Abstract
Acheron (Achn) is a new member of the Lupus Antigen family of RNA binding proteins. Previous studies have shown that Achn controls developmental decisions in neurons and muscle. In the human mammary gland, Achn expression is restricted to ductal myoepithelial cells. Microarray analysis and immunohistochemistry have shown that Achn expression is elevated in some basal-like ductal carcinomas. To study the possible role of Achn in breast cancer, we engineered human MDA-MB-231 cells to stably express enhanced green fluorescent protein-tagged wild-type Achn (AchnWT), as well as Achn lacking either its nuclear localization signal (AchnNLS) or its nuclear export signal (AchnNES). In in vitro assays, AchnWT and AchnNES, but not AchnNLS, enhanced cell proliferation, lamellipodia formation, and invasive activity and drove expression of the elevated expression of the metastasis-associated proteins MMP-9 and VEGF. To determine if Achn could alter the behavior of human breast cancer cells in vivo, Achn-engineered MDA-MB-231 cells were injected into athymic SCID/Beige mice. AchnWT and AchnNES-expressing tumors displayed enhanced angiogenesis and an approximately five-fold increase in tumor size relative to either control cells or those expressing AchnNLS. These data suggest that Achn enhances human breast tumor growth and vascularization, and that this activity is dependent on nuclear localization.
Keywords: invasion, metastasis, VEGF, MDA-MB-231 cells, xenograft, MMP9
INTRODUCTION
Breast cancer is the leading cause of cancer among women in the United States. Although early diagnosis and treatment have significantly improved long-term survival, patients with metastatic disease have not realized the same benefits. Improvements in both clinical diagnosis and treatment will require a better understanding of the molecular mechanisms responsible for tumor progression.
While many processes influence the ability of solid tumors to mediate pathology, the two main factors are metastasis and angiogenesis1–4. During metastasis, tumor cells must acquire a number of new capabilities that allow them to leave the primary tumor, travel to ectopic locations, and seed the formation of secondary colonies. In order for tumor cells to move through solid tissues they must first degrade extracellular matrix (ECM) proteins with a class of gelatinase family enzymes known as matrix metalloproteinases (MMPs)5–7. In this regard, the induction of MMPs such as MMP-2, MMP-3 and/or MMP-9, is directly correlated with the incidence of tumor metastasis8–10.
MMPs are usually secreted as inactive pro-enzymes that require the proteolytic removal of the N-terminus for activation. MMP-2 and MMP-9 are implicated in the invasiveness of numerous human cancers since they can degrade the ECM and activate a wide range of molecules including growth factors and cytokines11, 12. Release of these factors in turn induces tumor cell proliferation, adhesion, migration, angiogenesis, and host defense evasion13, 14. Recent data has demonstrated that MMP-9 can exert a pro-angiogenic function directly via vascular endothelial growth factor (VEGF) during tumor progression2, 15, 16.
Angiogenesis is another essential step in tumor survival and metastasis. Angiogenic factors, derived predominantly from tumor cells and infiltrating inflammatory cells, include: fibroblast growth factor-2 (FGF-2 or bFGF), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), angiopoietins and ephrins, with VEGF emerging as one of the most potent17–19. Indeed, the groundbreaking work of Folkman has led to the development of anti-angiogenic therapies that have the potential to effectively suppress tumor growth and attenuate metastasis20–23.
The Acheron (Achn) gene (also known as La Related Protein 6; LARP6) was recently discovered in our laboratory in a molecular screen for genes that are induced when insect skeletal muscles become committed to die at the end of metamorphosis24. Molecular and phylogenetic analysis has demonstrated that Achn is highly conserved throughout evolution and defines a new sub-family of Lupus Antigen (La) proteins. Human Achn encodes a 55 KDa protein that contains three highly conserved N-terminal La motifs, an imperfect RNA binding domain, and putative nuclear localization (NLS) and nuclear export (NES) signals. Achn binds to the CASK/Lin-2 signal transduction protein, which can regulate membrane signaling and gene expression25, 26. It also binds to stem loop sequences in the 5’ region of type I collagen mRNA and co-localizes these transcripts to non-muscle myosin, where it may facilitate translation27, 28.
