Abstract
Background
Guidelines recommend informed decision-making regarding prostate specific antigen (PSA) screening for men with at least 10 years of remaining life expectancy (RLE). Comorbidity measures have been used to judge RLE in previous studies, but assessments based on other common RLE measures are unknown. We assessed whether screening rates varied based on four clinically relevant RLE measures, including comorbidities, in a nationally-representative, community-based sample.
Methods
Using the National Social Life, Health and Aging Project (NSHAP), we selected men over 65 without prostate cancer (n=709). They were stratified into three RLE categories (0–7 years, 8–12 years, and 13+ years) based on validated measures of comorbidities, self-rated health status, functional status, and physical performance. The independent relationship of each RLE measure and a combined measure to screening was determined using multivariable logistic regressions.
Results
Self-rated health (OR = 6.82; p < 0.01) most closely correlated with RLE-based screening, while the comorbidity index correlated the least (OR = 1.50; p = 0.09). The relationship of RLE to PSA screening significantly strengthened when controlling for the number of doctor visits, particularly for comorbidities (OR= 43.6; p < 0.001). Men who had consistent estimates of less than 7 years RLE by all four measures had an adjusted PSA screening rate of 43.3%.
Conclusions
Regardless of the RLE measure used, men who were estimated to have limited RLE had significant PSA screening rates. However, different RLE measures have different correlations with PSA screening. Specific estimates of over-screening should therefore carefully consider the RLE measure used.
Keywords: prostate cancer, older adults, screening, PSA, remaining life expectancy, comorbidity
Introduction
Prostate-specific antigen (PSA) screening for prostate cancer (PCa) is prevalent,1–4 despite ongoing uncertainty about the mortality benefits of PSA screening and consequent concern over the negative impacts from testing.5, 6 Disappointingly, recently-published, long-anticipated randomized controlled trials of PSA screening did not resolve this uncertainty.7–10 Trials do suggest, however, that mortality benefits from treating localized PCa are not evident until approximately 10 years following definitive treatment.9, 10 Consequentially, older men in poor health are unlikely to live long enough to experience the potential benefits of early treatment, risking over-screening.11 However, with the increasing proportion of healthy older men expected to live more than 10 years,12 excluding all men from PSA screenings could result in at-risk, otherwise healthy men not receiving screening.13 Deciding which men to screen should therefore rely on more than age alone.
As a response to the uncertain benefits of screening, PCa screening recommendations have been based on estimated remaining life expectancy (RLE) rather than age, and they emphasize informed, shared decision-making. Two physicians’ groups, the American Cancer Society (ACS) and the American Urological Association (AUA), recommend that men with a RLE of at least 10 years have an opportunity to make an informed decision about whether to be screened for PCa.14, 15 In contrast, preliminary U.S. Preventive Services Task Force (USPSTF) guidelines recommend against PSA-based screening for PCa.7, 16 Despite the differences, all guidelines recommend against screening for those with limited RLE. Unfortunately, little guidance has been given to physicians on systematic approaches to estimating RLE, and currently, little is known about how RLE estimations are applied to actual clinical practice.
Prior studies examining the relationship of RLE to PSA screening rates did not include several potential measures known to estimate RLE in community-dwelling, older adults.11, 13, 17–20 Previous studies have instead mainly used comorbidities as their measure of RLE.11 Given this, we use a recent, nationally-representative sample of community-dwelling older adults in the United States to assess four well-known and clinically-relevant measures of RLE, including comorbidities, self-rated health, functional status, and a performance assessment. We examine whether the relationship of RLE to PSA screening varies based on which RLE measure is used, as well as the relationship of a combined RLE measure to PSA screening.
Methods
Study Design
Using data from the National Social Life, Health, and Aging Project (NSHAP), a nationally-representative survey of 3,005 community-dwelling individuals aged 57–85 in the United States.21 Data collection took place from July, 2005 through March, 2006, with a response rate of 75.5%. In-person interviews were conducted by trained professionals.22 A full account of sampling procedures, instrument development, and interview procedures for NSHAP are published elsewhere.21, 22 All men over 65 years old are included for analysis. After excluding 89 individuals for missing information on primary variables and 129 individuals with a prior history of PCa, the final sample size was 709 men.
PSA screening
The primary outcome measure is the self-reported use of a screening PSA test.1–3, 23 Individuals were asked, “About how long has it been since you last had a Prostate Specific Antigen test, also called a PSA test?” Response categories were: less than 1 year ago, less than 5 years ago, ever, and never. Receiving a PSA test within the last year indicates an appropriate screening for otherwise healthy men at the time of the data collection.15, 24
RLE Measures
Men were grouped into RLE categories of 0–7 years, 8–12 years, and 13+ years. These categories were chosen based on a 10-year RLE cut-off found in most guidelines,19, 24 then conservatively allowing for a two-year RLE “window” on either side of 10.25 Four specific measures to estimate RLE were assessed: a comorbidity index, self-rated health, physical performance, and functional health. A Charlson comorbidity index was chosen because it is well-validated, relatively simple to use, and provides objective information on clinically significant conditions from a questionnaire form.26, 27 Self-rated health, which has been consistently shown to be a predictor of RLE, was measured using the question, “Would you say your health is excellent, very good, good, fair, or poor?”28 Functional status was assessed by asking if the individual can walk one block, and by the ability to perform activities of daily living (ADL).29 Finally, the timed up and go (TUG) test, a common clinical tool that is associated with mortality,30–32 was also used as a performance assessment to place individuals into RLE categories. The TUG test was performed only on an NSHAP subsample (n=327).
