Abstract
Exopolysaccharides (EPS) and internal (intracellular) polysaccharides (IPS) obtained from the Pleurotus ostreatus M2191 and PBS281009 cultivated using the batch system revealed an average of between 0.1–2 (EPS) and 0.07–1.5 g/L/day (IPS). The carbohydrate analysis revealed that the polysaccharides comprised 87–89% EPS and 68–74% IPS. The investigation of antioxidant activity in vitro revealed a good antioxidant potential, particularly for the IPS and EPS isolated from PBS281009, as proved by the EC50 value for DPPH, ABTS scavenging activity, reducing power, and iron chelating activity.
1. Introduction
P. ostreatus is an edible mushroom commonly occurring all over Europe. Currently, it is being cultivated as food on an industrial level. Oyster mushroom (P. ostreatus) is currently gaining popularity over the other edible mushrooms that grow in spontaneous flora, like penny bun (Boletus edulis). This is because it has the great advantage that it cannot be mistaken for any poisonous variety of mushroom. Oyster mushrooms are rich in vitamins, amino acids, and minerals, besides being significantly involved in human detoxification processes. Recent research has revealed the important role they play in reducing blood cholesterol [1]. The lovastatin, which is present in large amounts, is assumed to play an appreciable part in controlling hypercholesterolemia [2]. Interestingly, significant amounts of gallic acid, flavonoids, chlorogenic acid, lycopene, and β-carotene have been identified [3].
P. ostreatus is a low-energy food, high in fiber, but low in lipids. The mycelium contains anticarcinogenic components. Thus, the Pleurotus species are often used for their nutritional value and medicinal properties. Such effects are also possible because of the presence of biologically active compounds with therapeutic activity isolated from the mycelium [4, 5].
The polysaccharides found in mushrooms belonging to the genus Pleurotus have revealed important biological activities. Production of the mycelium biomass and polysaccharides (exopolysaccharides and internal polysaccharides) by P. ostreatus, in submerged culture, depends on the strains used, growth parameters, growth rhythm, as well as their nutritional requirements. Generally, research is focused on optimizing the culture medium in bioreactor cultivation, to maximize the productivity of the mycelium and production of polysaccharide [6]. The present investigation was aimed at revealing the mycelium production, in batch system, from P. ostreatus M2191 and PBS281009. The tests included the isolation and characterization of the exopolysaccharides and internal polysaccharides, as well as evaluation of the in vitro antioxidant activities.
2. Materials and Methods
2.1. Chemicals
All chemicals and reagents were purchased from Sigma Aldrich GmbH (Sternheim, Germany). All other unlabelled chemicals and reagents were of analytical grade.
2.2. Microorganism and Media
Mushrooms belonging to P. ostreatus M2191 were purchased from Mycelia-Belgia, while those of PBS281009 were isolated from the Baneasa forest, Romania. This was authenticated by Dr. D. Pelinescu, Faculty of Biology, University of Bucharest, Romania. The mycelia were maintained on the medium of potato dextrose agar (PDA) at 4°C. The microorganisms were subcultured at regular intervals (45 days) to maintain viability. The inoculum was prepared by growing the mushrooms on a LabTech rotary shaker at 150 rpm, for five days, at 25°C, in 500 mL Erlenmeyer flasks containing 250 mL of the synthetic medium containing 6.0 g glucose, 100.0 g malt extract, 20.0 g yeast extract, 1.0 g KH2PO4, and 0.5 g MgSO4 ·7H2O, per liter. The medium was adjusted to a pH of 5.5 with 0.2 M NaOH [7].
2.3. Fermentations
The second inoculum was performed in a 500 mL flask containing 300 mL of the medium after inoculating with 10% (v/v) of the first inoculum culture under the conditions described above. The fermentation medium (KH2PO4 0.2%, CaSO4 0.5%, MgSO4 0.05%, Na2HPO4 0.01%, corn extract (dry substance 40%) 1% in 5% solution of corn starch) was inoculated with 10% (v/v) of the second inoculum culture and then cultivated in a 5-l New Brunswick BioFlo 310 bioreactor. Fermentations were conducted under the following conditions: temperature 25°C, aeration rate 1 vvm, agitation speed 150 rpm, pH 5.5–6, and working volume 4 L. The inoculum culture was then transferred to the fermentation medium and cultivated for 10 days [7, 8].
2.4. Mycelial Growth and Polysaccharide Production
Next, samples of the fermented medium were centrifuged at 10,000 ×g for 15 min. The precipitated biomass was washed twice with ultrapure water and dried in a Memmert oven at 60°C up to a constant weight [9, 10].
The supernatant was mixed with three volumes of pure ethanol and left for 24 hours at 4°C. The resulting precipitate was then separated by centrifugation at 8000 ×g for 10 min. The precipitate (exopolysaccaharides—EPS) was washed with ultrapure water and subsequently lyophilized for quantitative assessment and analysis.
