Abstract
Micro-CT is currently used in preclinical studies to provide anatomical information. But, there is also significant interest in using this technology to obtain functional information. We report here a new sampling strategy for 4D micro-CT for functional cardiac and pulmonary imaging.
Rapid scanning of free-breathing mice is achieved with fast prospective gating (FPG) implemented on a Field Programmable Gate Array. The method entails on-the-fly computation of delays from the R peaks of the ECG signals or the peaks of the respiratory signals for the triggering pulses. Projection images are acquired for all cardiac or respiratory phases at each angle before rotating to the next angle. FPG can deliver the faster scan time of retrospective gating (RG) with the regular angular distribution of conventional prospective gating (PG) for cardiac or respiratory gating. Simultaneous cardio-respiratory gating is also possible with FPG in a hybrid retrospective/prospective approach. We have performed phantom experiments to validate the new sampling protocol, and we have compared the results from FPG and RG in cardiac imaging of a mouse. Additionally, we have evaluated the utility of incorporating respiratory information in 4D cardiac micro-CT studies with FPG. A dual source micro-CT system was used for image acquisition with pulsed x-ray exposures (80 kVp, 100 mA, 10 ms). The cardiac micro-CT protocol involves the use of a liposomal blood pool contrast agent containing 123 mg I/ml delivered via a tail vein catheter in a dose of 0.01 ml/g body weight.
The phantom experiment demonstrates that FPG can distinguish the successive phases of phantom motion with minimal motion blur, and the animal study demonstrates that respiratory FPG can distinguish inspiration and expiration. 4D cardiac micro-CT imaging with FPG provides image quality superior to RG at an isotropic voxel size of 88 microns and 10 ms temporal resolution. The acquisition time for either sampling approach is less than 5 minutes. The radiation dose associated with the proposed method is in the range of a typical micro-CT dose (256 mGy for the cardiac study). Ignoring respiration does not significantly affect anatomic information in cardiac studies.
FPG can deliver short scan times with low-dose 4D micro-CT imaging without sacrificing image quality. FPG can be applied in high-throughput longitudinal studies in a wide range of applications, including drug safety and cardiopulmonary phenotyping.
Keywords: cardiac, small animal imaging, lung, micro-CT
Introduction
Micro-CT is widely used for small animal imaging in preclinical studies, and there is substantial interest in using these systems to obtain functional information. The relatively recent development of functional clinical CT has highlighted the value of the fourth dimension—time—in characterizing cardiac function and blood flow (Johnson et al., 2006; Vembar et al., 2003). Translation of the technology to preclinical studies has enormous potential to help understand critical pathways in genetic models and to highlight potential concerns in drug safety evaluation Ritman, 2004; Badea et al., 2008a). 4D cardiac micro-CT faces formidable challenges due to the small size of the mouse heart (the diameter of which is only 5 mm) and rapid heart rates (up to 600 beats/minute), thus requiring both high spatial resolution and high temporal resolution.
To minimize the influence of physiological motion, cardio-respiratory gating strategies play an important role during scanning. 4D micro-CT typically employs either prospective gating (PG) or retrospective gating (RG). Both strategies have advantages and disadvantages. PG ensures equiangular distribution of projections, but it requires long acquisition times, since each x-ray exposure is triggered by a coincidence of selected phases of the respiratory and ECG cycles, e.g. end-expiration and diastole. RG is fast, but it produces projections with irregular angular distribution that results in streaking artifacts in the reconstructed images. We have previously introduced a cine cardiac micro-CT protocol based on PG (Badea et al., 2005). Other groups have implemented RG on a slip-ring flat panel based micro-CT system for cardiac studies in mice (Drangova et al., 2007; Bartling et al., 2007).
In this work, we explore a novel sampling strategy, fast prospective gating (FPG), that combines the advantages of RG and PG, and we present its implementation with a LabVIEW (National Instruments, Austin TX) field-programmable gate array module. FPG can deliver the faster scan time of RG with the regular angular distribution of PG for cardiac or respiratory gating. Simultaneous cardio-respiratory gating is also possible with FPG in a hybrid retrospective/prospective approach. We have performed phantom experiments to validate the new sampling protocol. We have also compared the results from FPG and RG in cardiac imaging of a mouse. Additionally, we have evaluated the utility of incorporating respiratory information in 4D cardiac micro-CT studies with FPG.
Materials and Methods
1 .Micro-CT system
Image data is acquired with a dual source micro-CT system developed explicitly for dynamic applications that has been described in detail elsewhere (Badea et al., 2008b). Our dual source system contains two imaging chains, which are arranged orthogonally. Each imaging chain is composed of one x-ray tube and one detector. At each time point, two orthogonal cone beam projections are acquired simultaneously. Thus, the dual source system reduces the sampling time by half. The system contains two G-297 x-ray tubes (Varian Medical Systems, Palo Alto, CA) with 0.3/0.8 mm focal spot size, two Epsilon High Frequency X-ray generators by EMD Technologies (Quebec, Canada), and two CCD-based detectors with a Gd2O2S phosphor (XDI-VHR 2 Photonic Science, East Sussex, UK) with 22 micron pixels, which we typically bin to 88 microns. The data acquisition is controlled by a sequencing application written in LabVIEW. We use pulsed x-rays with a short exposure time of 10 ms to limit motion blur.
