Abstract
Antifungal activity of petroleum ether extract of Psoralea corylifolia L. seed, tested against Fusarium sp. namely, Fusarium oxysporum, Fusarium moniliforme, and Fusarium graminearum, was evaluated by agar well diffusion assay. The chromatographic fractionation of the extract yielded a new phenyl derivative of pyranocoumarin (PDP). The structure of the PDP was confirmed using spectroscopic characterization (GC-MS, IR, and NMR), and a molecular mass of m/z 414 [M-2H]+ with molecular formula C27H28O4 was obtained. The PDP had a potent antifungal activity with a minimum inhibitory concentration of 1 mg/mL against Fusarium sp. Molecular docking using Grid-Based Ligand Docking with Energetics (GLIDE, Schrodinger) was carried out with the Tri101, trichothecene 3-O-acetyltransferase, as target protein to propose a mechanism for the antifungal activity. The ligand PDP showed bifurcated hydrogen bond interaction with active site residues at TYR 413 and a single hydrogen bond interaction at ARG 402 with a docking score −7.19 and glide energy of −45.78 kcal/mol. This indicated a strong binding of the ligand with the trichothecene 3-O-acetyltransferase, preventing as a result the acetylation of the trichothecene mycotoxin and destruction of the “self-defense mechanism” of the Fusarium sp.
1. Introduction
All over the world, a significant portion of agriculture production is lost due to fungal diseases and different mycotoxins produced by the phytopathogens like Aspergillus sp., Fusarium sp., and Penicillium sp. To overcome these losses in agricultural field, the common procedure is to use synthetic pesticides and fungicides [1, 2]. These synthetic pesticides and fungicides cause several side effects which include carcinogenicity, teratogenicity, and residual toxicity [3, 4]. Plant metabolites and plant-based pesticides and fungicides appear to be one of the better alternatives as they are known to have minimal environmental impact and danger to consumers in contrast to the synthetic pesticides [5, 6]. To promote the proper use of botanicals and to determine their potential as a source for new fungicides, it is essential to study plants of medicinal value. Natural products of higher plants may be a new source of antimicrobial agents with possibly novel mechanism of action contrary to synthetic drugs [7]. The seed of Psoralea corylifolia L. (Fabaceae), well known as traditional Chinese medicine “Buguzhi,” is widely used for the treatment of various kinds of human disorders and contains good antioxidative, antimicrobial, antiinflammatory, antitumor, antimutagenic, and insect hormonal activities [8]. P. corylifolia L. seed has been reported to contain several phytoconstituents mainly coumarins, flavone components [9], and lactone as well as terpenoids like psoralen, isopsoralen, psoralidin, and bavachalcone [10].
With the aim of obtaining a bioactive principle, antifungal screening and activity-based fractionation of seed extract of P. corylifolia were carried out, which led to the isolation of the new phenyl derivative of pyranocoumarin (PDP). The compound had in vitro antifungal activity against the Fusarium sp. and molecular docking studies carried out with the X-ray crystal structures of Tri101, trichothecene 3-O-acetyltransferase (PDB ID: 2RKV) available in the Protein Data Bank (PDB; http://www.pdb.org/pdb/) proposed a hypothetical mechanism for antifungal activity against Fusarium sp.
The target protein selected for the molecular docking studies was a Tri101 gene product which catalyzes the transfer of an acetyl group from acetyl coenzyme A to the C3 hydroxyl moiety of several trichothecene mycotoxins. It has been reported that Tri101 acetylation was the primary defense mechanism against 3-hydroxylated trichothecenes in Fusarium sp. and that a mutation or a modification, resulting in a loss of Tri101, would be lethal to the organism [11].
When the pathway of the mycotoxin production in Fusarium sp. was analyzed, Tri101 gene product, that is, trichothecene 3-O-acetyltransferase catalyzes the acetylation of the C3 hydroxyl group of the toxin. The acetylation at C3 had been proposed to protect the Fusarium sp. through the remainder of the biosynthetic pathway. Tri101 will acetylate and reduce the toxicity of the final products of the biosynthetic pathway including trichothecene (T-2 toxin), nivalenol and deoxynivalenol [12–14]. The last step in the biosynthetic pathway is removal of the protecting C3 acetyl group before being secreted by the organism [15]. Control of the toxicity of trichothecene mycotoxins by modification of the C3 hydroxyl moiety has been identified as a self-defense mechanism of the organism.