Achn is predominantly expressed in neurons, striated skeletal muscle and cardiac muscle24. It plays essential roles in differentiation of several lineages, including neurons and muscles29. Blockade of endogenous Achn in zebrafish embryos with antisense morpholinos greatly reduces the formation of forebrain neurons and differentiated striated skeletal muscles. In vitro analysis in the mouse myoblast cell line C2C12 has shown that Achn is required for cells to both differentiate into striated skeletal muscles and initiate apoptosis following growth factor removal. As well, Achn has been shown to regulate myoblast migration and adhesion30, properties that are essential for both normal development and pathogenesis.
In the present study we extended these analyses to determine if Achn might play a role in the behavior of human breast cancer, since preliminary data (presented below) demonstrates that Achn is highly expressed in some basal-like ductal carcinomas in human. Expression of ectopic Achn in human MDA-MB-231 breast cancer cells enhances proliferation, lamellipodia formation, invasion and the expression of both MMP-9 and VEGF in vitro, and tumor angiogenesis and growth in an in vivo mouse xenograft model. The ability of Achn to mediate these behaviors appears to be dependent upon its ability to enter the nucleus, since loss of the NLS abrogates all of these effects. Taken together, these data support the hypothesis that Achn may play a role in the development and aggressive behavior of some human breast cancers.
MATERIAL AND METHODS
Immunohistochemistry and quantification
For IHC staining of Achn in human cancer samples, anonymous cancer samples were obtained from the tissue archives at the Department of Pathology, Baystate Medical Center under the auspices of an approved Internal Review Board protocol. Formalin fixed, paraffin-embedded human breast tissues with invasive carcinoma were processed for immunohistochemical (IHC) using our rabbit anti-human Achn antiserum (1:100) as described below. A detailed description of the anti-Achn antibody is presented elsewhere (Schwartz et al., in preparation).
Mouse tumor samples were processed for both paraffin-embedding and frozen sectioning, and 10 µm sections were randomly selected for IHC staining using antisera against VEGF, CD31, cytokeratins CK1/5/10/14, smooth muscle actin (SMA), and estrogen receptor alpha (ERα). All procedures were performed at room temperature. In brief, the samples were incubated in 3% H2O2 for 30 min to block endogenous peroxidase activity followed by incubation with blocking buffer (Vector Laboratories, Burlingame, CA) containing 10% goat serum for 1 hr. Slides were incubated with anti-VEGF polyclonal antibody (1:50, Santa Cruz), anti-CD31 monoclonal antibody (1:400, BD Biosciences, San Jose, CA), CK1/5/10/14 polyclonal (1:500, Dako, Carpinteria, CA), SMA monoclonal (1:500, Sigma), or ERα (1:200, Santa Cruz) for 2 hr and goat anti-rabbit/or rat secondary antibody (1:100) conjugated with HRP was added for 30 min. Finally, DAB substrate (Dako) was introduced for several minutes, and after washing, methyl green was used for counterstaining. For quantification of VEGF expression, the staining intensity was evaluated: no staining = 0 points, weak staining = 1 point, moderate staining = 2 points, and strong staining = 3 points. A final average value for each tumor sample was used for statistical analysis.
To quantify blood vessel density, we analyzed an average of CD31 density from six to eight fields in each section using the NIH Image J analysis program. Data are expressed as mean ± SE and “n” = the numbers of individual experiments performed. Differences among groups were determined using one-way analysis of variance followed by the Newman-Keuls procedures. The 0.05 level of probability was used as the criterion of significance.
Achn labeled with different tag proteins and Achn mutants
The human Achn coding sequence was subcloned into the pCMV-neo retroviral vector to create pCMV-Achn. The same coding region was subcloned into pCMV-GFP to create a C-terminal enhanced green fluorescent protein (eGFP) epitope tag (Achn-eGFP). In separate constructs, N-terminal HA or C-terminal 6X-His epitope tags were generated via PCR to create HA-Achn and Achn-His, respectively. PCR-based mutagenesis was used to delete the NLS (amino acids 271–305) or NES (amino acids 186–218) to generate AchnNLS and AchnNES, respectively.