To extract the intracellular polysaccharides (IPS), the mycelial biomass was then subjected to extraction with boiling water for an hour and the mixture was filtered through Whatman no. 1 filter paper. The filtrate was allowed to precipitate using pure ethanol and left overnight at 4°C. The polysaccharides thus precipitated were separated by centrifugation at 8000 ×g for 10 min. The precipitate, which contained the exopolysaccharides (EPS), was washed with ultrapure water and subsequently lyophilized for quantitative assessment and analysis [11].
Polysaccharides were purified by removing the soluble phenolic compounds, a procedure achieved by precipitation with 70% ethanol. The solvent was removed by filtration, and the sediment was dried at 60°C in a drying chamber [12].
Total cellular protein concentrations were determined by use of the Bio-Rad Protein Assay kit [13].
The amount of total phenolic compounds was determined colorimetrically with Folin-Ciocalteu reagent. Gallic acid was used as the reference standard and the results (total phenolic content) were expressed as gallic acid equivalents (GAE) in grams per 100 gram extract [14].
The total carbohydrates resulting from IPS and EPS were measured by the phenol-sulfuric acid method [15].
2.5. DPPH Radical Scavenging Assay
The DPPH scavenging activity was measured using spectrophotometry. First, 0.05 mL of the samples dissolved in ethanol were added to an ethanolic solution of DPPH (200 μm) at different concentrations (varying from 2–10 mg/mL). An equal amount of ethanol was added to the control. After 20 min, the absorbance was read at 517 nm and the inhibition was calculated using the formula: DPPH scavenging effect (%) = A0 − AP/A0 × 100, where A0 was the absorbance of the control and AP was the absorbance in the presence of the sample [16]. Ascorbic acid was used as the control.
2.6. ABTS Radical Scavenging Assay
ABTS●+ was obtained by adding 7 mM of the ABTS stock solution to 2.45 mM potassium persulfate. The mixture was left to stand in the dark, at room temperature, for 12–16 h before use. The ABTS●+ solution (stable for two days) was then diluted with 5 mM phosphate-buffered saline (pH 7.4) to an absorbance at 730 nm of 0.70 ± 0.02. After adding 10 μL of the sample to 4 mL of the diluted ABTS●+ solution, the absorbance was measured at 30 min. All samples were analyzed in triplicate. The ABTS●+ radical-scavenging activity of the samples was expressed as S% = ((Acontrol − Asample)/Acontrol) × 100, where Acontrol is the absorbance of the blank control (ABTS●+ solution without test sample) and Asample is the absorbance of the test sample [17]. Ascorbic acid was used as control.
2.7. Reducing Power
First, 200 μL of the samples were mixed with sodium phosphate buffer (pH 6.6), 1 mM FeSO4, and 1% potassium ferricyanide. After incubation of the mixture for 20 min at 50°C, trichloroacetic acid was added and the mixtures were centrifuged. Next, 2.5 mL of the resulting supernatant was mixed with an equal volume of water and 0.5 mL 0.1% FeCl3. The absorbance was measured at 700 nm [18].
2.8. Ferrous Ion Chelating Assay
1 mL of the sample (2–10 mg/mL) was mixed with 3.7 mL of ultrapure water, following which the mixture was reacted with ferrous chloride (2 mmol/L, 0.1 mL) and ferrozine (5 mmol/L, 0.2 mL) for 20 min. The absorbance at 562 nm was determined spectrophotometrically. EDTA was used as positive control. The chelating activity on the ferrous ion was calculated using the equation: chelating activity (%) = [(Ab − As)/Ab] × 100, where Ab is the absorbance of the blank without the extract or ascorbic acid and As is the absorbance in the presence of the extract or ascorbic acid [19].
2.9. Determining the Composition of the Exopolysaccharides by Thin Layer Chromatography (TLC)
Chromatographic plates with Merck's G silica gel were used of 0.25 mm layer thickness. Water/methanol/glacial acetic acid/1,2-dicloroethane were used as the developer (10/15/25/50, v/v/v/v) [20].
2.10. Statistical Analysis
All the assays for fermentation and antioxidant activity were assessed in triplicate, and the results were expressed as mean ± SD values of the three sets of observations (P < 0.05). The mean values and standard deviation were calculated using the EXCEL program from Microsoft Office 2010 package.
3. Results and Discussion
3.1. Mycelial Growth and Production of Polysaccharides
Obtaining the biomass was done with reference to the typical profile of the growth curve for a cultivated species (Figure 1). Maintaining constant pH, temperature, and stirring produced maximum accumulation, compared with the cultivation in shaken flasks. Generally, the amount of mycelium was observed to increase by about 20%. Also, the biomass accumulation was enhanced by the aeration rate that occurred within the bioreactor and which positively influenced mycelial growth. This parameter of batch cultivation assumes significance because it also favors the agitation, which proved to be a very important parameter in bioreactor fermentation [21]. Mycelium obtained by the submerged cultivation process in the batch system entered into a stationary phase, after the tenth day. Significantly, PBS281009 showed a better increase when the two strains were compared. The maximum difference was observed during the 4–6 day fermentation period, with a maximum difference of about 50%.
Figure 1.