2. Materials and Methods
2.1. Plant Material
The plant specimen with the seed of Psoralea corylifolia was identified and certified by the Botanical Survey of India, Southern Circle, Coimbatore, Tamil Nadu, India. Voucher specimens were maintained in laboratory for future reference.
2.2. Microorganisms
The fungal isolates of Fusarium sp. namely, Fusarium oxysporum, Fusarium graminearum, and Fusarium moniliforme from infected maize, paddy, and sorghum seeds were used for the study.
2.3. Extract Preparation
The dried and powdered seed samples of P. corylifolia L. were extracted by overnight percolation with ethanol (polar solvent), chloroform (medium polar solvent) and petroleum ether (least polar solvent), at the rate of 1 : 5 at room temperature. The extracts were then filtered with country filter paper and concentrated under vacuum in a rotary evaporator to obtain a gummy residue [16].
2.4. Antifungal Studies by Agar Well Diffusion Assay
The antifungal activity was tested for the prepared extracts against the selected pathogens. The sterilized sabouraud dextrose agar medium was poured into the petri plates and allowed to solidify. The fungal culture 200 μL was transferred to the petri plates. Using a sterile cork borer, wells of 6 mm in diameter were made in the plate containing the media. For each organism, 20 μL of the prepared sample was loaded in each well using sterilized dropping pipette. Three replications were maintained for each treatment. For each microorganism, the positive control (ketoconazole) and the negative control (ethanol) were also loaded in a separate well. The plates were incubated for 2-3 days and the observations were taken. The diameter of inhibition zone (DIZ) was measured and the mean D1Z was calculated [17, 18].
2.5. Fractionation and Antifungal Assay
The fractionation was carried out by column chromatography using a 22 cm long column having 1.6 cm diameter packed with 20 g of silica gel (60–120 mesh) using petroleum ether solvent. The active extract was loaded to the packed column and eluted with a 90 : 10 mixture of petroleum ether and ethyl acetate at a rate of 2.6 mL min−1. The polarity of mobile phase was gradually increased using ethyl acetate to give different fractions. Fractions with similar thin layer chromatogram were pooled together which were labeled serially and then subjected to antifungal assay [17, 18] to obtain the active fraction.
2.6. Minimum Inhibitory Concentration (MIC) by Tube Dilution Method
The assay was performed to obtain the MIC of the active fraction isolated from petroleum ether extract of P. corylifolia L. seed against the selected fungal pathogens using tube dilution method. The MIC is defined as the lowest concentration of antibiotics or plant extracts that did not show any growth of tested pathogens. The entire test sample was dissolved in ethanol and diluted to the highest concentration (10 mg min−1) to be tested and then serial dilutions were made in a concentration range from 1 mg min−1 to 0.01 mg min−1 in sterile test tubes containing standardized inoculums. The growth of the organism for each dilution was observed and thus the MIC was evaluated. Ketoconazole and ethanol were used as positive and negative control, respectively [16].
2.7. Spectroscopic Studies
The active fraction obtained was subjected to GC-MS, NMR, and IR spectroscopic studies to identify the structure of the active compound. GC-MS was done using THERMO GC-TRACE ULTRA VER: 5.0, THERMO MS DSQ II. The column used was TR 5-MS Capillary Standard Non-Polar Column with a dimension of 30 Mts, Id of 0.25 mm, film of 0.25 μm and helium as a carrier gas. The flow maintained was 1.0 mL min−1 with oven temperature 80°C raised to 250°C at the rate of 8°C min−1. The volume of sample injected was 1 μL. 1H-NMR and 13C-NMR were recorded at 400 and 100 MHz, respectively, in CDCl3. The NMR spectroscopic studies were done by Bruker 400 spectrometer. IR confirmation studies were carried out in BRUKER OPTIK GMBH with Model no-TENSOR 27, opus version 6.5 software, resolution 4 cm−1, sample scan time 64 scans and data taken from 4000 cm−1–400 cm−1.