Cell lines
Fugene 6 (Roche, Indianapolis, IN) was used to transfect both the pCL 10A1 vector and one of the pCMV-neo Achn-expression constructs into 293T retroviral packaging cells. Forty-eight hours after transfection, the supernatants were harvested, filtered through 0.45-µm pore size filters and the virus-containing medium used to infect human MDA-MB-231 and MDA-MB-435 breast cancer cells and non-tumorigenic human HBL-100 breast epithelial cells. Infected cells were selected with 800 µg/ml of G418 starting 48 hr after infection and the drug-resistant cell populations were used for subsequent studies. Cells were cultured with 5% CO2 at 37°C in Dulbecco’s modified Eagle medium (DMEM) containing 10% Fetal Bovine Serum (FBS) and penicillin/streptomycin. Ectopic Achn-eGFP and the various targeting mutants were observed via fluorescence microscopy on a Nikon ECLIPSE TE2000-U.
Cell Proliferation Assays
Cells were grown sub-confluently in 6-well plates and then the culture medium was replaced with serum-free medium in the presence of 1 µCi of 3H-thymidine. The cells were incubated for 6 hrs and then washed extensively in PBS. The cells were scraped off the plate and their nucleotides were precipitated in 200 µl of 10% trichloroacetic acid and then dissolved in 0.3 ml of 0.3 M NaOH. Radioactivity was quantified by liquid scintillation counting.
Lamellipodia Assay/Membrane Ruffling Assay
Cells were treated with 0.25% trypsin, rapidly re-plated in 6-well plates and then cultured for approximately 75 minutes at 37°C in DMEM containing 10% FBS. All subsequent steps were performed at room temperature. The cells were fixed in 4% paraformaldehyde for 10 minutes, washed in 0.1% Triton-X100 in PBS for an additional 5 minutes, blocked in 1% of BSA in PBS for 30 minutes and then stained with 0.165 µM rhodamine-phalloidin (Invitrogen) for 20 minutes and visualized via fluorescence microscopy. Membrane ruffling was scored in a manner consistent with previous work31. Briefly, individual cells were scored on a scale of 0–2, with: no ruffling = 0; ruffling confined to one area of the cell = 1; and ruffling in two or more discrete areas = 2. The ruffling index was recorded as the sum of ruffling scores of 100 cells.
Cell Invasion Assays
Cells (2 × 105) were pre-incubated with serum-free medium for 24 hr and transferred onto transwells (24-well plates) pre-loaded with 50 µl of Matrigel (1 mg/ml) (Beckton Dickinson Labware, Bedford, MA). After 18-hr incubation the gel, including non-migrating cells, was removed and cells invading into the membrane were fixed and stained with hematoxylin.
MMP-9 promoter construct, transient transfection and luciferase activity
A fragment of human genomic DNA containing the first −710 bp upstream of the start of transcription for the MMP-9 gene was amplified via PCR and then subcloned into pCAT3-promoter vector containing a luciferase reporter gene (Promega, Madison,WI). The resulting pCAT3-promoter plasmid was introduced into cells using lipofectamine (Invitrogen) according to the manufacture’s instruction. Following 48 hr incubation, the cells were incubated in lysis buffer provided with the Luciferase Report System kit (Promega). The lysates were centrifuged and the resulting supernatant was tested for luciferase activity.
Western Blot Analysis
Cells were collected by scraping and extracted in a lysis buffer (pH 7.4) containing 0.25 mM HEPES, 14.9 mM NaCl, 10 mM NaF, 2 mM MgCl2, 0.5% NP-40, 0.1 mM PMSF, 20 µM pepstatin A and 20 µM leupeptin. The lysates were centrifuged at 10,000g for 10 min at 4°C and the resulting supernatant collected for 10% SDS-PAGE. Proteins were transferred to a nylon membrane (Invitrogen, Carlsbad, CA) and incubated with one of several antisera: rabbit anti-Achn (1:100; Schwartz laboratory), rabbit anti-GFP (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-MMP-9 (1:500), (VWR, West Chester, PA), VEGF (1:200) (Sigma, St. Loius, MO), and actin (1:1000) (Sigma). HRP-labeled goat anti-rabbit or mouse antisera (1:10,000) and an ECL kit (VWR) were used for detection.
Xenograft tumor models
Four week old female SCID/Beige mice (Charles River, Wilmington, MA) were injected subcutaneously with Achn-engineered MDA-MB-231 cells (2 × 106) in 0.2 ml of Hank’s balanced buffer without calcium or magnesium. The growth of solid tumors from the injected cells was monitored daily for up to 7 weeks before the animals were sacrificed to remove tumors for analysis. The tumors’ volumes were calculated based on the following equation: volume = length × width2 × 0.52.