2. Fast Prospective Gating
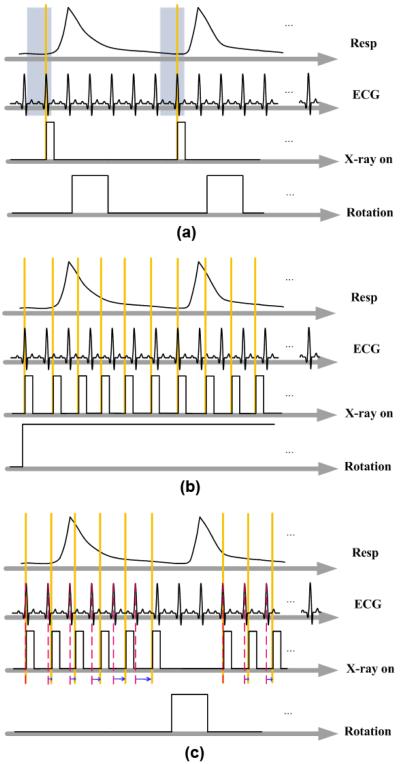
The goal of 4D micro-CT imaging is to reconstruct a series of volumes from a set of x-ray projections of an object that rapidly changes in shape or composition over time. Two gating strategies are used in small animal cardiopulmonary imaging, PG and RG. In PG, acquisition is triggered by the coincidence of a selected respiratory phase and a selected cardiac phase, as shown in Fig. 1(a). This produces a set of projections with a constant angular step, resulting in reconstructed images that are free of streaking artifacts. However, because of the time spent waiting for the coincidence of cardiac and respiratory events, the scan time can take as long as 1 hour to cover 10 different phases of the cardiac cycle (Badea et al., 2005).
Fig. 1.
Schematic drawing of different gating strategies. In PG (a), acquisition waits for the coincidence of the desired respiratory and cardiac phases. In RG (b), acquisition occurs at a fast and constant rate while ECG and respiratory signals are recorded. In FPG (c), projections of all phases are acquired at each angle before rotation, and the delays between each acquisition are computed in real time.
In RG, the projection images are acquired at a rapid and constant rate without waiting for cardiac and respiratory coincidence, as shown in Fig. 1(b). Respiratory and cardiac motion are monitored and saved in synchrony with the acquisition of the projections. Using these signals, the projections are retrospectively sorted into different subsets corresponding to different cardiac and respiratory phases. With this protocol, the scan time can be shortened to 50 seconds, when using a slip ring gantry (Drangova et al., 2007). However, the irregular angular distribution has a deleterious effect on the quality of the reconstructed images.
FPG combines the regular angular distribution of PG with the fast scan time of RG when the focus is on respiratory or cardiac imaging. In FPG, we acquire multiple projections at the same angle, corresponding to all cardiac or respiratory phases to be reconstructed, before the cradle is rotated to the next angle. FPG requires on-the-fly computation of the triggering events, which are delayed from the peaks of the respiratory or cardiac signals. The idea of adjusting imaging parameters during sampling has been also applied in MR imaging to deal with motion artifacts (Haacke and Patrick, 1986; Pelc and Glover, 1987; Bailes et al., 1985).
As shown in Fig.1(c) for cardiac gating only, a different delay of the x-ray trigger is required in successive cardiac cycles. To calculate this delay, the ECG signal is continuously monitored, the R peaks are detected based on their amplitude, and the RR period is found. A running average of the previous RR periods is used to predict the next RR period. In our study, the RR period is divided into Nφ = 10 intervals corresponding to different cardiac phases (i.e. from 0% to 90%) of the RR period, and the starting time of the interval of the desired phase in the predicted RR period determines the next delay. FPG can be similarly applied to respiratory imaging if the ECG signal is replaced with a respiratory signal. FPG can also be used for combined cardio-respiratory gating if respiratory information is considered and projections are sorted post-reconstruction, similar to the RG approach. However, as shown in this paper, respiratory information may not be necessary for cardiac micro-CT studies. A comparison of the three different gating strategies implemented on our system is shown in Table I.
Table I.
Parameters and advantages for three different scan protocols. Note that implementation of these protocols vary between different system designs; we have selected two typical versions of PG and RG on our own system to compare with the proposed FPG.