2.8. Molecular Modelling Studies
Induced Fit Docking studies have been carried out using GLIDE [19] software v5.5, developed by Schrodinger, running on Red Hat Enterprise Linux 5 (RHEL5) workstation and Maestro v9.0 Graphical User Interface (GUI) workspace was used for all the steps involved in ligand preparation, protein preparation, and Induced Fit Docking (IFD).
2.8.1. Preparation of the Ligand “Phenyl Derivative of Pyranocoumarin” and Protein “Trichothecene 3-O-Acetyltransferase”
The ligand used in this study was prepared using Ligprep module of v2.3 of Schrodinger Suite 2009. Ligprep follows OPLS-AA (Optimized Potential Liquid Simulations for All Atoms) force fields for energy minimization. The protein taken for the study was 2RKV (Tri101 Complexed with Coenzyme A and T-2 Mycotoxin) retrieved from PDB database. The optimized structure was then energy minimized to remove the steric clashes between the atoms. The energy minimization was done till it reached a Root Mean Square Deviation (RMSD) cutoff of 0.18 Å and the resulting structure was used for docking [20].
The native ligand used was ZBA, a T2 mycotoxin which readily binds with protein trichothecene 3-O-acetyltransferase during the mycotoxin production and activates the self-defense mechanism of the Fusarium sp.
2.8.2. Induced Fit Docking (IFD)
IFD of the prepared ligand with the prepared protein was performed using IFD protocol of GLIDE v5.5 from Schrodinger Suite 2009 [20]. Both the ligand and the receptor were flexible which enabled the ligand to dock at the receptor's binding site and generate multiple poses of the receptor-ligand complex. Each docking included unique structural conformations of the receptor needed to fit the ligand pose. The IFD gives the best structure of the docked complex based on the Glide score (G-score) of the dockings.
2.8.3. Visualization and Analysis
The hydrophobic interactions and hydrogen bond interactions were obtained as ligplot diagram by submitting the docked complexes to the online PDB sum server (http://www.ebi.ac.uk/pdbsum/).
3. Results and Discussion
The seed of P. corylifolia L. possesses several secondary metabolites with remarkable biological properties [9]. With this background the present research has been initiated to identify the active secondary metabolite compound produced by P. corylifolia L. seed against Fusarium sp.
3.1. Antifungal Activity and Fractionation
Among the three extracts tested, petroleum ether extract exhibited highest zone of inhibition when compared to chloroform and ethanol extract at a concentration of 100 mg/mL against the selected pathogens. The DIZ produced by the petroleum ether extract (Table 1) was comparable to that of the positive control ketoconazole. The petroleum ether extract was fractionated and repeated fractionations yielded five different secondary fractions. The secondary fraction four (SF4) showed potent antifungal activity when compared to other fractions (Table 2). There is growing evidence that most of the secondary metabolites from plants are involved in the defense of the plant from plant pests and diseases. Thus, secondary compounds represent a large reservoir of chemical structures with biological activity. This resource is largely untapped for use as pesticides and fungicides [1].
Table 1.
Antifungal activity of seed extracts of Psoralea corylifolia L. against plant pathogens.
| Extracts | Zone of inhibition (diameter in cm) | ||
|---|---|---|---|
| Fusarium moniliforme | Fusarium oxysporum | Fusarium graminearum | |
| Petroleum ether extract (100 mg/mL) | 2.5 (±0.06) | 2.9 (±0.06) | 3.03 (±0.03) |
| Chloroform extract (100 mg/mL) | 1.8 (±0.07) | 2.13 (±0.03) | 1.5 (±0.03) |
| Ethanol extract (100 mg/mL) | 1.6 (±0.09) | 2.03 (±0.03) | 1.30 (±0.06) |
| Ketoconazole (1 mg/mL) | 3.2 (±0.03) | 3.16 (±0.03) | 2.97 (±0.03) |
| Ethanol (100%) | 0.33 (±0.03) | 0.26 (±0.03) | 0.33 (±0.03) |
*Mean of three replications.