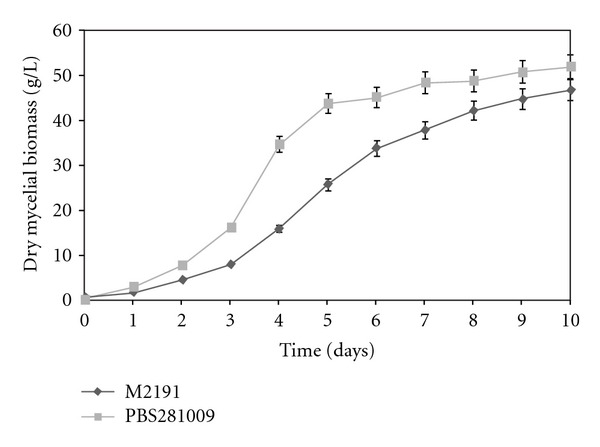
Mycelial growth by submerged culture of Pleurotus ostreatus M2191 and PBS281009 in a New Brunswick BioFlo 310 bioreactor.
The maximum productivity was observed to be higher in PBS281009, with a maximum of 3.15 g/L/day. PBS281009 productivity showed 42.5% higher for EPS. However, the M2191 EPS productivity was higher by 30%. Data were confirmed by the product yield of each strain as well as by the values obtained for each type of polysaccharide determined (Table 1). However, a higher difference was observed in the carbohydrate content of the polysaccharides isolated in the batch system submerged cultivation (Figure 2).
Table 1.
EPS and IPS production from the submerged culture of Pleurotus ostreatus M2191 and PBS281009 after 10 days of batch fermentation.
| Polysaccharide | Polysaccharide productivity (g/L/day) |
Polysaccharide (g)/dry biomass (g) | Carbohydrates (%) | Product yield (mg polysaccharide/g carbon source) |
|---|---|---|---|---|
| Pleurotus ostreatus M2191 | ||||
|
| ||||
| IPS | 0.07 ± 0.05 | 0.002 ± 0.002 | 68 ± 0.23 | 30 ± 0.64 |
| EPS | 0.1 ± 0.03 | 0.04 ± 0.002 | 87 ± 0.16 | 32.2 ± 0.25 |
|
| ||||
| Pleurotus ostreatus PBS281009 | ||||
|
| ||||
| IPS | 1.15 ± 0.1 | 0.03 ± 0.005 | 74 ± 0.45 | 31.6 ± 0.16 |
| EPS | 2.0 ± 0.07 | 0.06 ± 0.001 | 89 ± 0.32 | 34.8 ± 0.24 |
Figure 2.
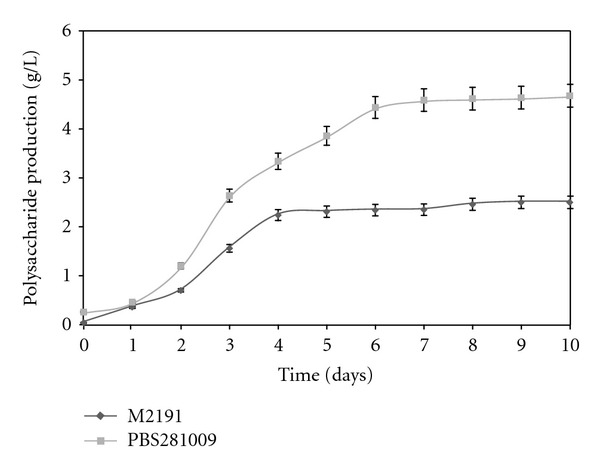
Polysaccharide production by submerged culture Pleurotus ostreatus M2191 and PBS281009 in a New Brunswick BioFlo 310 bioreactor.
The molecular weight of EPS was higher than 2000 kDa, whereas that of the IPS was less than 2000 kDa. Thus, the EPS values obtained were 2538 kDa for PBS281009 and 2133 kDa for M2191. In the case of IPS, the molecular weight was 1323 kDa for PBS281009 and 1078 kDa for M2191. The protein composition of the polysaccharides was noted to be less than 15% in both cases [21]. The estimated content of phenols in IPS was approximately 8% for M2191 and 13% for PBS281009. In EPS, only insignificant quantities were identified, which probably resulted from the mycelium disintegrated during fermentation, due to mechanical stirring. The values did not exceed 0.1% of the analyzed content of polysaccharide. The sugar content, however, varied depending on the polysaccharide type and strain. The monomer composition of EPS included glucose, galactose, mannose, and arabinose for both strains. The percentage ratio of the sugar components for EPS M2191 was: 88% glucose, 6% galactose, 2% mannose, and 2% arabinose, 2% xylose, and for EPS PBS281009 it was 91% glucose, 3% galactose, 4% arabinose, 1% mannose, and 1% xylose. The monomer composition of IPS showed the absence of xylose. The percentage ratio of the sugar components was: 84% glucose, 10% galactose, 4% arabinose, and 2% manose for IPS M2191 and 89% glucose, 7% galactose, 2% arabinose, and 2% manose for IPS PBS281009 [21, 22]. The EPS content, expressed as percentages of anhydrous glucose, was found to include 53.85 ± 1.65% and 44.07 ± 0.86% for IPS. The degree of polymerization revealed was 50.7 ± 0.59% for EPS and 36.7 ± 0.51% for IPS.