| PG | RG | FPG | |
|---|---|---|---|
| Respiratory signals |
Intubation and ventilation or free breathing with pneumatic pillow |
Free breathing with pneumatic pillow |
Intubation and ventilation or free breathing with pneumatic pillow |
|
| |||
| Cardiac signals | ECG | ECG | ECG |
|
| |||
| Gating Strategy | Cardiac and respiratory gating |
Cardiac and respiratory gating |
Cardiac or respiratory gating |
| Acquisition triggered by the coincidence between the appropriate respiratory and cardiac phases using fixed RR percentage for time delays |
Continuous rotation and acquisition of projections |
Real time prediction of RR and computation of the time delays |
|
| ECG and respiration signals saved during sampling; |
Multiple projections acquired at each angle |
||
| One projection is acquired at each rotation angle |
Post-sampling sorting of projections |
||
| Cardiac phases are acquired sequentially |
|||
|
| |||
| Number of rotations during scanning |
Equal to the number of sampled phases |
4 or 5 rotations | Single rotation |
|
| |||
| Acquisition time | > 30 minutes | 5 minutes | 5 minutes |
|
| |||
| Advantage | Equiangular distribution of projections results in good image quality |
Fast acquisition | Equiangular distribution of projections results in good image quality |
| Fast acquisition | |||
| No registration errors between cardiac phases |
|||
|
| |||
| Disadvantage | Long acquisition times | Irregular angular distribution of projections results in streaking artifacts in reconstruction |
Only respiration or ECG considered in acquisition |
| The sequential acquisition of all cardiac phases results in registration errors |
If both cardiac and respiratory gating are considered, the distribution of projections becomes irregular |
||
3. FPG Implementation
To meet the requirements of FPG for real-time processing, we implemented the new acquisition protocol on a reconfigurable data acquisition card (PCI-7811R, National Instruments, Texas). The card has R-series digital reconfigurable input/outputs (RIOs) at rates up to 40MHz with a Virtex-II 1M Field-Programmable Gate Array chip for onboard processing. The chip is integrated with LabVIEW, and can be programmed using the LabVIEW field-programmable gate array module, which creates VHDL code from the graphical code. The VHDL code is then transferred to the Xilinx tool chain to create a bit file, which is uploaded to the field-programmable gate array chip. Since multiple control loops and computations can be executed independently, we can achieve high loop rates and precise counting. During sampling, all physiological signals are processed with Coulbourn modules (Coulbourn Instruments, Allentown, PA) and displayed on a computer monitor using a custom LabVIEW application. The Coulbourn modules include a comparator that generates a transistor-transistor logic(TTL) signal based on the R peaks in the ECG signal. This digital TTL is directed to the digital I/O port of the PCI-7811R card. When a rising edge is detected, the time elapsed since the previous rising edge determines the RR period. To predict the next RR period used to calculate the phase delay, we use an exponential moving average (Peterson and Silberschatz, 1985; Hwang and Wu, 2000). The predicted period is computed with a recursive formula:
| (1) |
where a is a constant proportionality coefficient between 0 and 1, RRn+1 is the next predicted value, RRn is the previous predicted value, and RR is the most recent measured value. The predicted RR value is used to calculate the current phase delay. The parameter a determines the relative weight of recent measurements in the prediction. If a = 0, then RRn+1 = RRn and the most recent measured RR value has no effect. If a = 1, then RRn+1 = RR and the prediction only takes into account the most recent measured value and ignores previous values, and may be more sensitive to the influence of noise. In our implementation, we use a = 0.5, so that the previous value and the most recent value are weighted equally. This recursive method allows us to adapt the sampling rate over the course of the scan for animals with irregular heart rates.
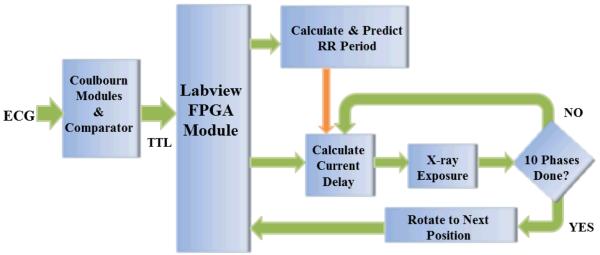
Using the chip’s 40-MHz timer on the PCI-7811R board, the delay for each phase can be calculated by a Single Cycle Timed Loop (SCTL) in 25 ns with a loop timer. When the time elapsed since the last R peak equals the delay, the field-programmable gate array generates a predefined output to trigger the exposure of the x-ray tube. Once the projection images corresponding to all of the desired cardiac phases are acquired, the sampling program sends a trigger signal to the rotation stage to rotate to the next angular position and start the acquisition process over again. This is repeated until the full acquisition arc is complete. A flowchart of FPG focused on cardiac imaging is shown in Fig. 2.
Fig. 2.
Diagram of the FPG implementation focused on cardiac imaging. The Coulbourn modules, including a band pass filter and a comparator, detect the R peaks in the ECG signals and generate TTL pulses. The TTL signal is directed to the digital I/O input port of the PCI-7811R LabVIEW field-programmable gate array module. After detecting the rising edge of a pulse, the RR period is calculated, and this value is used to continuously determine the current phase of the heart. When the phase is equal to the next phase that must be acquired, a trigger is sent and a projection is obtained. Once the projections for all of the desired cardiac phases are acquired, a trigger is sent to the rotation stage to move to the next angular position and start the acquisition process over again. This is repeated until the full acquisition arc is complete.
In FPG, the time spent at each view angle depends on the number of phases acquired and the RR interval of the mouse. In our experiments, the heart rate is approximately 625 beats/min with an average RR of 96 ms. To acquire 10 phases, we need 10 successive ECG cycles, i.e. 960 ms. Once these are acquired, the cradle rotates to the next angle. The time of rotation depends on the speed of our rotation stage and the step angle. In our case, the typical rotation speed is 16°/s and the step angle is 1.2°, which results in a rotation time of 75 ms for a single step. In practice, to limit the accumulation of heat on our x-ray tubes, we wait 10 ECG cycles before acquiring projections at the next angle.
4. Retrospective Gating
In this study, we compared FPG with standard RG. We have previously implemented both conventional PG and RG, and proposed combinations of the two approaches (Badea et al., 2008c). In RG, all input and output signals, including the ECG, ECG TTL, x-ray exposure, and all triggers can be monitored in synchrony with acquisition and stored in the host computer. Post-acquisition, the projection images are sorted and associated with angle, cardiac, and respiratory phase information using a MATLAB (MathWorks, Natick, MA) script. This script detects the R peaks in the ECG signal and the maxima in the respiratory signal. Each cardiac cycle is divided into Nφ intervals (each equal to 10% of the RR interval in our case for Nφ = 10). Each projection is temporally registered with the ECG signal by finding the temporal distance from the previous R peak that was closest to the projection sampling time, and dividing the distance by the RR period. This information is kept in lists that are saved in files and used during reconstruction. Note that we have also monitored the signals and sorted the projections for validation of FPG in phantom studies, but in practice, no monitoring or sorting is necessary for FPG if only cardiac or respiratory gating is needed.