Table 2.
Antifungal activity of secondary fraction of petroleum ether extract against plant pathogens.
| Secondary fractions | Zone of inhibition (diameter in cm) | ||
|---|---|---|---|
| Fusarium moniliforme | Fusarium oxysporum | Fusarium graminearum | |
| SF1 (10 mg/mL) | 0.26 (±0.03) | 0.46 (±0.03) | — |
| SF2 (10 mg/mL) | — | 0.4 9 (±0.06) | — |
| SF3 (10 mg/mL) | 0.33 (±0.03) | 1.6 (±0.10) | — |
| SF4 (10 mg/mL) (PDP) | 1.8 (±0.06) | 2.37 (±0.07) | 2.43 (±0.03) |
| SF5 (10 mg/mL) | 1.3 (±0.06) | 1.43 (±0.03) | 1.13 (±0.09) |
| Ketoconazole (1 mg/mL) | 3.2 (±0.03) | 3.16 (±0.03) | 2.97 (±0.03) |
| Ethanol (100%) | 0.33 (±0.03) | 0.26 (±0.03) | 0.33 (±0.03) |
*Mean of three replications, (—): no zone.
3.2. Minimum Inhibitory Concentration
The bioactive fraction SF4 showed inhibition at a concentration of 10 mg/mL and 1 mg/mL against the selected pathogens, while growth was observed in the other two dilutions 0.1 mg/mL and 0.01 mg/mL (Table 3). For the management and protection from plant diseases, exploitation of naturally available chemicals from plants would be a ecologically sound method and a prominent commercial fungicide can be obtained in future, replacing toxic fungicides [21, 22].
Table 3.
Minimum inhibitory concentration of PDP against fungal plant pathogens.
| Compound | Fungal plant pathogens | ||
|---|---|---|---|
| Fusarium moniliforme | Fusarium oxysporum | Fusarium graminearum | |
| Phenyl derivative of pyranocoumarin | |||
| 10 mg/mL | − | − | − |
| 1 mg/mL | − | − | − |
| 0.1 mg/mL | + | + | + |
| 0.01 mg/mL | + | + | + |
| Ketoconazole | |||
| 10 mg/mL | − | − | − |
| 1 mg/mL | − | − | − |
| 0.1 mg/mL | + | + | + |
| 0.01 mg/mL | + | + | + |
| Solvent control | + | + | + |
| Cells | + | + | + |
“+” Growth, “–”: no growth.
3.3. Spectroscopic Studies
The antifungal compound isolated from the seed of P. corylifolia L. was obtained as whitish slight yellow colored compound. The spectroscopic studies confirmed the presence of new phenyl derivative of pyranocoumarin. The molecular formula was determined as C27H28O4 by GC-MS (Figure 1) m/z 414[M-2H]+. The fragmentation pattern confirmed the presence of pyranocoumarin [23]. The IR spectrum showed absorption at λmax 1717 and 1174 cm−1 indicating the presence of C=O and C–O–C groups, which was confirmed by the presence of δC 161.2 in 13C spectrum. Moreover, the 13C NMR spectra displayed 29 carbon signals differentiated as five methyl, one methoxyl, 10 methine (including olefins) and 11 quaternary carbons (including oxygenated at δC 68.9, 154.6, 145.9 and characteristic coumarin carbonyl at 161.2) indicating that the structure should be a pyranocoumarin. The 1H NMR spectrum suggested the presence of four methyls (δ 1.65 (3H, s), δ 1.69 (3H, s), 1.32 (3H, s), 1.28 (3H, s), 1.21 (3H, s)), olefinic protons δH 5.83 and 9.93 (each 1H, d, J = 8.56 Hz), a methoxyl δ 3.8 (s) and two 1H singlets were observed at about δH 6.33 and 6.23 and down field 6 aromatic protons observed between δH 6.78 and 7.28. On the basis of these observations and spectral data, the compound was predicted as “(E)-5-methoxy-8, 8-dimethyl-3-(2-methylpent-3-en-2-yl)-6-phenylpyrano [3, 2-g] chromen-2(8H)-one”, a PDP (Figure 2) [24–26].