3.2. DPPH Radical Scavenging Activity
DPPH radicals are most often used to assess the antioxidant capacity of an extract, because they are extremely stable and are easy to use. This has become a recognized test, based on the color change of the substrate from mauve to yellow, depending on the capacity of the product tested. The samples were subsequently read spectrophotometrically at 517 nm [23, 24]. The DPPH scavenging activity of EPS and IPS of the two types of mycelium that were isolated after submerged cultivation in the batch system was directly correlated with the concentration of the sample (Figure 3). The EPS of the two strains showed a higher DPPH scavenging activity than the samples from IPS. EPS synthesized by the strain PBS281009 showed a difference of 19.1% more when compared with the EPS synthesized by M2191 with a maximum of 78.06% at 10 mg/mL. For the IPS isolated from the mycelium, the difference was 22.42% in favor of PBS281009, with a maximum of 74.6%, for the same concentration. From the data presented above, the IPS for both strains were found to show a higher DPPH scavenging activity than those of the EPS synthesized.
Figure 3.
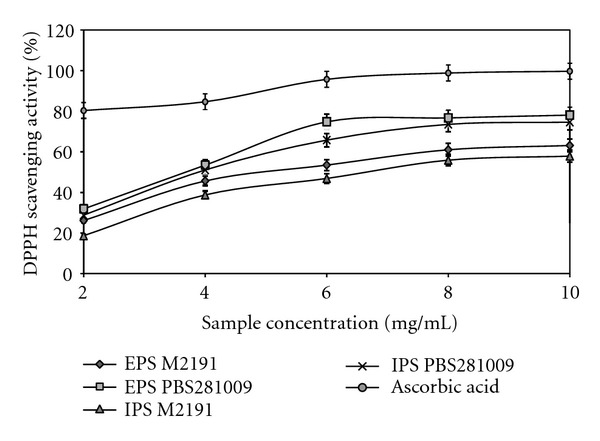
Scavenging effect on 1,1-diphenyl-2-picrylhydrazyl radical of EPS and IPS from submerged culture of Pleurotus ostreatus M2191 and PBS281009.
The results obtained show that the polysaccharides of both the strains are good DPPH radical scavengers. This statement is also supported by the EC50 value, which was found to be 4.2 mg/mL for EPS and 4.75 mg/mL for IPS in PBS281009. The M2191 values were 5.87 mg/mL for EPS and 7.2 mg/mL for IPS. Compared with ascorbic acid, EC50 0.05 mg/mL, the values were over 10 times higher which reveals the direct connection between the scavenging activityand the EC50 value.
3.3. ABTS Radical Scavenging Activity
The inhibition percentage of the ABTS radical by the two types of polysaccharides was found to depend upon the concentration. In Figure 4, a correlation is evident between the DPPH radical scavenger and that of the ABTS radical. The scavenging effect of all the extracts increased with increasing concentration. At a concentration of 10.0 mg/mL, the percentage inhibition of EPS had a value which was relatively similar in both strains with an additional difference of 1.2% for PBS281009. The same trend was also observed for IPS, the only change being that the difference increased up to 10.3%. By contrast, ascorbic acid at similar concentrations showed lower values. High concentrations of the EPS and IPS are able to more effectively quench the free radicals in the system. The results indicated that the EPS and IPS from both the strains possessed a strong scavenging power for the ABTS radicals and could certainly be explored as new potential antioxidants. The results are correlated with the antioxidant effect generated by such polysaccharides, including those obtained by the polysaccharides from medicinal plants [11].
Figure 4.
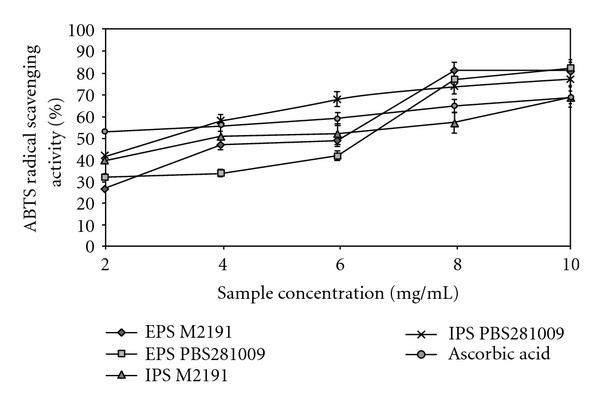
Scavenging effect on ABTS radical of EPS and IPS from submerged culture of Pleurotus ostreatus M2191 and PBS281009.
3.4. Reducing Power Determination
Reducing power represents the ability of the two types of polysaccharides to donate electrons. Interestingly, the redution of Fe3+ to Fe2+ was spectrophotometrically determined, at 700 nm. Therefore, by using this method, a greater absorbance will result in a much higher reduction power [25]. Thus, the reduction capacity was compared with that of ascorbic acid used as the standard.