In cases where simultaneous cardio-respiratory gating is of interest, a retrospective respiratory sorting approach can be applied in FPG. Two respiratory phases are considered, inspiration and expiration, and each projection is associated with one of these phases using amplitude-based detection of the inspiration events in the monitoring file.
5. Image Reconstruction
Analytical reconstruction algorithms, such as filtered backprojection (FBP) (Feldkamp et al., 1984), suffer from streaking artifacts when the projections have an irregular or sparse angular distribution. As we have shown previously (Song et al., 2007), FBP reconstruction with undersampling produces fewer artifacts when the distribution of the projections is equiangular. If focused solely on respiratory or cardiac gating, FPG ensures a regular angular distribution of projections, and is thus more amenable to angular undersampling. In studies where cardio-respiratory gating is desired, the retrospective sorting reduces the number of views and introduces irregularities in the distribution of the projections. The reconstructed images from the RG scans suffered from more streaking artifacts than the FPG scans.
6. Phantom Validation
We performed phantom studies to validate the accuracy of the phase selection in our FPG implementation. We constructed a phantom consisting of a plastic container filled with a dense liquid soap solution, in which we immersed a balloon connected to a mechanical ventilator system (Hedlund et al., 1986). We adjusted the exhalation/inhalation ratio of the ventilator to set the inflation/deflation cycle of the balloon at a rate of 200 cycles/minute. In this experiment, the exposure parameters of the micro-CT scanner were set to 80 kVp, 100 mA, and 10 ms for both imaging chains. The scanner acquired a total of 2000 projections (10 phases acquired at each angle) with a step angle of 3.8°. The ventilation and x-ray exposure trigger signals were recorded during the acquisition. For each projection, we compared the phase determined by FPG with the phase determined by RG at the post-sampling level.
7. Animal Experiments
We performed animal studies on Sprague Dawley (SD) rats and C57BL/6 mice. All animal studies were conducted under the protocol approved by the Duke University Institutional Animal Care and Use Committee (IACUC). To provide the necessary blood/tissue contrast, we used a liposomal blood pool contrast agent containing 123 mg I/ml delivered by injection via a tail vein catheter at a dose of 0.01 ml/g body weight. Post-injection, the contrast between blood and myocardium was about 500 HU. Animals were anesthetized with isoflurane (1.5%) mixed with 50% oxygen and balanced with nitrogen. ECG was monitored with electrodes (Blue Sensor, Medicotest, UK) taped to the footpads, and body temperature was maintained with heat lamps, a rectal probe, and feed-back controller (Digi-Sense®, Cole Parmer, Chicago, IL).
We first performed a respiratory FPG scan with an SD rat. The animal was intubated and mechanically ventilated during imaging. The tidal volume was 2.5 ml. The respiratory cycle was divided into two phases, inspiration and expiration, with 360 projections acquired for each respiratory phase. The precise control over respiration provided by the intubation and ventilation allowed us to verify the correctness of the FPG phase determination.
In the mouse studies, we used a free breathing protocol. A pneumatic pillow on the thorax was used to monitor respiration. During a typical experiment, the average heart rate of the mouse reached as much as 625 beats/minute, while the respiration rate was approximately 260 breaths/minute.
We then performed cardiac imaging with both FPG and RG. The exposure parameters of the micro-CT scanner were set to 80 kVp, 100 mA, and 10 ms per exposure for both imaging chains. In the RG scan, 1800 projections were acquired over 4 complete 360° rotations. In the FPG scan, 1600 projections were acquired over a half scan with a 96° arc (192° from the combined imaging chains) and a step angle of 1.2°, with 10 projections acquired at each angle corresponding to 10 cardiac phases. The total acquisition times for the two scans were less than 5 minutes. The radiation dose was 288 mGy in the RG scan and 256 mGy in the FPG scan.
To quantitatively assess the results of the different gating and reconstruction techniques, both the standard deviation (STD) and contrast-to-noise ratio (CNR) were computed in selected regions of interest (ROIs) in the reconstructed images. In this study, the CNR was computed as:
| (2) |
where Hb and Hs are the average attenuation coefficients in the ROIs in the left ventricle and the myocardium, measured in Hounsfield units, and σb and σs are the STDs in the same ROIs.
Results
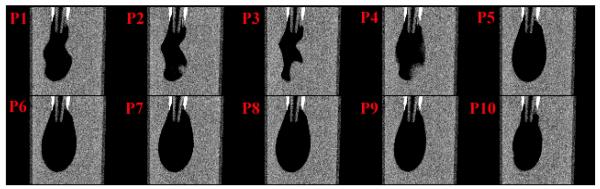
The results from the physical phantom study are shown in Fig. 3. Ten different phases of the dynamic balloon phantom are distinguished. Note that this experiment involves sharp contrast between the balloon and the surrounding medium, highlighting the presence of motion blur. The blur in Phase 4 was mostly caused by irregularities in the inflation/deflation pattern of the balloon. According to the monitoring files, the accuracy of the FPG phase identification was 98%.
Fig. 3.