Figure 1.
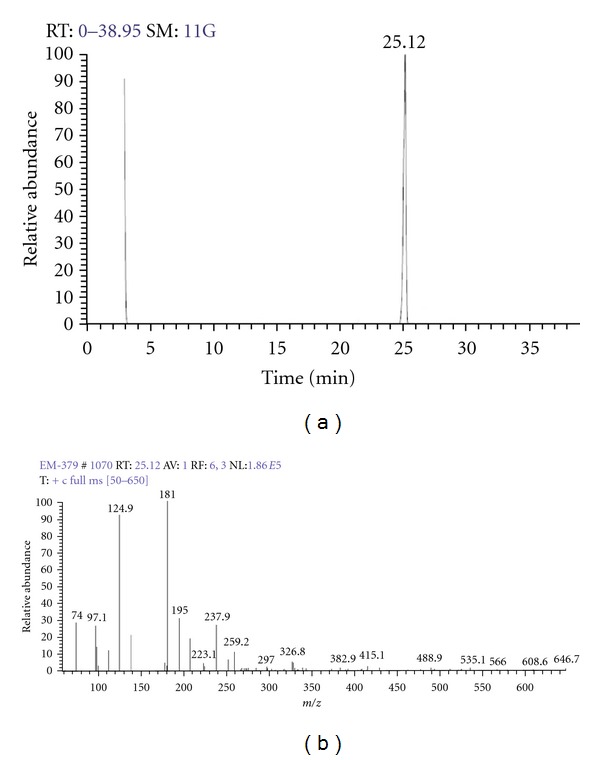
(a) Gas chromatogram of the PDP. (b) Mass fragmentation spectrum of PDP.
Figure 2.
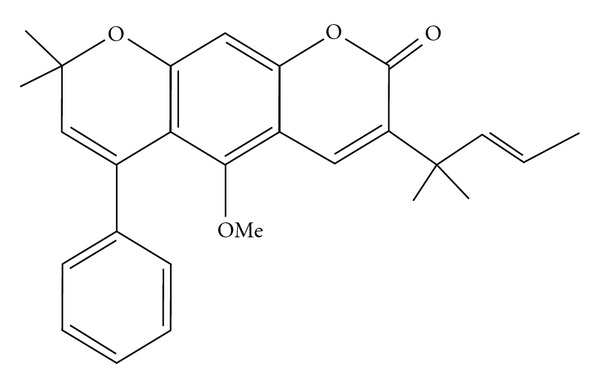
Phenyl derivative of pyranocoumarin (PDP).
3.4. Docking Studies
The native T2 mycotoxin exhibited docking score of −7.79 and glide energy of −48.81 kcal/mol (Table 4) with hydrogen bond interactions TYR 413 and HIS 156 (Figure 3). The PDP was found to exhibit favorable bifurcated hydrogen bond interactions with the active site residue TYR 413 and ARG 402. It exhibited a docking score of −7.19 and glide energy of −45.78 kcal/mol. In the ligplot image, the hydrophobic interactions with twelve protein residues LEU 16, GLN 310, LEU 365, ALA 292, MET 312, HIS 156, SER 364, ILE 19, THR 121, LEU122, PHE 405, and THR 366 surrounding the PDP were also observed, indicating a stable docked complex of the protein and ligand (Figures 4 and 5).
Table 4.
IFD results of ligand PDP and native ligand ZBA with the target protein trichothecene 3-O-acetyltransferase.
| Sl. no. | Ligand name | Docking score | Glide energy kcal/mol | Hydrogen bond D–H⋯A | Distance Å |
|---|---|---|---|---|---|
| (1) | Phenyl derivative of pyranocoumarin (PDP) | −7.19 | −45.78 | (TYR 413)O–H⋯O (TYR 413)O–H⋯O (ARG 402)N–H⋯O |
3.03 3.33 3.06 |
| (2) | Native ligand ZBA | −7.79 | −48.81 | O–H⋯N(HIS 156) (TYR 413)O–H⋯O |
2.67 2.89 |
Figure 3.