Estimating RLE
We used two literature-supported approaches for RLE estimates: one based on life table calculations and the other on national RLE data (Table 1). Life tables were used for the self-rated health and functional status measures. Questionnaire responses were stratified by the age of the respondent, and then compared with appropriate life tables to estimate RLE.29, 33, 34 Individuals were then placed into appropriate RLE categories. National RLE data was used for the comorbidity index and the TUG test. Percentages of men expected to live 0–7 years, 8–12 years, and 13+ years were calculated for each age group based on the United States 2005 Life Tables from the National Center for Health Statistics.12 Then, men were placed into the appropriate RLE categories by matching the percentages expected in each category to the distribution of the RLE variable (Table 2).35 For example, for comorbidities, those aged 65 were matched into RLE categories as follows: those with a comorbidity score of 4 or more were placed into the RLE category 0–7 years, those with a score of 3 were placed into RLE category 8–12 years, and those with a score of 0–2 were place into the RLE category of 13+ years. For the TUG test, distributions of TUG completion times within each age group were matched to corresponding distributions for the three relevant RLE categories. The time cut-off intervals used to place individuals into RLE groups correspond with suggested TUG clinical cut-offs.30, 36
Table 1.
Self-rated health and Functional Health Remaining Life Expectancy Life Table Calculations
| Age (years)
|
||||
|---|---|---|---|---|
| 65 | 70 | 75 | 80 | |
| Self-Rated Health Life Table (years)1 | ||||
| Excellent | 18.1 | 14.7 | 11.3 | 8.0 |
| Very good | 17.5 | 14.2 | 10.9 | 7.8 |
| Good | 16.7 | 13.3 | 10.2 | 7.3 |
| Fair | 15.1 | 11.6 | 8.7 | 6.2 |
| Poor | 11.7 | 8.2 | 5.6 | 3.8 |
|
| ||||
| Functional Health Life Table (years)2 | ||||
| Independent | 17.5 | 12.1 | 9.4 | 7.2 |
| Mobility disabled | 14.7 | 10.7 | 7.9 | 5.7 |
| ADL disabled | 9.5 | 6.5 | 4.4 | 3.1 |
|
| ||||
| National RLE Data (%)3 | ||||
| 0–7 years RLE | 14.9 | 26.9 | 34.8 | 50.3 |
| 8–12 years RLE | 15.6 | 17.9 | 27.4 | 29.1 |
| 13+years RLE | 69.5 | 55.2 | 37.8 | 20.6 |
Table 2.
Remaining life expectancy cutoffs and distributions for comorbidity index, performance, self-rated health, and functional health
| Comorbidity Index1 | Performance status1 | Self-Rated Health2 | Functional Health3 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Range | % in range | Range (sec) | % in range | Ratings | % in group | Ratings | % in group | |
| 65 Years | ||||||||
| 13+yrs RLE | ≧0–2 | 72.2 | ≧13 | 71.2 | F, G, V, E | 92.8 | M, I | 99.2 |
| 8–12 yrs RLE | 3 | 10.7 | 14–16 | 16.1 | P | 7.2 | A | 0.8 |
| 0–7 yrs RLE | 4 | 17.1 | ≦17 | 12.7 | - | - | - | |
| 70 Years | ||||||||
| 13+yrs RLE | ≧0–1 | 47.4 | ≧11 | 54.3 | G, V, E | 75.7 | M, I | 93.8 |
| 8–12 yrs RLE | 2–3 | 31.3 | 12–13 | 21 | P, F | 24.3 | A | 6.2 |
| 0–7 yrs RLE | 4 | 21.4 | ≦14 | 24.7 | - | - | - | |
| 75 Years | ||||||||
| 13+yrs RLE | ≧0–1 | 36.4 | ≧10 | 32 | - | - | ||
| 8–12 yrs RLE | 2 | 21.6 | 11–13 | 26.4 | F, G, V, E | 93.8 | M, I | 98.8 |
| 0–7 yrs RLE | 3 | 42 | ≦14 | 41.7 | P | 6.2 | A | 1.2 |
| 80 years | ||||||||
| 13+yrs RLE | ≧0 | 17.2 | ≧10 | 19.7 | - | - | ||
| 8–12 yrs RLE | 1 | 17.2 | 11–12 | 25 | V, E | 31.6 | - | |
| 0–7 yrs RLE | 2 | 65.5 | 13 | 55.4 | P, F, G | 68.4 | A, M, I | 100.0 |
Comorbidity index and performance status distributions were matched to national mortality data from the 2005 Life Tables of United States [12]
Self-rated health responses were matched to a self-rated health life table published by Diehr et al. 1998, Table 5 [34]
Functional status responses were matched to life tables published by Lynch et al. 2003 [33], Table 2 and Keeler et al. 2010, Table 1 [29]
Abbreviations: RLE - Remaining life expectancy, TUG - Timed Up and Go test, yrs - years, SRH - Self-rated health, FH - Functional health P - Poor, F - Fair, G - Good, V - Very good, E - Excellent; A - ADL Disabled, M - Mobility Disabled, I - Independent
Combined Measure of RLE
In addition to evaluating the relationship of individual RLE measures to PSA screening, the relationship of a combined measure utilizing all four RLE measures to PSA screening was assessed. There are inherent limitations in the accuracy of individual RLE estimations, therefore the purpose of this additional analysis was to understand rates of screening in men for whom informed decision making would be very unlikely to be useful (i.e. when all measures indicate RLE of 0–7 years) or when there is a possibility of benefit requiring further conversation (all measures indicate RLE of 13+ years). Men were grouped into categories of 0–7 years RLE if all measures indicated 0–7 years of RLE, or 13+ years RLE if all measures indicated 13+ years of RLE. For simplicity, all other men were grouped into the category “Discordant RLE estimation.” Because TUG was administered only to a subsample of NSHAP, a combined analysis was done separately for men with available TUG data (4 RLE measures) and men for whom TUG data was unavailable (3 RLE measures).