The coronal reconstructed micro-CT slices of the balloon phantom during 10 phases of the motion cycle. An animation of these images confirms the ability of FPG to distinguish each temporal phase. The blurring in Phase 4 (P4) was associated with irregularities in the inflation/deflation pattern of the balloon.
To investigate the robustness of our prediction of the RR interval, we applied our technique to a set of ECG data recorded during an RG experiment. This set consisted of 16000 RR intervals. At each interval, we applied equation (1) to predict the RR length, and then calculated the percentage difference between the predicted length and the actual length. The average percentage error was 0.8%. Since we aim to distinguish cardiac phases that are 10% of the cardiac cycle, we consider the predictions sufficiently robust. In the final reconstruction, we expect these errors to result in small blurring around moving structures.
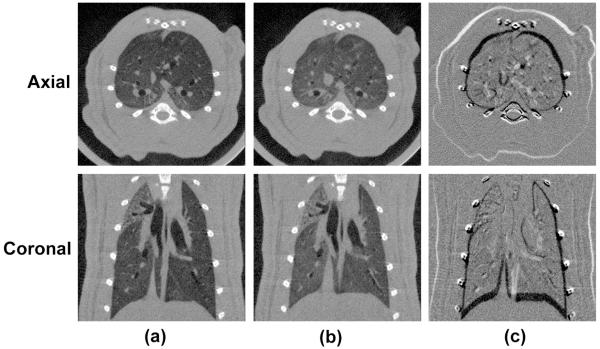
Fig. 4 displays pulmonary micro-CT images of a rat acquired with FPG and reconstructed with FBP. Both axial and coronal planes during end inspiration are shown in Fig. 4(a) and end expiration is shown in Fig. 4(b). To enhance the visualization of the differences between the two respiratory phases, subtraction images are shown in Fig. 4(c). With FPG, lung structures such as the airways and diaphragm are clear and sharp. The diaphragm and lung structures appear to change position between inspiration and expiration.
Fig. 4.
Pulmonary micro-CT images of a rat acquired with FPG. Both end-inspiration (a) and end-expiration (b)images in axial and coronal orientations are shown. The subtraction between two different respiratory phases is shown in (c). The images were reconstructed with FBP using 360 projections.
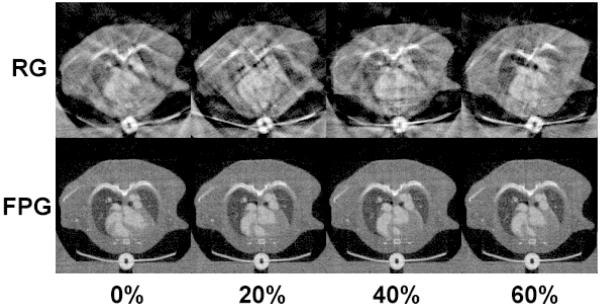
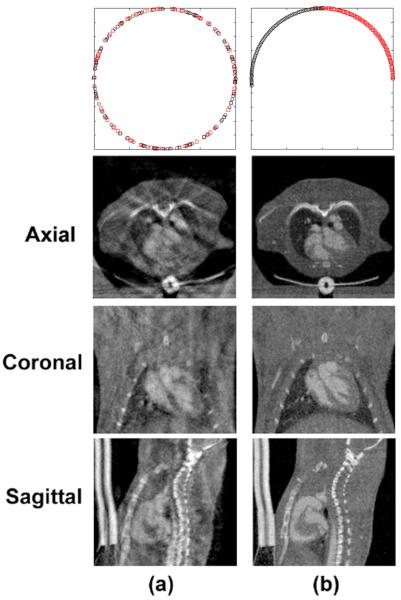
Fig. 5 compares images acquired with RG and FPG, which were reconstructed with FBP. Axial micro-CT images are shown at 0%, 20%, 40%, and 60% of the RR interval, scaled from −900 HU to 1700 HU. We note that this is just a subset of four phases out of the total of ten phases that were reconstructed. In both scans, respiratory information was ignored. For these cardiac phases using RG, there were 202, 168, 178, and 172 projections. Since the total number of projections in the RG scan was 1800, the efficiency ratios (i.e. the numbers of projections used for each cardiac phase over the total number of projections acquired) were 11.22%, 9.33%, 9.89%, and 9.56%. In the FPG scan, the total number of projections for each cardiac phase was consistently 160 and the total number of projections was 1600, so the efficiency ratios were constant and equal to 10%. But, more important than the variations in efficiency ratios is the irregular angular distributions of these projections in RG compared with FPG. This is illustrated in Fig. 6 (top), where each projection is represented as a circle or square and color-coded in red and black for the two imaging chains. Fig. 6 also illustrates the volumetric nature of each cardiac dataset. Micro-CT images are shown in axial, coronal, and sagittal slices at 0% of the RR interval and scaled from −900 HU to 1700 HU. The images acquired with RG are compromised by streaking artifacts, and the boundaries of the anatomical structures (especially the myocardium) appear blurry and indistinct. Images acquired with FPG, on the other hand, are relatively free of streaking artifacts.
Fig. 5.
Comparison of axial images acquired with RG and FPG, which are reconstructed with FBP. Images are shown for 4 different cardiac phases: 0%, 20%, 40%, and 60% of the RR interval. In FPG, the reconstruction results show much sharper boundaries and less streaking artifacts.
Fig. 6.