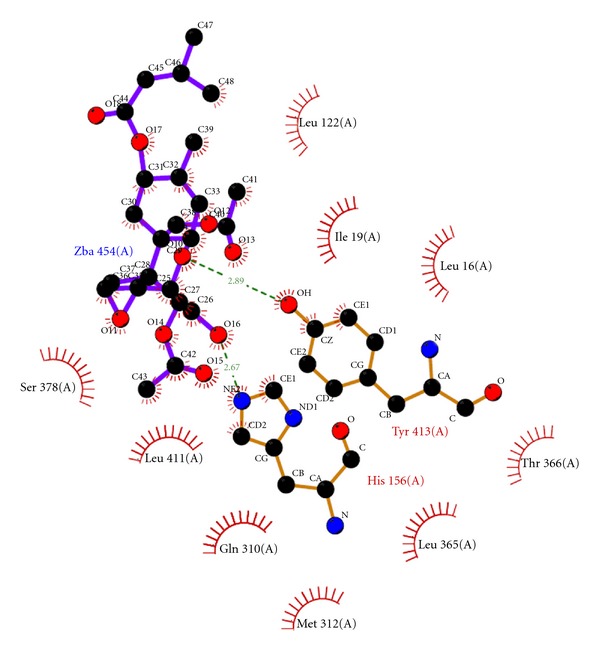
Ligplot image of interaction between ZBA (T2 mycotoxin) with trichothecene 3-O-acetyltransferase. Green dotted lines are hydrogen bond interactions and red semicircles are the hydrophobic interactions.
Figure 4.
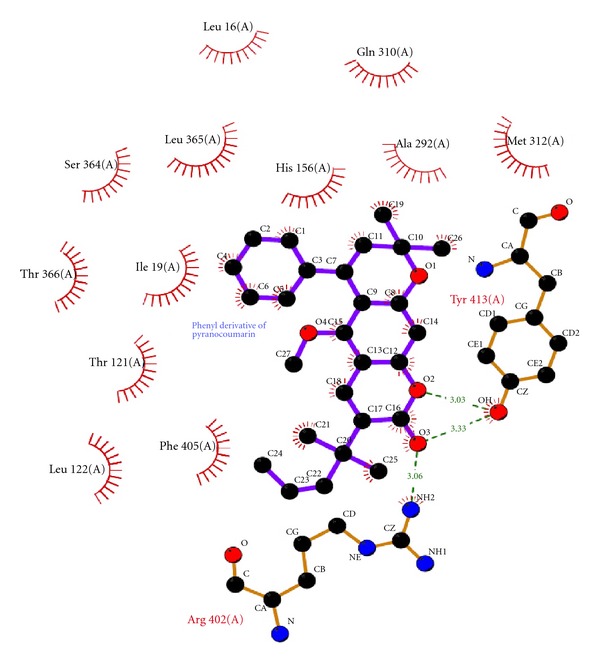
Ligplot image of interaction between PDP with trichothecene 3-O-acetyltransferase. Green dotted lines are hydrogen bond interactions and red semicircles are the hydrophobic interactions.
Figure 5.

The docked complex of the ligand PDP in the active site residue cleft of the protein trichothecene 3-O-acetyltransferase.
This docking study proposes that the PDP has a similar affinity towards the trichothecene 3-O-acetyltransferase as that of the ZBA (T2 mycotoxin). Hence PDP can easily dock in the active site residue of the protein and form a docked complex and as a result prevent the activation of Tri101. This leads to the inhibition of acetylation of the T2 mycotoxin and disturbs the self-defense mechanism of the Fusarium sp.
Coumarins possess several biological activities, but they also play an important role in defense mechanisms against insects and plant pathogens. An increasing body of evidence indicates that the chemical interactions between plant-pathogenic fungi and higher plants are both complex and highly integrated. For instance, as fungi have developed toxins that increase their virulence on plant tissues, plants have developed a variety of ways to limit the effectiveness of these fungal toxins. Biosynthesis of fungal toxins can, however be blocked in vitro by the addition of certain naturally occurring plant metabolites at concentrations inhibitory to fungal growth [27, 28].