The reducing powers of EPS and IPS from both the mushroom strains using submerged culture, determined at 700 nm, are shown in Figure 5. The data show that the two types of polysaccharides from both strains possessed the reducing capacity. By comparison, the ascorbic acid revealed better values than the samples analyzed. The reducing powers of EPS and IPS increased as the concentration increased. The reducing powers of EPS and IPS were 1.02 and 0.595 at 10 mg/mL, and 1.25 and 0.875 for PBS281009 and M2191, respectively. The IPS showed a greater reducing power than the EPS. Values achieved at 10 mg/mL were 8% lower for PBS281009 and 25% for M2191. The reducing power of EPS and IPS implies that the reductone-associated and hydroxide groups of polysaccharides can act as electron donors to react with free radicals to form stable products. The EPS and IPS isolated from both mushroom strains might contain reductones, which could react with certain precursors of peroxides to prevent peroxide formation, confirming the earlier studies conducted using the Armillaria mellea strain [11].
Figure 5.
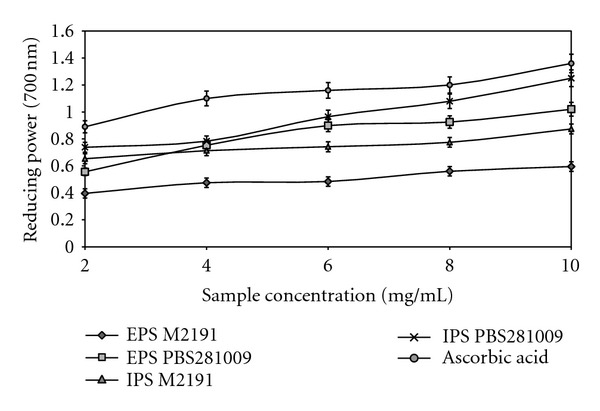
Reducing power of EPS and IPS from submerged culture of Pleurotus ostreatus M2191 and PBS281009.
3.5. Iron Chelating Activity
The Fe2+ ion is the most powerful prooxidant among the various species of metal ions, being able to form complexes with ferrozine [26]. Fe2+ generates lipid peroxidation through the Fenton reaction or by accelerating the dissociation of lipid hydroperoxides. The capacity of the samples to bind with Fe2+ in the presence of ferrozine was compared with EDTA [27]. The chelating ability (%) of the polysaccharides from both mushrooms was found to be dose-dependent (Figure 6). At a maximum concentration of 10 mg/mL, the order of samples with respect to their chelating ability was as follows: IPS PBS281009 > EPS PBS281009 > IPS M2191 > EPS M2191. Thus, from the data obtained, the IPS and EPS from the two P. ostreatus mushrooms using submerged culture can be termed as good sources of chelators for ferrous ions. The value obtained at a maximum quantity of 10 mg/mL was lower when compared with that of EDTA, with values between 19.79% (M2191)–10.4% (PBS281009) for EPS. For IPS, the values were lower by 13.13% (M2191)–5.75% (PBS281009).
Figure 6.
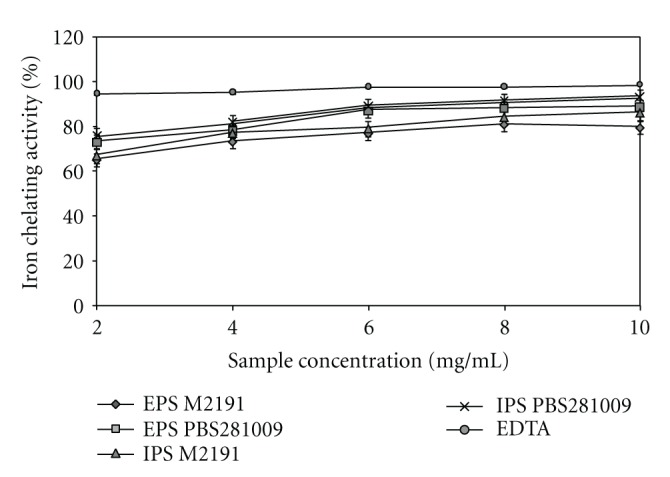
Ferrous ion-chelating ability of EPS and IPS from submerged culture of Pleurotus ostreatus M2191 and PBS281009.
Table 2 gives a general perspective on the antioxidant activity of the two types of polysaccharides isolated from the batch cultivation of P. ostreatus M2191 and PBS281009. The low values of EC50 are a valid indicator of the effectiveness of the tested samples. If they are less than 10 mg/mL, they indicate a strong effect [11, 28].
Table 2.