Covariates
Several factors other than RLE known to be related to PSA screening were included as covariates. Age was stratified into 5 year age groups for descriptive statistics, and used as a continuous variable in regressions. Ethnicity, marital status, and socioeconomic status, including education and income, have all been associated with PSA screening.2, 3, 11, 23, 37–40 Ethnicity was self-reported. Marital status was defined as currently married or unmarried. Education was divided into four categories: “less than high school,” “high school diploma/GED,” “some college/vocational degree,” and “bachelors or more.” Income was defined based on percentage of the federal poverty level (FPL) for single adult and 2 adult households,41,42 with the categories “poor” (0–100% FPL), “near-poor” (101–200% FPL), “not poor” (201+% FPL), and “unknown” if they did not respond. Health care access was assessed by using the number of visits to a doctor’s office in the last 12 months.
Individuals with certain health-related behaviors, such as low alcohol use, low smoking rates, and regular exercise, have higher rates of PSA screening.2 Therefore, three dichotomous health behavior variables were included: tobacco use, alcohol consumption, and exercise.43, 44 These variables were included as covariates due to the likelihood of patient preferences for “prevention” influencing the screening process. Tobacco use was based on whether the individual smokes cigarettes, a pipe, cigars, or uses snuff or chewing tobacco. Individuals who consumed 14 or more alcoholic beverages a week and had at least three episodes of drinking more than four drinks in one night in the last three months were coded as “high alcohol consumption.”43, 44 Individuals who did not exercise at least 1–2 times per week were rated as physically inactive.
Statistical Analysis
First, descriptive statistics were calculated to characterize the distributions and rates of PSA screening for each variable (Tables 3 and 4).21 Multivariable logistic regression models were used to test the independent relationship of RLE based on each measure to rates of PSA utilization (Table 5). Estimated rates of PSA screening in the last year for each logistic regression model were calculated (Figure 1). In the combined analysis, we conducted bivariate analysis to illustrate the degree of concordance and discordance between different RLE estimations (Table 6). Finally, the adjusted PSA screening rates for the combined measures of RLE were calculated (Figure 2). All statistical analyses were performed using NSHAP sample weights in Stata 11.0 (StataCorp, College Station, TX). The study was approved by the Institutional Review Board at the University of Chicago.
Table 3.
Sociodemographic, health characteristics, and national estimates of PSA screening rates of men from the National Social Life, Health, and Aging Project sample, age 65–85
| Variables | Total (%) | PSA Screening Rates
|
||
|---|---|---|---|---|
| N=709 | (95% CI) | <1 yr | (95% CI) | |
| Age groups | ||||
| 65–69 | 35.7 | (31.9–39.5) | 58.4 | (51.0–65.4) |
| 70–74 | 28.1 | (24.6–31.5) | 61.7 | (50.9–71.4) |
| 75–79 | 21.9 | (18.3–25.6) | 63.8 | (55.2–71.6) |
| 80–85 | 14.3 | (11.4–17.2) | 43.3 | (33.5–53.6) |
| Ethnicity | ||||
| White | 83.8 | (80.1–87.6) | 60.5 | (55.4–65.3) |
| African-American | 7.8 | (5.5–10.1) | 49.4 | (35.2–63.6) |
| Hispanic | 5.8 | (2.8–8.9) | 49.2 | (31.5–67.1) |
| Other | 2.5 | (1.3–3.8) | 35.3 | (14.3–64.0) |
| Marital status | ||||
| Married | 74.7 | (70.7–78.7) | 61.3 | (55.2–67.1) |
| Not married | 25.3 | (21.3–29.3) | 49.5 | (41.5–57.5) |
| Education | ||||
| Less than high school | 19.7 | (16.1–23.4) | 42.6 | (32.7–53.0) |
| High school/equivalent | 25.3 | (21.9–28.7) | 65.1 | (59.0–70.7) |
| Certification/some college | 26.5 | (22.5–30.6) | 63.8 | (55.5–71.4) |
| Bachelors or more | 28.4 | (23.9–32.9) | 58.1 | (47.2–68.3) |
| Income level1 | ||||
| Poor (0–100% FPL) | 4.9 | (2.9–7.0) | 24.2 | (14.6–37.3) |
| Near poor (101–200% FPL) | 12.7 | (9.8–15.6) | 54.9 | (41.7–67.4) |