4. Conclusions
The seed of P. corylifolia L. is a commonly used botanical medicine. The present study has explored the use of P. corylifolia in the field of agriculture, by using it against plant pathogen Fusarium sp. A new antifungal compound, PDP, was identified with a minimum inhibitory concentration of 1 mg/mL against the selected pathogens namely, Fusarium oxysporum, Fusarium moniliforme, and Fusarium graminearum.
The molecular docking study with trichothecene 3-O-acetyltransferase proposes a hypothesis that the PDP has the affinity towards the target protein and it is able to bind with the protein and inhibit the acetylation mechanism of the protein leading to death of the Fusarium sp. The mechanism of how the antifungal compound PDP interacts with the 2RKV protein is revealed here. Nonetheless, this data require confirmation by appropriate experimental data. Our theoretical prediction may lead to the establishment of future molecular biology approaches.
Exploitation of naturally available chemicals from plants, which retards the reproduction of undesirable microorganisms, would be a more realistic and ecologically sound method for plant protection and will have a prominent role in the development of future commercial pesticides and fungicides for crop protection strategies, with special reference to the management of plant diseases [22, 29]. The PDP can be used for developing a commercial formulation based on its field trial and toxicological experiments in future.
Acknowledgment
The authors are grateful to the Bioinformatics Infrastructure Facility (BIF), University of Madras, Guindy Campus, Chennai, India, for providing the infrastructure facility to carry out molecular docking studies.
References
- 1.Kiran B, Lalitha V, Raveesha KA. In vitro evaluation of antifungal activity of Psoralea corylifolia L. (seeds) and its different fractions on seed borne fungi of maize. Journal of Chemical and Pharmaceutical Research. 2011;3(4):542–550. [Google Scholar]
- 2.Reddy KRN, Nurdijati SB, Salleh B. An overview of plant-derived products on control of mycotoxi genie fungi and mycotoxins. Asian Journal of Plant Sciences. 2010;9(3):126–133. [Google Scholar]
- 3.Satish S, Raghavendra MP, Raveesha KA. Antifungal potentiality of some plant extracts against Fusarium sp. Archives of Phytopathology and Plant Protection. 2009;42(7):618–625. [Google Scholar]
- 4.Fathima SK, Bhat SS, Girish K. Efficacy of some essential oils against Phomopsis azadirachtae the incitant of die-back of neem. Journal of Biopesticides. 2009;2(2):157–160. [Google Scholar]
- 5.Dubey NK, Srivastava B, Kumar A. Current status of plant products as botanical pesticides in storage pest management. Journal of Biopesticides. 2008;1(2):182–186. [Google Scholar]
- 6.Satish S, Mohana DC, Raghavendra MP, Raveesha KA. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. Journal of Agricultural Technology. 2007;3(1):109–119. [Google Scholar]
- 7.Farooqi AA, Sreeramu BS. Cultivation of Medicinal and Aromatic Plants. Hyderabad, India: Universities Press (India) Limited; 2003. History, importance, present status and future prospects of medicinal plant; pp. 1–18. [Google Scholar]
- 8.Katsura H, Tsukiyama RI, Suzuki A, Kobayashi M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrobial Agents and Chemotherapy. 2001;45(11):3009–3013. doi: 10.1128/AAC.45.11.3009-3013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Hong C, Zhou C, Xu D, Qu HB. Screening antitumor compounds psoralen and isopsoralen from Psoralea corylifolia L. seeds. Evidence-based Complementary and Alternative Medicine. 2011;2011:7 pages. doi: 10.1093/ecam/nen087. Article ID 363052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Li J, Wang X, et al. Psc-AFP, an antifungal protein with trypsin inhibitor activity from Psoralea corylifolia seeds. Peptides. 2006;27(7):1726–1731. doi: 10.1016/j.peptides.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 11.McCormick PS, Stanley MA, Stover AN, Alexander JN. Trichothecenes: from simple to complex mycotoxins. Toxins. 2011;3(7):802–814. doi: 10.3390/toxins3070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohn TM, van Middlesworth F. Purification and characterization of the sesquiterpene cyclase trichodiene synthetase from Fusarium sporotrichioides. Archives of Biochemistry and Biophysics. 1986;251(2):756–761. doi: 10.1016/0003-9861(86)90386-3. [DOI] [PubMed] [Google Scholar]