Reconstructed micro-CT images shown in axial, coronal, and sagittal orientations. The images were generated with different gating strategies, RG (a) and FPG (b). The first row illustrates the angular distributions of the projections for each gating strategy, where each projection is represented as a circle or square and color-coded in black and red for the two imaging chains. In this set, RG acquired 202 projections, while FPG acquired 160 projections at this phase (0% of RR interval).
Table II shows the STD and CNR from the different sampling protocols. We selected ROIs in the air, myocardium and in the blood in the left ventricle, and found that the CNR of FPG is greater than that of RG. Even though the number of projections in the RG scan is higher than in the FPG scan, the noise levels are still higher in RG images.
Table II.
A comparison of figures of merit for FPG and RG.
| RG | FPG | |
|---|---|---|
| CNR | 4.23 | 6.20 |
| STD of air [HU] | 139.15 | 89.17 |
| STD of blood [HU] | 132.47 | 73.99 |
| STD of myocardium [HU] | 123.89 | 85.74 |
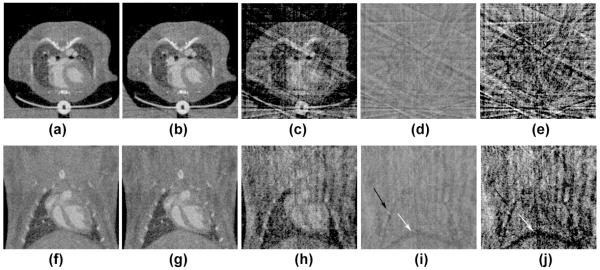
The effect of including or ignoring the respiratory information in cardiac FPG micro-CT is illustrated in Fig. 7. Both expiration and inspiration-biased reconstructions were produced by retrospectively sorting projections in two respiratory sets. When respiratory information is ignored, the cardiac micro-CT images display lower noise and no streaking artifacts (Figs. 7 (a), (f)). For the micro-CT images corresponding to phase 0% of the RR, most projections (i.e. 130 out of 160) occurred during expiration. Consequently, the expiration-biased images (Figs. 7 (b), (g)) show fewer artifacts than inspiration-biased images (Figs.7 (c), (e)). Subtraction of these expiration or inspiration-biased images from the reconstructions where respiration information was ignored are shown in Figs. 7 (d), (i) and Figs.7 (e), (j). A shadow around the diaphragm is apparent in the subtraction images. However, much less structural detail appears around the heart. Consequently, respiratory information may not be important in mouse cardiac studies.
Fig. 7.
The effect of respiration on cardiac micro-CT images (Phase 0% of RR) acquired with FPG. Axial and coronal slices are shown in which respiratory information was considered or ignored in the selection of projections used for reconstruction. In (a, f), all 160 projections independent of respiratory phase were used. In (b, d) the projections during inspiration were discarded. 130 projections were available for this expiration-biased reconstruction. Similarly, (c, h) presents inspiration-biased reconstruction using only the remaining 30 projections. Subtraction images are shown between the respiration-independent set, and inspiration-biased (d, i) or the expiration-biased (e, j) sets. Note that structured details around the ribs (black arrows) and the diaphragm (white arrows) can be discerned in the subtracted images, but are less apparent around the heart.
Discussion and Conclusions
We have demonstrated the potential of FPG to combine the equiangular distribution of PG with the short scan time of RG. The images are relatively free of streaking artifacts. FPG works best, if the focus is only on respiratory or cardiac imaging. Combining the two in sampling, as in the cardio-respiratory gating strategy that we have presented previously (Badea et al., 2004), increases the scan time for FPG to levels similar to conventional PG. In fact, we are not the first to propose a cardiac imaging protocol in the mouse that ignores respiratory motion. Bucholz et al. (Bucholz et al., 2008) proposed such a protocol for 4D magnetic resonance imaging. Respiratory information can be obtained in an extrinsic or intrinsic approach and used to sort and discard projections retrospectively. In such cases, the angular distribution of the FPG projections becomes irregular, but the irregularities are less pronounced and the streaking artifacts less severe than with conventional RG. We have investigated the importance of respiratory information for cardiac imaging. The number of projections for expiration-biased reconstruction was reduced by approximately 19%, resulting in an irregular angular distribution. We subtracted the reconstructions that used this strategy from the reconstructions with the full set of projections that ignored respiratory information. The subtracted images show structural details around the diaphragm, but much less around the heart, suggesting that respiratory information may not be essential in cardiac studies. We have previously proposed weighting the projections during reconstruction to favor a certain respiratory phase (Johnston et al., 2010b). This alternative strategy can allow us to maintain the number of projections with FPG for simultaneous cardiac and respiratory gating.
FPG has additional advantages over PG and RG. Since conventional PG acquires the information for each cardiac phase in one contiguous set at a time, physiological and mechanical changes might occur over the course of a study between the different sets, which can distort the results of post-processing segmentation and cardiac function estimation (Badea et al., 2008d). Both RG and FPG interleave the acquisition of the sets, ensuring that the physiological state and the position of the animal are consistent across all sets. FPG is also more robust than PG in acquiring consistent data from scans of animals with respiratory or cardiac rates that vary over the course of the scan, since PG assumes a single value for the period of the cycle, while FPG measures and predicts this value throughout the scan. However, if the predicted RR is very different from the real RR (due to a sudden irregular heart beat), FPG will not calculate the correct phase delays.
Since RG acquires projections over all phases of all physiological cycles, most of these projections are wasted if only a few phases are needed. This is often the case, if the goal of the study is to compare end-inspiration and end-expiration in the respiratory cycle, or systole and diastole in the cardiac cycle. Consequently, FPG can provide higher dose efficiency than RG.