RESULTS
Differential Expression of Acheron in Some Human Breast Cancers
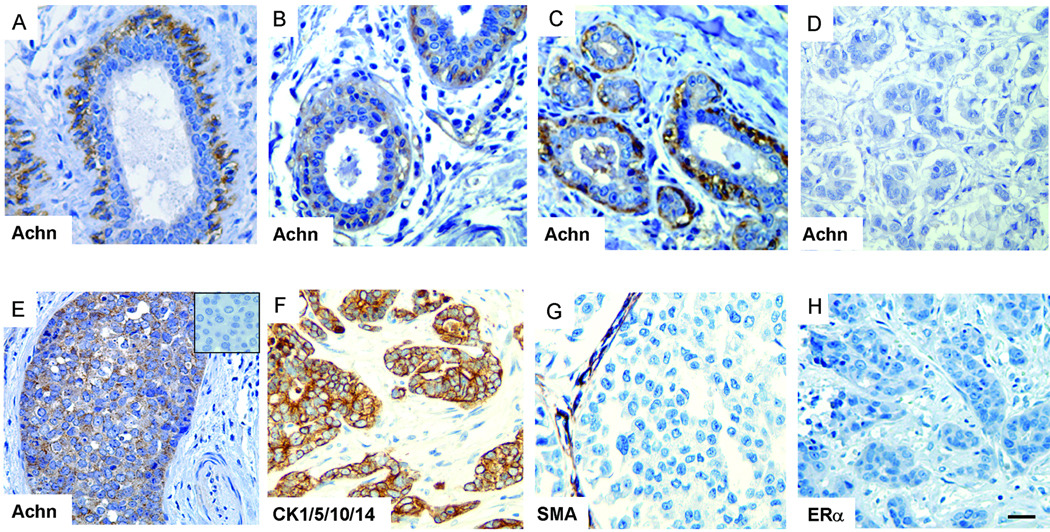
As part of our survey of the tissue distribution of Acheron expression in normal human tissues, we examined the mammary gland and observed strong expression in the myoepithelial cells surrounding ductal epithelium (Figure 1A). No signal was detected in other cell types within the tissue. To extend this analysis to determine if Achn is differentially expressed in breast cancer, we performed immunohistochemical analysis of ductal carcinoma in situ (DCIS) and infiltrating ductal carcinomas (IDC). The normal mammary tissue adjacent to tumors displayed strong Achn staining in the myoepithelial cells (Figure 1B). In two of the five cases of DCIS, Achn was highly expressed in basal layer of the ducts (Figure 1C), moderately expressed in two others (data not shown) and undetectable in the fifth (data not shown). In the 21 cases of IDCs examined, Achn staining was normal in 15 individuals (Figure 1D). However, in 4 of the 21 cases, Achn expression was highly and uniformly expressed throughout the diseased tissue (Figure 1E). Moderate expression was detected in the remaining two cases (data not shown). To facilitate diagnosis in these Achn-positive mammary tumors, we stained tissues for the expression of diagnostic markers. These tumors were positive for cytokeritins 1/5/10/14 (Figure 1F) and negative for smooth muscle actin or the estrogen receptor alpha subtype (Figure 1G & H). Taken together, these data suggest that Achn is elevated in some breast cancers that display markers that are basal-like in nature.
Figure 1. Achn staining of human ductal carcinoma.
A. Immunohistochemical analysis with an anti-Achn antibody demonstrated that Achn expression in the mammary gland is essentially restricted to the myoepithelial cells surrounding ductal epithelium. B. IHC staining of normal ducts adjacent to ductal carcinoma revealed high levels of Achn expression. C. DCIS also demonstrated strong expression of Achn in the basal layer of the ducts. D. A representative of Achn-negative IDC samples was shown. E. A representative Achn-positive IDCs expressed a high level of Achn. A negative control in the absence of anti-Achn antibody in IHC was shown in the insert. F–H. A representative staining of ductal carcinoma with anti-CK1/5/10/14, SMA, and ERα. Scale bar = approximately 250 µm.