| Not poor (>200% FPL) | 58.6 | (53.8–63.4) | 62.5 | (56.8–67.8) |
| Unknown | 23.7 | (19.9–27.5) | 57.1 | (48.5–65.2) |
| Health Behaviors | ||||
| Regular tobacco use2 | ||||
| Yes | 19.2 | (14.7–23.8) | 49.9 | (41.1–58.7) |
| No | 80.8 | (76.2–85.3) | 60.3 | (54.7–65.7) |
| High alcohol consumption3 | ||||
| Yes | 40.9 | (36.8–45.0) | 50.3 | (42.0–58.5) |
| No | 59.1 | (55.0–63.2) | 63.9 | (57.8–69.6) |
| Low Exercise4 | ||||
| Yes | 14.6 | (11.8–17.5) | 54.0 | (42.6–65.0) |
| No | 85.4 | (82.5–88.2) | 59.1 | (54.1–63.8) |
| Doctor visits in last 12 months | ||||
| 0 | 6.9 | (4.7–9.1) | 7.0 | (2.4–18.7) |
| 1 | 9.9 | (6.7–13.1) | 48.9 | (36.7–61.2) |
| 2 or 3 | 31.2 | (27.6–34.9) | 63.0 | (55.9–69.5) |
| 4 to 9 | 33.8 | (30.2–37.3) | 61.3 | (53.8–68.3) |
| 10+ | 18.2 | (14.0–22.4) | 69.6 | (61.5–76.6) |
Income is measured as % of 2006 Federal Poverty Level (FPL), in 2006 the FPL for a one-adult household was $9,669, and $12,201 for a 2-adult household [41]
Regular tobacco use includes use of cigarettes, a pipe, cigars, or snuff or chewing tobacco [44]
High alcohol is defined as drinking ≥ 14 drinks/week and having 3 or more instances of drinking ≥4 drinks in one night in the last 3 months [44]
Low exercise is defined as exercising less than 1–2 per week [44]
Abbreviations: PSA - Prostate-specific antigen, CI - confidence interval, FPL - Federal poverty level
Table 4.
National estimates of 1-year PSA screening rates by comorbidities, self-rated health, timed up and go test, and functional health
| Variables | Total (%) | PSA Screening Rates
|
||
|---|---|---|---|---|
| N=709 | (95% CI) | <1 yr | (95% CI) | |
| Comorbidity Index Score | ||||
| 0 | 19.2 | (16.3–22.1) | 50.1 | (39.0–61.1) |
| 1 | 23.4 | (20.2–26.6) | 62.8 | (55.5–69.5) |
| 2 | 21.9 | (18.5–25.3) | 58.7 | (48.9–67.9) |
| 3 | 12.3 | (9.1–15.6) | 60.2 | (46.5–72.4) |
| 4 | 9.5 | (6.9–12.1) | 63.1 | (47.9–76.0) |
| 5+ | 13.7 | (10.8–16.6) | 56.7 | (43.6–68.9) |
| Self-rated health | ||||
| Excellent | 14.1 | (11.2–16.9) | 54.1 | (43.3–64.6) |
| Very good | 29.6 | (26.4–32.8) | 67.0 | (58.7–74.3) |
| Good | 31.3 | (27.6–35.0) | 60.0 | (52.8–66.8) |
| Fair | 18.7 | (15.1–22.3) | 53.2 | (42.3–63.7) |
| Poor | 6.4 | (4.9–7.9) | 34.5 | (21.5–50.2) |
| TUG test time (seconds)1 | ||||
| 0–10 | 37.7 | (31.4–44.0) | 73.2 | (63.6–81.1) |
| 11–15 | 44.7 | (38.4–51.0) | 57.1 | (46.9–66.7) |
| 16+ | 17.7 | (12.3–23.0) | 38.6 | (27.4–51.3) |
| Functional Health | ||||
| Not impaired | 96.2 | (94.5–97.9) | 59.1 | (53.8–64.2) |
| Mobility impaired | 3.0 | (1.4–4.7) | 37.8 | (19.6–60.3) |
| ADL impaired | 0.8 | (0.3–1.4) | 41.1 | (17.5–69.7) |
Timed up and Go test only performed on n=327 subsample
Abbreviations: PSA - Prostate-specific antigen, TUG - Timed Up and Go test ADL - Activities of Daily Living, CI - confidence interval
Table 5.
Adjusted multivariate logistic regression for receiving a PSA screening test in the last year by remaining life expectancy (RLE) groups
| OR | (95% CI) | p-value | |
|---|---|---|---|
| Comorbidity RLE | |||
| 0–7 years | Ref | - | - |
| 8–12 years | 1.31 | (0.78–2.19) | 0.30 |
| 13+ years | 1.50 | (0.94–2.40) | 0.09 |
| Self-Rated Health RLE | |||
| 0–7 years | Ref | - | - |
| 8–12 years | 2.79 | (1.31–5.94) | 0.01 |
| 13+ years | 6.82 | (2.48–18.82) | <0.01 |
| Timed Up and Go test RLE | |||
| 0–7 years | Ref | - | - |
| 8–12 years | 1.39 | (0.55–3.54) | 0.48 |
| 13+ years | 2.75 | (1.32–5.72) | 0.01 |
| Functional Status RLE | |||
| 0–7 years | Ref | - | - |
| 8–12 years | 2.39 | (1.19–4.79) | 0.02 |
| 13+ years | 3.17 | (1.00–10.02) | 0.05 |
Model is adjusted for age, ethnicity, marital status, education, income, exercise, tobacco, alcohol, and visits to the doctor
Timed up and go test only performed on a N=327 subsample
Reference group is the 0–7 yr RLE group and has OR=1.00
Abbreviations: RLE - Remaining life expectancy, CI - confidence interval, AA - African-American, SES - socioeconomic status, OR - odds ratio, Ref - Reference
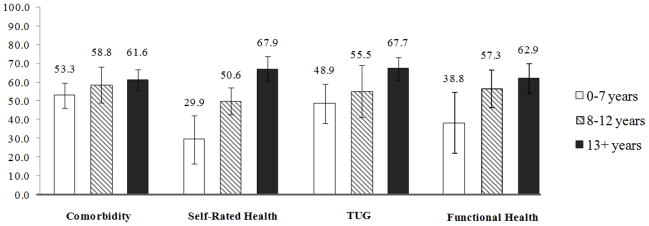
Figure 1.