Our studies used extrinsic gating, in which the cardiac and respiratory signals were acquired with dedicated monitoring devices, i.e. ECG leads and a pneumatic respiratory pillow. As an alternative to extrinsic gating, an intrinsic image-based gating approach without any external devices has been developed initially for a clinical cone-beam spiral CT scanner (Kachelriess et al., 2002). In intrinsic gating, a post-processing algorithm evaluates the center of mass of certain regions of interest within each projection to detect the respiratory and cardiac motion (Dinkel et al., 2008; Bartling et al., 2008; Sawall et al., 2011). While intrinsic respiratory gating is straightforward, intrinsic cardiac gating is more challenging and may require very high sampling rates to support the high heart rates in the mouse. In the work of Sawall et al.(Sawall et al., 2011), for example, due to the limitation of the detector’s readout rate, only 6 projections could be acquired during each cardiac cycle of the mouse, whose heart rate was only 260 beats/minute. In our experience, the heart rate can reach up to 600 beats/minute. Our micro-CT system can acquire up to 20 frames/second and limits our ability to perform intrinsic cardiac gating. Dynamic CT scans can involve large numbers of projections and high radiation dose. When the numbers of projections and the dose are reduced, the images reconstructed via analytic algorithms such as FBP suffer from high noise and streaking artifacts. This noise can be reduced when reconstruction is performed with other algorithms. Together, FPG combined with improved iterative reconstruction algorithms could be used to perform dynamic CT studies with fewer projections, shorter scan times, and lower radiation dose without a loss of image quality in the future.
In conclusion, this novel gating strategy for 4D micro-CT demonstrates promise for low-dose, high-throughput imaging in longitudinal studies, with immediate applications in a wide range of preclinical studies, such as in cardiopulmonary phenotyping and drug safety. While our dual source micro-CT system is unique in many ways, the FPG technique we describe here can be used in commercial systems (Drangova et al., 2007).
Acknowledgements
All work was performed at the Duke Center for In Vivo Microscopy, an NIH/NCRR national Biomedical Technology Research Center (P41 RR005959), with additional support from NCI (U24 CA092656 and K08 CA114176). Xiaolian Guo has been supported by a grant provided by China Scholarship Council (CSC). Liposomal contrast agent was provided by Ketan Ghaghada and Ananth Annapragrada (University of Texas). We also want to thank Sally Zimney for the editorial assistance.
References
- Badea C, Hedlund LW, Johnson GA. Micro-CT with respiratory and cardiac gating. Med Phys. 2004;31:3324–9. doi: 10.1118/1.1812604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea CT, Drangova M, Holdsworth DW, Johnson GA. In vivo small-animal imaging using micro-CT and digital subtraction angiography. Physics in Medicine and Biology. 2008a;53:R319–R50. doi: 10.1088/0031-9155/53/19/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea CT, Fubara B, Hedlund LW, Johnson GA. 4-D micro-CT of the mouse heart. Mol Imaging. 2005;4:110–6. doi: 10.1162/15353500200504187. [DOI] [PubMed] [Google Scholar]
- Badea CT, Johnston S, Johnson B, Lin M, Hedlund LW, Johnson GA. A dual micro-CT system for small animal imaging - art. no. 691342. P Soc Photo-Opt Ins. 2008b;6913:91342–2081. doi: 10.1117/12.772303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea CT, Schreibmann E, Fox T. A registration based approach for 4D cardiac micro-CT using combined prospective and retrospective gating. Med Phys. 2008c;35:1170–9. doi: 10.1118/1.2868778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea CT, Wetzel AW, Mistry N, Pomerantz S, Nave D, Johnson GA. Left ventricle volume measurements in cardiac micro-CT: The impact of radiation dose and contrast agent. Comput Med Imag Grap. 2008d;32:239–50. doi: 10.1016/j.compmedimag.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes DR, Gilderdale DJ, Bydder GM, Collins AG, Firmin DN. Respiratory ordered phase encoding (ROPE): a method for reducing respiratory motion artefacts in MR imaging. J Comput Assist Tomogr. 1985;9:835–8. [PubMed] [Google Scholar]
- Bartling SH, Dinkel J, Stiller W, Grasruck M, Madisch I, Kauczor HU, Semmler W, Gupta R, Kiessling F. Intrinsic respiratory gating in small-animal CT. Eur Radiol. 2008;18:1375–84. doi: 10.1007/s00330-008-0903-3. [DOI] [PubMed] [Google Scholar]
- Bartling SH, Stiller W, Grasruck M, Schmidt B, Peschke P, Semmler W, Kiessling F. Retrospective motion Gating in small animal CT of mice and rats. Invest Radiol. 2007;42:704–14. doi: 10.1097/RLI.0b013e318070dcad. [DOI] [PubMed] [Google Scholar]