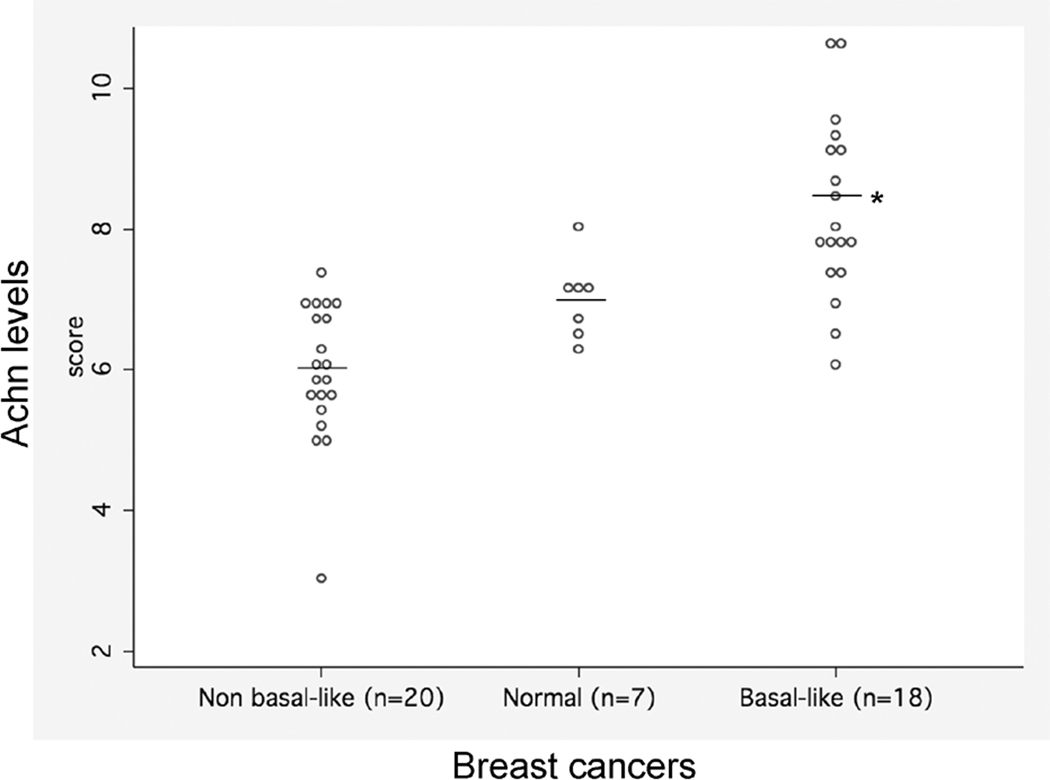
As an independent test for the differential expression of Achn in breast cancer, we queried the GEO (Gene Expression Omnibus) database (http://www.ncbi.nlm.nih.gov/geo/) for microarray data sets that included Achn probes. We analyzed a study of a data set that included samples from 7 normal glands, 20 non-basal like tumors and 18 basal-like breast tumors32. There were group differences in the expression of Achn such that non-basal breast cancer < normal tissue < basal-like (Figure 2). Overall, these group differences were statistically significant (Kruskal-Wallis test on 2 degrees of freedom, p = .0001), as were all pairwise comparisons of groups (Rank Sum test p-value ≤ .01 in all three instances). These data agree with the immunohistochemical analyses suggesting that elevated levels of endogenous Achn expression are associated with the more aggressive basal-like breast cancers.
Figure 2. Comparison of mRNA levels from microarray analysis of non-basal like vs basal-like breast cancer tissues and normal controls.
Dot plots of individual scores reveal a trend in values: non-basal like < normal < basal-like (medians: 5.9, 7.1 and 7.9, respectively. Kruskal Wallis p-value = .0001, Test of trend p-value < .0001). Data from32.