Adjusted marginal mean PSA screening rates across remaining life expectancy groups.
Model is adjusted for age, ethnicity, marital status, education, income, exercise, tobacco, alcohol, and visits to the doctor. Bars indicate a 95% confidence interval.
Abbreviations: RLE - Remaining Life expectancy, TUG - Timed up and go test
Table 6.
Concordance and discordance between different remaining life expectancy estimations
| Comorbidity RLE
|
Performance status RLE
|
Functional Health RLE
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–7 yrs | 8–12 yrs | 13+ yrs | 0–7 yrs | 8–12 yrs | 13+ yrs | 0–7 yrs | 8–12 yrs | 13+ yrs | |
| Self-Rated Health RLE | |||||||||
| 0–7 years | 8.3 | 1.6 | 1.2 | 6.7 | 3.3 | 2.3 | 9.7 | 1.3 | 0.0 |
| 8–12 years | 14.5 | 8.5 | 11.3 | 14.3 | 7.7 | 11.8 | 5.0 | 21.6 | 7.7 |
| 13+ years | 9.0 | 10.6 | 35.1 | 5.3 | 10.3 | 38.4 | 0.0 | 0.3 | 54.4 |
| Comorbidity RLE | |||||||||
| 0–7 years | - | - | - | 15.4 | 6.7 | 14.0 | 9.9 | 10.1 | 11.9 |
| 8–12 years | - | - | - | 3.4 | 4.8 | 10.9 | 2.2 | 5.9 | 12.5 |
| 13+ years | - | - | - | 7.5 | 9.7 | 27.6 | 2.7 | 7.3 | 37.6 |
| Performance status RLE | |||||||||
| 0–7 years | - | - | - | - | - | - | 7.7 | 10.2 | 8.3 |
| 8–12 years | - | - | - | - | - | - | 4.7 | 5.7 | 10.9 |
| 13+ years | - | - | - | - | - | - | 3.9 | 8.0 | 40.6 |
Abbreviations: Remaining life expectanct (RLE), years (yrs)
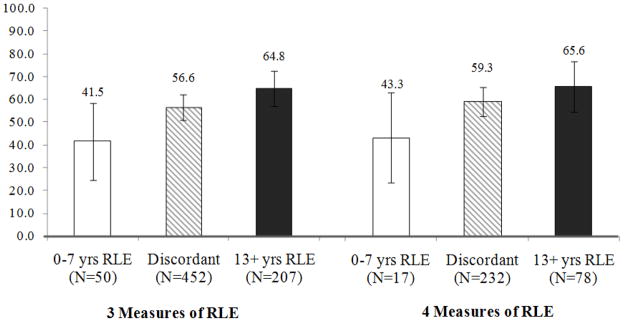
Figure 2.
Adjusted marginal mean PSA screening rates using combined RLE measures.
Model is adjusted for age, ethnicity, marital status, education, income, exercise, tobacco, alcohol, and visits to the doctor. Bars indicate a 95% confidence interval. 4 measure analysis was performed on a subsample having the TUG information (n=327), and includes self-rated health, functional health, comorbidities, and TUG/performance status. 3 measure analysis was performed on the entire sample (n=709), and includes self-rated health, functional health, and comorbidities.
Abbreviations: RLE - Remaining Life expectancy, yrs – years, TUG – Timed up and go test
Results
Sample characteristics and PSA screening rates (Tables 3 and 4)
The PSA testing rates for each variable were consistent with previous studies examining factors related to PSA screening.2, 3, 37 The weighted overall rate of PSA screening in the last year for men between 65–85 years old was 58.3%, which is comparable to other national estimates.1, 4 The weighted proportion of men receiving a PSA test in the last year differed by age group, with individuals aged 80–85 having a rate of 43.3% in the last year, compared to men aged 65–69, who had a rate of 58.3%. Men who visited the doctor more often were much more likely to receive a PSA screening test, with 48.9% receiving a test if going to the doctor 1 time and 69.6% receiving a test if visiting a doctor more than 10 times. Individuals with better self-rated health had more PSA screenings in the last year, with 54.1% in the “excellent health” group receiving a screening, compared to 34.5% in the “poor health” group. This was similar for the TUG test results. In contrast, men with higher co-morbidity scores received more screenings in the last year compared to individuals with low co-morbidity scores.