EC50 values of EPS and IPS from submerged culture of Pleurotus ostreatus M2191 and PBS281009.
| Activity | EC50 (mg/mL) | |||||
|---|---|---|---|---|---|---|
| EPS | IPS | Ascorbic acid | EDTA | |||
| M2191 | PBS281009 | M2191 | PBS281009 | |||
| DPPH radical scavenging activity | 6.11 ± 0.07 | 4.81 ± 0.11 | 7.32 ± 0.04 | 3.85 ± 0.25 | 23.04 ± 1.54 | — |
| ABTS radical scavenging activity | 7.66 ± 0.55 | 5.42 ± 0.06 | 5.97 ± 0.22 | 5.48 ± 0.59 | 0.21 ± 0.32 | — |
| Reducing power | 7.02 ± 0.23 | 4.49 ± 0.09 | 5.03 ± 0.17 | 4.25 ± 0.43 | 0.25 ± 0.65 | — |
| Iron chelating activity | 1.53 ± 0.64 | 1.36 ± 0.02 | 1.49 ± 0.2 | 1.31 ± 0.08 | — | 0.05 ± 2.43 |
The EC50 value, in the case of assessment of the antioxidant capacity, has demonstrated the efficiency of the IPS when compared with that of the EPS. This finding has been determined for both strains and is consistent with the results found for other strains of fungi such as A. mellea [11], Grifola frondosa [21], as well as tested strains belonging to the genus Pleurotus [6]. As the amount of the biomass and synthesized polysaccharides depends directly on the strain used, the tests regarding submerged cultivation assume significance because of the short time taken to concomitantly obtain the mycelium and EPS. Another important aspect that emerges from such research is that of optimizing the parameters to maximize the production of mycelium and polysaccharides, although further tests will be required to demonstrate the biological value of the products isolated. To achieve these objectives, the tests performed in the batch system have revealed the importance of a fresh inoculum and the carbon and nitrogen source [29].
The biological value of the polysaccharides is directly proportional to the molecular weight. This fact is demonstrated by the data submitted and confirmed by the earlier studies [28]. High-molecular-weight EPS is correlated with maximum antitumor activity [21, 30]. This observation is supported by other studies that have shown that the polysaccharides isolated at the end of a fermentative process possess a greater molecular weight than those isolated in the lag or the exponential growth phases. A rise in the biological properties resulted simultaneously in the entry into the final phase of the fermentative process [11, 21, 31].
The correlation (r2) between the methods of antioxidant activity was found to be 0.5739 for EPS and 0.9952 for IPS in the case of PBS281009. However, the correlation coefficients (0.8676 for EPS and 0.8261 for IPS) determined for the polysaccharides isolated from M2191 cultivation suggested that the two methods show a highly similar predictive capacity for antioxidant activities [32]. The specific differences between the two methods are determined by the heterogeneous composition of the two types of polysaccharides.
Thus, the protein content of IPS and EPS from both stems displayed a weak correlation with the previously presented antioxidant activity. Between the protein content and the DPPH/ABTS radicals scavenging activity, the value r2 was between 0.09 and 0.132; for the reducing power, r2 was between 0.09 and 0.117, while the value r2, related to iron chelating activity, displayed values between 0.125 and 0.248.
On the other hand, the content of polyphenols identified in IPS displayed a good correlation with the DPPH/ABTS radicals scavenging activity (r2 = 0.751–0.867), reducing power (r2 = 0.792–0.847), and iron chelating activity (r2 = 0.73–0.81). If in the case of EPS the entire antioxidant activity is attributable to the polysaccharide, in the case of IPS, it is split between polyphenols and polysaccharide. In the case of the polysaccharide extracted from fruit bodies of several edible mushrooms, the ratio of polyphenols/polysaccharide was relatively constant [33]. The cultivation of mycelium by batch system determined a different ratio in favor of PBS281009. These results demonstrate that the source of origin of the tested samples is very important. Even between stems of the same species, differences may occur, because the quantity of polysaccharides and proteins, as well as the ratio between them, are different. A direct relation between the antioxidant activity and the polyphenols/polysaccharide ratio in IPS was noticed. The relation between the content of polyphenols and polysaccharides, expressed by the r2 value of IPS, corresponded to previous studies [33] reaching the value of 0.749 for M2191 and 0.871 for PBS281009. A higher positive correlation was found between the polysaccharides content and antioxidant activity, displaying higher values as in the case of polyphenols. The r2 value corresponding to the DPPH/ABTS radicals scavenging activity was between 0.747 and 0.967, for the reducing power 0.719–0.898, and for the iron chelating activity 0.763–0.852. Maximum values were obtained in the case of EPS for DPPH/ABTS scavenging activity while the reducing power and the iron chelating activity had maximum values for IPS. The results determined by IPS demonstrate the fact that these reduce the concentration of the transition metal that catalyzes lipid peroxidation [34]. The removal of the phenolic component in IPS indicated a decrease of approximately 20–25% in case of the values of the antioxidant activity. By removing the polyphenolic component and inactivation of proteins in IPS, values are reached, in average 5% lower than the values of the antioxidant activity of EPS. Also, it was noticed that the main sugar in the structure of the mushrooms' polysaccharides is generally represented by glucose, without any direct relation between this component and the antioxidant activity [15, 35].
Submerged cultivation in the batch system has proven to be efficient in obtaining polysaccharides with good biological activity in the tested strains, confirming the earlier studies performed with strains already known for their favorable pharmaceutical effects, such as Ganoderma lucidum [10, 16]. The importance of the immune stimulating and antitumor properties of the polysaccharides synthesized by the fungi has triggered an increase in their demand in recent years. Growing requirements for valuable biological material demonstrate that it is more productive during submerged cultivation, at the bioreactor level, as the cultivation parameters can be more easily attained and are more economically efficient. Growing the mycelium in a fermentation system is currently accepted, because it can be a fully automated process, which becomes a good way to achieve the required amount with the same biological value. Due to the advancement in the technology in this area, the cost can be significantly reduced. Current research is being directed toward finding new strains which can produce a valuable mycelium by submerged cultivation to manufacture functional products [6, 8, 36, 37].