- 13.Kimura M, Kaneko I, Komiyama M, et al. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Journal of Biological Chemistry. 1998;273(3):1654–1661. doi: 10.1074/jbc.273.3.1654. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Shingu Y, Yoneyama K, Yamaguchi I. Features of TRI101, the tricothecene-3-o-acetyltransferase gene, related to self defense mechanism in Fusarium graminearum. Bioscience, Biotechnology and Biochemistry. 1998;62(5):1033–1036. doi: 10.1271/bbb.62.1033. [DOI] [PubMed] [Google Scholar]
- 15.McCormick SP, Alexander NJ. Fusarium Tri8 encodes a trichothecene C-3 esterase. Applied and Environmental Microbiology. 2002;68(6):2959–2964. doi: 10.1128/AEM.68.6.2959-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geyid A, Abebe D, Debella A, et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. Journal of Ethnopharmacology. 2005;97(3):421–427. doi: 10.1016/j.jep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Jamil A, Shahid M, Khan MM, Ashraf M. Screening of some medicinal plants for isolation of antifungal proteins and peptides. Pakistan Journal of Botany. 2007;39(1):211–221. [Google Scholar]
- 18.Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. Journal of Ethnopharmacology. 1998;62(2):183–193. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 19.Singh J, Randhawa V. Structure based drug designing of a novel antiflaviviral inhibitor for nonstructural 3 protein. Bioinformation. 2011;6(2):57–60. doi: 10.6026/97320630006057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinuchakkaravarthy T, Kumaravel KP, Ravichandran S, Velmurugan D. Active compound from the leaves of Vitex negundo L. shows anti-inflammatory activity with evidence of inhibition for secretory phospholipase A2 through molecular docking. Bioinformation. 2011;7(4):199–206. doi: 10.6026/97320630007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preetha JP, Karthika K, Rekha NR, Elshafie K. Formulation and evaluation of in situ ophthalmic gels of Diclofenac sodium. Journal of Chemical and Pharmaceutical Research. 2010;2(3):528–535. [Google Scholar]
- 22.Varma J, Dubey NK. Prospectives of botanical and microbial products as pesticides of tomorrow. Current Science. 1999;76(2):172–179. [Google Scholar]
- 23.Bhat VS, Nagasampagi AB, Meenakshi S. Chemistry of Natural Products. Berlin, Germany: Springer; 2005. [Google Scholar]
- 24.Su CR, Yeh SF, Liu CM, et al. Anti-HBV and cytotoxic activities of pyranocoumarin derivatives. Bioorganic and Medicinal Chemistry. 2009;17(16):6137–6143. doi: 10.1016/j.bmc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Ramesh P, Das TA, Mohandass P, Nagasathya R. The structure of hantzsch coumarin. Indian Journal of Chemistry B. 2008;47(9):1447–1450. [Google Scholar]
- 26.Tandon S, Rastogi RP. Recent advances in naturally occurring coumarins. Journal of Science and Industrial Research. 1979;38:428–441. [Google Scholar]
- 27.Desjardins EA, Hohn TM, McCormick SP. Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiological Reviews. 1993;57(3):595–604. doi: 10.1128/mr.57.3.595-604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garvey GS, McCormick SP, Rayment I. Structural and functional characterization of the TRI101 trichothecene 3-O-acetyltransferase from Fusarium sporotrichioides and Fusarium graminearum: kinetic insights to combating Fusarium head blight. Journal of Biological Chemistry. 2008;283(3):1660–1669. doi: 10.1074/jbc.M705752200. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb OR, Borin MR, Brito NR. Integration of ethnobotany and phytochemistry: dream or reality? Phytochemistry. 2002;60(2):145–152. doi: 10.1016/s0031-9422(02)00088-2. [DOI] [PubMed] [Google Scholar]


