of yeast induced by amiodarone or intracellular acidification. Biochim. Biophys. Acta 1757 1366–1370 [DOI] [PubMed] [Google Scholar]
- Vande Walle L., Lamkanfi M., Vandenabeele P. (2008). The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ. 15 453–460 [DOI] [PubMed] [Google Scholar]
- Wang C., Youle R. J. (2009). The role of mitochondria in apoptosis*. Annu. Rev. Genet. 43 95–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewski M., Scorrano L. (2009). The changing shape of mitochondrial apoptosis. Trends Endocrinol. Metab. 20 287–294 [DOI] [PubMed] [Google Scholar]
- Wilkinson D., Ramsdale M. (2011). Proteases and caspase-like activity in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 39 1502–1508 [DOI] [PubMed] [Google Scholar]
- Wissing S., Ludovico P., Herker E., Büttner S., Engelhardt S. M., Decker T., Link A., Proksch A., Rodrigues F., Corte-Real M., Frohlich K. U., Manns J., Cande C., Sigrist S. J., Kroemer G., Madeo F. (2004). An AIF orthologue regulates apoptosis in yeast. J. Cell Biol. 166 969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Wang J., Gao Y., Lin H., Du L., Yang S., Long S., She Z., Cai X., Zhou S., Lu Y. (2010). The anthracenedione compound bostrycin induces mitochondria-mediated apoptosis in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 10 297–308 [DOI] [PubMed] [Google Scholar]
- Yamaki M., Umehara T., Chimura T., Horikoshi M. (2001). Cell death with predominant apoptotic features in Saccharomyces cerevisiae mediated by deletion of the histone chaperone ASF1/CIA1. Genes Cells 6 1043–1054 [DOI] [PubMed] [Google Scholar]
- Yang H., Ren Q., Zhang Z. (2008). Cleavage of Mcd1 by caspase-like protease Esp1 promotes apoptosis in budding yeast. Mol. Biol. Cell 19 2127–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ždralević M., Guaragnella N., Antonacci L., Marra E., Giannattasio S. (2012). Yeast as a tool to study signaling pathways in mitochondrial stress response and cytoprotection. Sci. World J. 2012 912147 [DOI] [PMC free article] [PubMed] [Google Scholar]