Logistic regression results (Table 5)
In the self-rated health RLE regressions, the adjusted model indicated that individuals in the highest RLE category have a significantly higher odds of receiving a PSA test than individuals in the 0–7 RLE group (OR=6.82, p<0.001). For functional health RLE and the TUG test RLE, there was a similar, but weaker, trend for PSA utilization in comparison to self-rated health. In contrast, for the co-morbidity index RLE, there was no significant RLE-related trend with the 13+ year RLE category having an OR of 1.50 (p=0.09). Visits to the doctor was strongly predictive of PSA screening in the adjusted logistic regression model for all RLE estimates (Results not shown). The relationship of RLE to PSA screening significantly strengthened when controlling visits to the doctor for both comorbidities (OR= 43.6; p < 0.001) and self-rated health (OR=45.7, p<0.001). In the fully-adjusted model, self-rated health RLE was most closely associated with PSA test use, where estimated rates of PSA test use were 29.9% for 0–7 yrs, 50.6% for 8–12 yrs, and 67.9% for 13+ yrs (Figure 1). Co-morbidity RLE had the least association with PSA test use for estimated rates.
Combined RLE analysis
Bivariate comparisons of each RLE variable show the concordance/discordance between each RLE estimation measure (Table 6). In the comparison of self-rated health and co-morbidity index RLE, 8.3% of men were placed in 0–7 years RLE for both estimations, 35.1% were placed in 13+ years RLE for both estimations, and 48.2% had discordant estimations. Interestingly, 9.0% of men were placed in the 0–7 years RLE category by the comorbidity index, but placed in 13+ years RLE by self-rated health. The self-rated health and functional health RLE estimations were most concordant, in which 85.7% of men were placed in the same category. Overall, 5.2% of men with four RLE measures and 7.1% of men with only three RLE measures available were placed in 0–7 years RLE by all measures. Men who were estimated to have 0–7 years RLE by all four measurements combined had an adjusted PSA screening rate of 43.3%, and men with 13+ years RLE by all measurements had an adjusted screening rate of 65.6% (Figure 2). Those with discordant RLE estimates were in-between.
Discussion
Consistent with guideline recommendations at the time of the NSHAP wave 1 survey (2005), RLE does modestly correlate with PSA screening rates, with some variation in correlation based on which RLE measure is used. However, our results provide additional evidence of high rates of inappropriate PSA screening regardless of how RLE is calculated.11 For men estimated to have a limited RLE by all four measures, after controlling for other related factors, over a third of individuals with an RLE estimated to be less than 7 years were screened in the last year. We believe this is the first article to compare the relationship of several clinically-appropriate RLE measures to PSA screening in a representative population.
Why there are high rates of apparently inappropriate PSA screening is unknown, but our results support two possibilities. First, the regression analysis suggests increasing exposure to the health care system is related to over-screening, since controlling for visits to the doctor significantly strengthened the relationship of RLE with guideline-recommended PSA screening for each RLE measure. This trend is consistent with data showing that geographic areas with more “technologically aggressive” care have more PSA utilization.45 Another consideration is the dynamic of patient-physician discussions regarding screening. Physicians are more likely to initiate discussions about PSA tests than patients (including with older patients and those with more comorbidities), and these discussions can take place without a careful consideration of both the harms and benefits of the PSA test.46, 47 Consequently, physician-initiated discussions, combined with poor communication regarding the overall utility of PSA screening tests, may lead to men who are very unlikely to receive benefit being tested.46, 48 Other explanations for increased screening may include a response to patient preferences or a consequence of sicker patients being more accepting of additional tests.11
A second explanation may be that certain measures for estimating RLE, while available for research purposes,42 are difficult to apply in practice and difficult for practicing physicians to use in estimating RLE. For example, we found that the RLE measurement based on an established comorbidity index was less correlated with guideline-based screening than other measures, such as self-rated health. In bivariate analysis, individuals with more comorbidities had higher rates of PSA testing than those with less, as some others have noted.11, 13 This is consistent with other studies which have shown that physicians often have a difficult time estimating 10-year RLE based on comorbidities and often report feeling uncertain when applying these RLE estimates to decisions.49 Additional longitudinal work is necessary to clarify which measures are the best clinical tools for guiding RLE-based screening recommendations.25
We acknowledge some limitations in our study. First, our study relied on self-reported PSA testing rates, which may be subject to recall bias or other inaccuracies.50, 51 Nevertheless, our national estimates of PSA test use are comparable to those from other studies.3, 4, 11 Second, our data is cross-sectional, which limits inferences regarding causality. Additional work incorporating a longitudinal component using future waves of the NSHAP data, which is currently being collected, could help address this limitation.
In conclusion, our results improve upon previous work assessing the relationship between clinically-relevant RLE measures and PSA screening. Men consistently estimated to have limited RLE by a variety of methods continue to receive significant amounts of PSA screening. Furthermore, alternative RLE estimators appear to provide different assessments of the relationship of RLE to PSA screening rates. Future research should focus on identifying the most appropriate RLE estimators in guiding appropriate discussions regarding informed, shared decision-making in cancer screening.
Acknowledgments
Funding: American Federation for Aging Research (AFAR) – Medical Student Training in Aging Research (AFAR); National Social Life Health and Aging Project (NSHAP) (NIH - 5R01 AG021487).