- Bucholz E, Ghaghada K, Qi Y, Mukundan S, Johnson GA. Four-dimensional MR microscopy of the mouse heart using radial acquisition and liposomal gadolinium contrast agent. Magn Reson Med. 2008;60:111–8. doi: 10.1002/mrm.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel J, Bartling SH, Kuntz J, Grasruck M, Kopp-Schneider A, Iwasaki M, Dimmeler S, Gupta R, Semmler W, Kauczor HU, Kiessling F. Intrinsic Gating for Small-Animal Computed Tomography A Robust ECG-Less Paradigm for Deriving Cardiac Phase Information and Functional Imaging. Circ-Cardiovasc Imag. 2008;1:235–43. doi: 10.1161/CIRCIMAGING.108.784702. [DOI] [PubMed] [Google Scholar]
- Drangova M, Ford NL, Detombe SA, Wheatley AR, Holdsworth DW. Fast retrospectively gated quantitative four-dimensional (4D) cardiac micro computed tomography imaging of free-breathing mice. Invest Radiol. 2007;42:85–94. doi: 10.1097/01.rli.0000251572.56139.a3. [DOI] [PubMed] [Google Scholar]
- Feldkamp LA, Davis LC, Kress JW. Practical Cone-Beam Algorithm. Journal of the Optical Society of America a-Optics Image Science and Vision. 1984;1:612–9. [Google Scholar]
- Haacke EM, Patrick JL. Reducing motion artifacts in two-dimensional Fourier transform imaging. Magn Reson Imaging. 1986;4:359–76. doi: 10.1016/0730-725x(86)91046-5. [DOI] [PubMed] [Google Scholar]
- Hedlund LW, Deitz J, Nassar R, Herfkens R, Vock P, Dahlke J, Kubek R, Effmann EL, Putman CE. A Ventilator for Magnetic-Resonance-Imaging. Invest Radiol. 1986;21:18–23. doi: 10.1097/00004424-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Hwang CH, Wu ACH. A predictive system shutdown method for energy saving of event-driven computation. Acm T Des Automat El. 2000;5:226–41. [Google Scholar]
- Johnson TRC, Nikolaou K, Wintersperger BJ, Leber AW, von Ziegler F, Rist C, Buhmann S, Knez A, Reiser MF, Becker CR. Dual-source CT cardiac imaging: initial experience. Eur Radiol. 2006;16:1409–15. doi: 10.1007/s00330-006-0298-y. [DOI] [PubMed] [Google Scholar]
- Johnston SM, Johnson GA, Badea CT. lGPU-based iterative reconstruction with total variation minimization for micro-CT. P Soc Photo-Opt Ins. 2010a:7622–1816. [Google Scholar]
- Johnston SM, Perez BA, Kirsch DG, Badea CT. Phase-selective image reconstruction of the lungs in small animals using Micro-CT. P Soc Photo-Opt Ins. 2010b:7622–1816. doi: 10.1117/12.844359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachelriess M, Sennst DA, Maxlmoser W, Kalender WA. Kymogram detection and kymogram-correlated image reconstruction from subsecond spiral computed tomography scans of the heart. Med Phys. 2002;29:1489–503. doi: 10.1118/1.1487861. [DOI] [PubMed] [Google Scholar]
- Kamphuis C, Beekman FJ. Accelerated iterative transmission CT reconstruction using an ordered subsets convex algorithm. Ieee T Med Imaging. 1998;17:1101–5. doi: 10.1109/42.746730. [DOI] [PubMed] [Google Scholar]
- Lange K. Convergence of Em Image-Reconstruction Algorithms with Gibbs Smoothing. Ieee T Med Imaging. 1990;9:439–46. doi: 10.1109/42.61759. [DOI] [PubMed] [Google Scholar]
- Leng S, Tang J, Zambelli J, Nett B, Tolakanahalli R, Chen GH. High temporal resolution and streak-free four-dimensional cone-beam computed tomography. Physics in Medicine and Biology. 2008a;53:5653–73. doi: 10.1088/0031-9155/53/20/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng S, Zambelli J, Tolakanahalli R, Nett B, Munro P, Star-Lack J, Paliwal B, Chena GH. Streaking artifacts reduction in four-dimensional cone-beam computed tomography. Med Phys. 2008b;35:4649–59. doi: 10.1118/1.2977736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelc NJ, Glover GH. Method of reducing image artifacts due to periodic signal variations in NMR imaging. U.S. Patent 4,663,591. 1987
- Peterson JL, Silberschatz A. Operating system concepts. Reading, Mass.; Addison-Wesley: 1985. [Google Scholar]
- Ritman EL. Micro-computed tomography-current status and developments. Annu Rev Biomed Eng. 2004;6:185–208. doi: 10.1146/annurev.bioeng.6.040803.140130. [DOI] [PubMed] [Google Scholar]
- Sawall S, Bergner F, Lapp R, Mronz M, Karolczak M, Hess A, Kacheliress M. Low-dose cardio-respiratory phase-correlated cone-beam micro-CT of small animals. Med Phys. 2011;38:1416–24. doi: 10.1118/1.3551993. [DOI] [PubMed] [Google Scholar]
- Segars WP, Tsui BMW, Frey EC, Johnson GA, Berr SS. Development of a 4-D digital mouse phantom for molecular imaging research. Mol Imaging Biol. 2004;6:149–59. doi: 10.1016/j.mibio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Song J, Liu QH, Johnson GA, Badea CT. Sparseness prior based iterative image reconstruction for retrospectively gated cardiac micro-CT. Med Phys. 2007;34:4476–83. doi: 10.1118/1.2795830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembar M, Garcia MJ, Heuscher DJ, Haberl R, Matthews D, Bohme GE, Greenberg NL. A dynamic approach to identifying desired physiological phases for cardiac imaging using multislice spiral CT. Med Phys. 2003;30:1683–93. doi: 10.1118/1.1582812. [DOI] [PubMed] [Google Scholar]