The polysaccharides (IPS and EPS) produced by submerged cultivation of P. ostreatus M2191 and PBS281009 revealed a certain antioxidant activity, demonstrated by the low values of EC50. The antioxidant effect is relatively similar to that of the ascorbic acid, particularly for PBS281009. The antioxidant activity of the polysaccharides included the scavenging activity against DPPH and ABTS radicals, the reducing power, as well the chelating ability. A good correlation between the DPPH and ABTS scavenging activity was determined. Evaluation of the antioxidant effect of the polysaccharides leads to a better understanding of the mechanism that enables the mycelium of these fungi to exert a functional effect on the human body. In the future, in vitro tests will be mandatory to clarify the antioxidant effect they exert when transiting the human digestive tract.
Acknowledgment
This work was supported by the Executive Agency for Higher Education, Research, Development and Innovation Funding-Human Resources, Theme 9/2010.
References
- 1.Alam N, Yoon KN, Lee TS, Lee UY. Hypolipidemic activities of dietary Pleurotus ostreatus in hypercholesterolemic rats. Mycobiology. 2011;39:45–51. doi: 10.4489/MYCO.2011.39.1.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam N, Yoon KN, Lee KR, et al. Antioxidant activities and tyrosinase inhibitory effects of different extracts from Pleurotus ostreatus fruiting bodies. Mycobiology. 2010;38:295–301. doi: 10.4489/MYCO.2010.38.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis FS, Pereira E, Barros L, Sousa MJ, Martins A, Ferreira ICFR. Biomolecule profiles in inedible wild mushrooms with antioxidant value. Molecules. 2011;16(6):4328–4338. doi: 10.3390/molecules16064328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavi I, Levinson D, Peri I, Tekoah Y, Hadar Y, Schwartz B. Chemical characterization, antiproliferative and antiadhesive properties of polysaccharides extracted from Pleurotus pulmonarius mycelium and fruiting bodies. Applied Microbiology and Biotechnology. 2010;85(6):1977–1990. doi: 10.1007/s00253-009-2296-x. [DOI] [PubMed] [Google Scholar]
- 5.Papaspyridi LM, Aligiannis N, Topakas E, Christakopoulos P, Skaltsounis AL, Fokialakis N. Submerged fermentation of the edible mushroom Pleurotus ostreatus in a batch stirred tank bioreactor as a promising alternative for the effective production of bioactive metabolites. Molecules. 2012;17(3):2714–2724. doi: 10.3390/molecules17032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregori A, Vagelf M, Pohleven J. Cultivation techniques and medicinal properties of Pleurotus spp. Food Technology and Biotechnology. 2007;45(3):238–249. [Google Scholar]
- 7.Lee BC, Bae JT, Pyo HB, et al. Biological activities of the polysaccharides produced from submerged culture of the edible basidiomycete Grifola frondosa. Enzyme and Microbial Technology. 2003;32(5):574–581. [Google Scholar]
- 8.Tang YJ, Zhu LL, Li DS, Mi ZY, Li HM. Significance of inoculation density and carbon source on the mycelial growth and Tuber polysaccharides production by submerged fermentation of Chinese truffle Tuber sinense. Process Biochemistry. 2008;43(5):576–586. [Google Scholar]
- 9.Fang QH, Zhong JJ. Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites—ganoderic acid and polysaccharide. Biochemical Engineering Journal. 2002;10(1):61–65. [Google Scholar]
- 10.Liu GQ, Wang XL. Selection of a culture medium for reducing costs and enhancing biomass and intracellular polysaccharide production by Agaricus blazei. Food Technology and Biotechnology. 2009;47(2):210–214. [Google Scholar]
- 11.Lung MY, Chang YC. In vitro antioxidant properties of polysaccharides from Armillaria mellea in batch fermentation. African Journal of Biotechnology. 2011;10(36):7048–7057. [Google Scholar]
- 12.Kozarski M, Klaus A, Niksic M, Jakovljevic D, Helsper JPFG, Van Griensven LJLD. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chemistry. 2011;129:1667–1675. [Google Scholar]
- 13.Mehmeti I, Kiran F, Osmanagaoglu O. Comparison of three methods for determination of protein concentration in lactic acid bacteria for proteomics studies. African Journal of Biotechnology. 2011;10(11):2178–2185. [Google Scholar]
- 14.Cosmulescu S, Trandafir I. Seasonal variation of total phenols in leaves of walnut (Juglans regia L.) Journal of Medicinal Plants Research. 2011;5:4938–4942. [Google Scholar]
- 15.Thetsrimuang C, Khammuang S, Sarnthima R. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. International Journal of Pharmacology. 2011;7(1):58–65. [Google Scholar]