This study was supported by the Medical Student Training in Aging Research Program (MSTAR) from the American Federation of Aging Research (AFAR). The National Social Life, Health, and Aging Project (NSHAP) is supported by grants from the National Institutes of Health, including the National Institute on Aging, the Office of Research on Women’s Health, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (5R01 AG021487), and by NORC, which was responsible for the data collection.
We greatly appreciate very helpful comments from Phil Schumm, PhD on the manuscript and data analysis, as well as comments from two anonymous reviewers.
The National Social Life, Health, and Aging Project (NSHAP) is supported by grants from the National Institutes of Health, including the National Institute on Aging, the Office of Research on Women’s Health, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (5R01 AG021487), and by NORC, which was responsible for the data collection.
Footnotes
None of the authors have any potential conflicts of interest with any aspect of the paper.
Author Contribution:
AK and WD designed research; AK and WD conducted data analysis; AK, WD, and SM interpreted data and wrote the manuscript. All authors read and approved the final manuscript.
Disclosures:
All authors have no potential conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sirovich B, Schwartz L, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003;289(11):1414. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 2.Ross L, Coates R, Breen N, Uhler R, Potosky A, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Preventive Medicine. 2004;38(6):732–744. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Finney Rutten L, Meissner H, Breen N, Vernon S, Rimer B. Factors associated with men’s use of prostate-specific antigen screening: evidence from Health Information National Trends Survey. Preventive Medicine. 2005;40(4):461–468. doi: 10.1016/j.ypmed.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhao G, Pollack L, Smith J, Joseph D. Use of the Prostate-Specific Antigen Test Among Men Aged 75 Years or Older in the United States: 2006 Behavioral Risk Factor Surveillance System. Preventing Chronic Disease. 2010;7(4):1–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma. Cancer. 2005;104(3):467–478. doi: 10.1002/cncr.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz D, Jarrard D, McHorney C, Hillis S, Wiebe D, Fryback D. Health perceptions in patients who undergo screening and workup for prostate cancer. Urology. 2007;69(2):215–220. doi: 10.1016/j.urology.2006.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the US Preventive Services Task Force. Annals of Internal Medicine. 2011 doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 8.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. The New England journal of medicine. 2009;360(13):1310. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine. 2009;360(13):1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 10.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. The Lancet Oncology. 2010;11(8):725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter L, Bertenthal D, Lindquist K, Konety B. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 12.Arias E, Rostron B, Tejada-Vera B. United States Life Tables, 2005. National Vital Statistics Reports. 2005;58(10) [PubMed] [Google Scholar]
- 13.Hoffman K, Chen M, Moran B, et al. Prostate cancer-specific mortality and the extent of therapy in healthy elderly men with high-risk prostate cancer. Cancer. 2010;116(11):2590–2595. doi: 10.1002/cncr.24974. [DOI] [PubMed] [Google Scholar]
- 14.Wolf A, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA: A Cancer Journal for Clinicians. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 15.AUA. Prostate-specific antigen (PSA) best practice policy. American Urological Association (AUA) Oncology (Williston Park) 2000;14(2):267–272. [PubMed] [Google Scholar]
- 16.USPSTF. Draft recommendation statement. 2011 http://www.uspreventiveservicestaskforce.org/draftrec3.htm.
- 17.de Groot V, Beckerman H, Lankhorst G, Bouter L, Babaian R. How to measure comorbidity: a critical review of available methods. Journal of Clinical Epidemiology. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 18.Mistry S, Mayer W, Khavari R, Ayala G, Miles B. Who’s too old to screen? Prostate cancer in elderly men. Canadian Urological Association Journal. 2009;3(3):205–210. doi: 10.5489/cuaj.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene K, Albertsen P, Babaian R, et al. Prostate Specific Antigen Best Practice Statement: 2009 Update. The Journal of Urology. 2009;182(5):2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 20.Extermann M, Overcash J, Lyman G, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. Journal of Clinical Oncology. 1998;16(4):1582. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 21.O’Muircheartaigh C, Eckman S, Smith S. Statistical Design and Estimation for the National Social Life, Health, and Aging Project. The Journals of Gerontology: Series B. 2009;64(Supplement 1):i12. doi: 10.1093/geronb/gbp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith S, Jaszczak A, Graber J, et al. Instrument development, study design implementation, and survey conduct for the National Social Life, Health, and Aging Project. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64(suppl 1):i20. doi: 10.1093/geronb/gbn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross L, Taylor Y, Richardson L, Howard D. Patterns in Prostate-Specific Antigen Test Use and Digital Rectal Examinations in the Behavioral Risk Factor Surveillance System, 2002–2006. Journal of the National Medical Association. 2009;101(4):317. doi: 10.1016/s0027-9684(15)30878-6. [DOI] [PubMed] [Google Scholar]