- 16.Baskar G, Shree Rajesh LK, Renganathan S. Statistical screening and optimization of exo-polysaccharide production by medicinal mushroom using design of experiments. Biotechnology, Bioinformatics and Bioengineering. 2011;1:47–58. [Google Scholar]
- 17.Li P, Huo L, Su W, et al. Free radical-scavenging capacity, antioxidant activity and phenolic content of Pouzolzia zeylanica. Journal of the Serbian Chemical Society. 2011;76(5):709–717. [Google Scholar]
- 18.Papuc C, Crivineanu M, Goran G, Nicorescu V, Durdun N. Free radicals scavenging and antioxidant activity of European mistletoe (Viscum album) and European birthwort (Aristolochia clematitis) Revista de Chimie. 2010;61(7):619–622. [Google Scholar]
- 19.Oyetayo VO, Dong CH, Yao YJ. Antioxidant and antimicrobial properties of aqueous extract from Dictyophora indusiata. The Open Mycology Journal. 2009;3:20–26. [Google Scholar]
- 20.Vamanu E, Vamanu A, Pelinescu D, Niţă S, Rusu N, Popa N. Influence of the culture medium composition on the exopolysaccharides synthesis by Streptococcus sp. IL5 strain. Acta Alimentaria. 2012;41:118–125. [Google Scholar]
- 21.Lee WY, Park Y, Ahn JK, Ka KH, Park SY. Factors influencing the production of endopolysaccharide and exopolysaccharide from Ganoderma applanatum. Enzyme and Microbial Technology. 2007;40(2):249–254. [Google Scholar]
- 22.Refaie FM, Esmat AY, Daba AS, Taha SM. Characterization of polysaccharopeptides from Pleurotus ostreatus mycelium: assessment of toxicity and immunomodulation in vivo. Micología Aplicada Internacional. 2009;21:67–75. [Google Scholar]
- 23.Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae) Food Chemistry. 2008;111(4):925–929. [Google Scholar]
- 24.Subramanion LJ, Zuraini Z, Sreenivasan S. Phytochemicals screening, DPPH free radical scavenging and xanthine oxidase inhibitiory activities of Cassia fistula seeds extract. Journal of Medicinal Plant. 2011;5:1941–1947. [Google Scholar]
- 25.Noorlidah A, Siti MI, Norhaniza A, Adawiyah SS, Beng FL. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. Evidence-Based Complementary and Alternative Medicine. 2012;2012:12 pages. doi: 10.1155/2012/464238.464238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong F, Zhang M, Liao S, Yu S, Chi J, Wei Z. Antioxidant activity of polysaccharide-enriched fractions extracted from pulp tissue of Litchi chinensis sonn. Molecules. 2010;15(4):2152–2165. doi: 10.3390/molecules15042152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rainha N, Lima E, Baptista J, Rodrigues C. Antioxidant properties, total phenolic, total carotenoid and chlorophyll content of anatomical parts of Hypericum foliosum. Journal of Medicinal Plants Research. 2011;5:1930–1940. [Google Scholar]
- 28.Liang CH, Syu JL, Mau JL. Antioxidant properties of solid-state fermented adlay and rice by Phellinus linteus. Food Chemistry. 2009;116(4):841–845. [Google Scholar]
- 29.Baskar R, Lavanya R, Mayilvizhi S, Rajasekaran P. Free radical scavenging activity of antitumour polysaccharide fractions isolated from Ganoderma lucidum (Fr.) P. Karst. Natural Product Radiance. 2008;7(4):320–325. [Google Scholar]
- 30.Lung MY, Huang WZ. Production, purification and tumor necrosis factor-α (TNF-α) release capability of exopolysaccharide from Laetiporus sulphureus (Bulliard: Fries) Bondartsev & Singer in submerged cultures. Process Biochemistry. 2011;46(2):433–439. [Google Scholar]
- 31.Zhang M, Cui SW, Cheung PCK, Wang Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends in Food Science and Technology. 2007;18(1):4–19. [Google Scholar]
- 32.Zeng L, Vrijmoed LLP. Antioxidant activities and phenolic constituents of Cephalotaxus oliveri Mast. Aerial part. Journal of the Serbian Chemical Society. 2011;76:1–24. [Google Scholar]
- 33.Wei S, Van Griensven LJLD. Pro- and antioxidative properties of medicinal mushroom extracts. International Journal of Medicinal Mushrooms. 2008;10(4):315–324. [Google Scholar]
- 34.Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complementary and Alternative Medicine. 2008;8, article 63 doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Xie MY, Nie SP, Li C, Wang YX. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chemistry. 2008;107(1):231–241. [Google Scholar]
- 36.Altaf SA, Umar DM, Muhammad MS. Production of xylanase enzyme by Pleurotus eryngii and Flamulina velutipes grown on different carbon sources under submerged fermentation. World Applied Sciences Journal. 2010;8:47–49. [Google Scholar]
- 37.Wu CY, Liang ZC, Lu CP, Wu SH. Effect of carbon and nitrogen sources on the production and carbohydrate composition of exopolysaccharide by submerged culture of Pleurotus citrinopileatus. Journal of Food and Drug Analysis. 2008;16(2):61–67. [Google Scholar]