- 24.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA: A Cancer Journal for Clinicians. 2009;59(1):27. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 25.Lewis C, Moore C, Golin C, Griffith J, Tytell-Brenner A, Pignone M. Resident Physicians? Life Expectancy Estimates and Colon Cancer Screening Recommendations in Elderly Patients. Medical Decision Making. 2008;28(2):254–261. doi: 10.1177/0272989X07311756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz J, Chang L, Sangha O, Fossel A, Bates D. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care. 1996;34(1):73. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation* 1. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Idler E, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. Journal of health and social behavior. 1997;38(1):21–37. [PubMed] [Google Scholar]
- 29.Keeler E, Guralnik J, Tian H, Wallace R, Reuben D. The Impact of Functional Status on Life Expectancy in Older Persons. Journal of Gerontology. 2010;65(7):727–733. doi: 10.1093/gerona/glq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montero-Odasso M, Schapira M, Soriano E, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(10):1304. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 31.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(2):142. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 32.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. Journal of the American Geriatrics Society. 2007;55(11):1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 33.Lynch S, Brown J, Harmsen K. The effect of altering adl thresholds on active life expectancy estimates for older persons. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58(3):S171. doi: 10.1093/geronb/58.3.s171. [DOI] [PubMed] [Google Scholar]
- 34.Diehr P, Patrick D, Bild D, Burke G, Williamson J. Predicting Future Years of Healthy Life for Older Adults. Journal of Clinical Epidemiology. 1998;51(4):343–353. doi: 10.1016/s0895-4356(97)00298-9. [DOI] [PubMed] [Google Scholar]
- 35.Walter L, Covinsky K. Cancer screening in elderly patients: a framework for individualized decision making. Jama. 2001;285(21):2750. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 36.Bohannon R. Reference values for the timed up and go test: a descriptive meta-analysis. Journal of Geriatric Physical Therapy. 2006;29(2):64. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Steele C, Miller D, Maylahn C, Uhler R, Baker C. Knowledge, attitudes, and screening practices among older men regarding prostate cancer. American journal of public health. 2000;90(10):1595. doi: 10.2105/ajph.90.10.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilligan T, Wang P, Levin R, Kantoff P, Avorn J. Racial differences in screening for prostate cancer in the elderly. Archives of Internal Medicine. 2004;164(17):1858. doi: 10.1001/archinte.164.17.1858. [DOI] [PubMed] [Google Scholar]
- 39.Lin K, Lipsitz R, Miller T, Janakiraman S. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the US Preventive Services Task Force. Annals of Internal Medicine. 2008;149(3):192. doi: 10.7326/0003-4819-149-3-200808050-00009. [DOI] [PubMed] [Google Scholar]
- 40.Manzoli L, Villari P. Marital status and mortality in the elderly: a systematic review and meta-analysis. Social Science & Medicine. 2007;64(1):77–94. doi: 10.1016/j.socscimed.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 41.Qato D, Alexander G, Conti R, Johnson M, Schumm P, Lindau S. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. Jama. 2008;300(24):2867. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daskivich T, Sadetsky N, Kaplan S, Greenfield S, Litwin M. Severity of Comorbidity and Non-Prostate Cancer Mortality in Men With Early-Stage Prostate Cancer. Archives of Internal Medicine. 2010;170(15):1396. doi: 10.1001/archinternmed.2010.251. [DOI] [PubMed] [Google Scholar]
- 43.Drum M, Shiovitz-Ezra S, Gaumer E, Lindau S. Assessment of Smoking Behaviors and Alcohol Use in the National Social Life, Health, and Aging Project. The Journals of Gerontology: Series B. 2009;64(Supplement 1):i119. doi: 10.1093/geronb/gbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coups E, Gaba A, Orleans C. Physician screening for multiple behavioral health risk factors. American journal of preventive medicine. 2004;27(2):34–41. doi: 10.1016/j.amepre.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Bynum J, Song Y, Fisher E. Variation in Prostate-Specific Antigen Screening in Men Aged 80 and Older in Fee-for-Service Medicare. Journal of the American Geriatrics Society. 2010;58(4):674–680. doi: 10.1111/j.1532-5415.2010.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han P, Coates R, Uhler R, Breen N. Decision Making in Prostate-Specific Antigen Screening:: National Health Interview Survey, 2000. American journal of preventive medicine. 2006;30(5):394–404. doi: 10.1016/j.amepre.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman RM, Couper MP, Zikmund-Fisher BJ, et al. Prostate cancer screening decisions: results from the National Survey of Medical Decisions (DECISIONS study) Archives of Internal Medicine. 2009;169(17):1611. doi: 10.1001/archinternmed.2009.262. [DOI] [PubMed] [Google Scholar]
- 48.Hudson S, Ohman-Strickland P, Ferrante J, Lu-Yao G, Orzano A, Crabtree B. Prostate- specific antigen testing among the elderly in community-based family medicine practices. The Journal of the American Board of Family Medicine. 2009;22(3):257. doi: 10.3122/jabfm.2009.03.080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson J, Clarke M, Ewings P, Graham J, MacDonagh R. The assessment of patient life-expectancy: how accurate are urologists and oncologists? BJU international. 2005;95(6):794–798. doi: 10.1111/j.1464-410X.2005.05403.x. [DOI] [PubMed] [Google Scholar]
- 50.Chan E, Vernon S, Ahn C, Greisinger A. Do men know that they have had a prostate-specific antigen test? Accuracy of self-reports of testing at 2 sites. American journal of public health. 2004;94(8):1336. doi: 10.2105/ajph.94.8.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.González H, West B, Underwood W., III PSA Testing in Office-Based Clinics: Are We Testing As Much As We Think? Journal of the American College of Surgeons. 2005;201(6):906–912. doi: 10.1016/j.jamcollsurg.2005.07.012. [DOI] [PubMed] [Google Scholar]