Subcellular targeting sequences in Achn
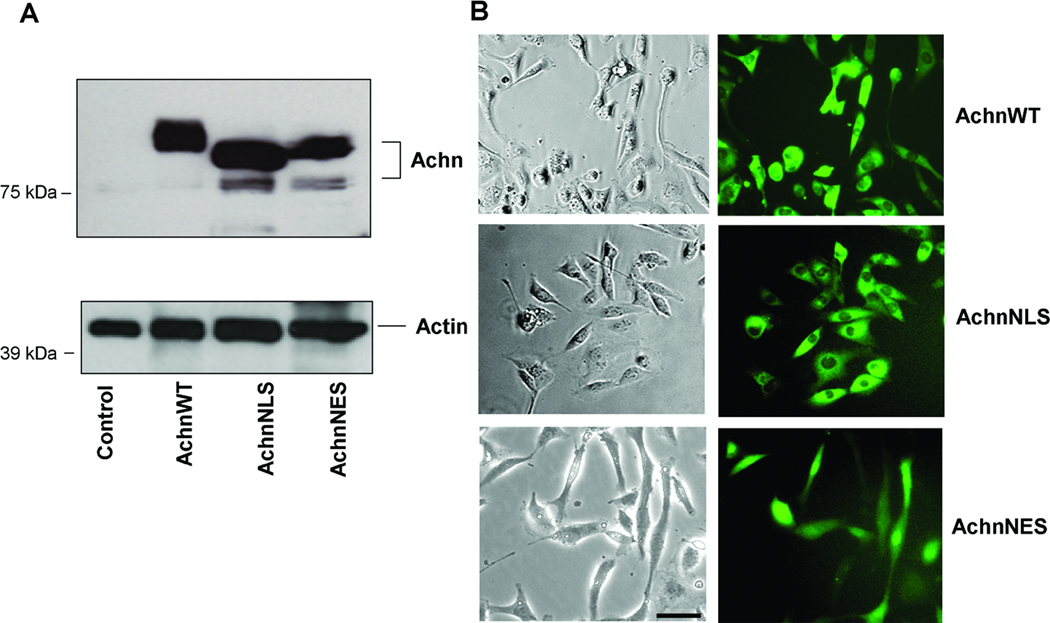
Given that Achn is differentially expressed in some mammary tumors, we wanted to determine if over-expression of Achn might regulate physiological processes associated with malignancy. We generated several retroviral constructs encoding Achn with and without a variety of epitope tags, including: C-terminal enhanced Green Fluorescent Protein (eGFP), C-terminal 6xHis, and N-terminal HA tag. These constructs were then used to infect human MDA-MB-231 breast cancer cells since this well characterized line would allow us to monitor both the enhancement and repression of metastasis-associated behavior33–36. MDA-MB-231 cells do not express detectable levels of endogenous Achn protein (Figure 3A). Infection with the empty vector served as a negative control for each experiment and these cells were likewise negative for Achn (Figure 3A). The effects of ectopic Achn described below were identical with or without epitope tags, suggesting that these modifications did not interfere with Achn function (data not shown).
Figure 3. Expression of ectopic Achn-GFP fusion proteins in MDA-MB-231 cells.
A. eGFP control and Achn-eGFP fusion DNA constructs were introduced into MDA-MB-231 cells by retroviral infection and ectopic Achn-eGFP fusion proteins from cell lysates were examined by immunoblotting using anti-Achn and anti-GFP antisera. B. MDA-MB-231 cells were infected with retroviral constructs encoding Achn constructs with C-terminal eGFP tags. The three constructs examined in this experiment were Achn wild-type (AchnWT), AchnNLS (Achn lacking the nuclear localization signal) and AchnNES (Acheron lacking the nuclear export signal). Live cells were examined by fluorescence microscopy to determine the subcellular localization of ectopic Achn. Bar = 20 µm.
Achn has putative nuclear localization (NLS) and nuclear export (NES) signals24. To determine if these sequences function to target Achn to the nucleus and cytoplasm respectively, we generated an additional series of C-terminal eGFP-tagged Achn retroviral constructs that carried deletions of the NLS (AchnNLS) and NES (AchnNES) (Figure 3A) (Achn has multiple putative phosphorylation sites24, which may account for the doublet of Achn protein observed with some constructs on Western blots). These constructs were introduced into MDA-MB-231 cells via retroviral infection and the subcellular localization of eGFP was examined. In agreement with immunohistochemical analysis of most human tissues, AchnWT localizes predominantly to the cytoplasm, although modest signal was also observed within the nucleus (Figure 3B). The nuclear eGFP signal was lost in cells expressing AchnNLS, while deletion of the NES sequence facilitated dramatic nuclear accumulation of Achn at the expense of cytoplasmic staining. MDA-MB-435 cells displayed the same cellular localization of Achn as MDA-MB-231 cells when transduced with different versions of Achn (Supplemental Figure 1). Taken together, these data support the hypothesis that Achn normally enters and exits the nucleus and depends on the NES and NLS signals for this translocation.
Effects of Achn on the invasive behavior of human breast cancer cells in vitro
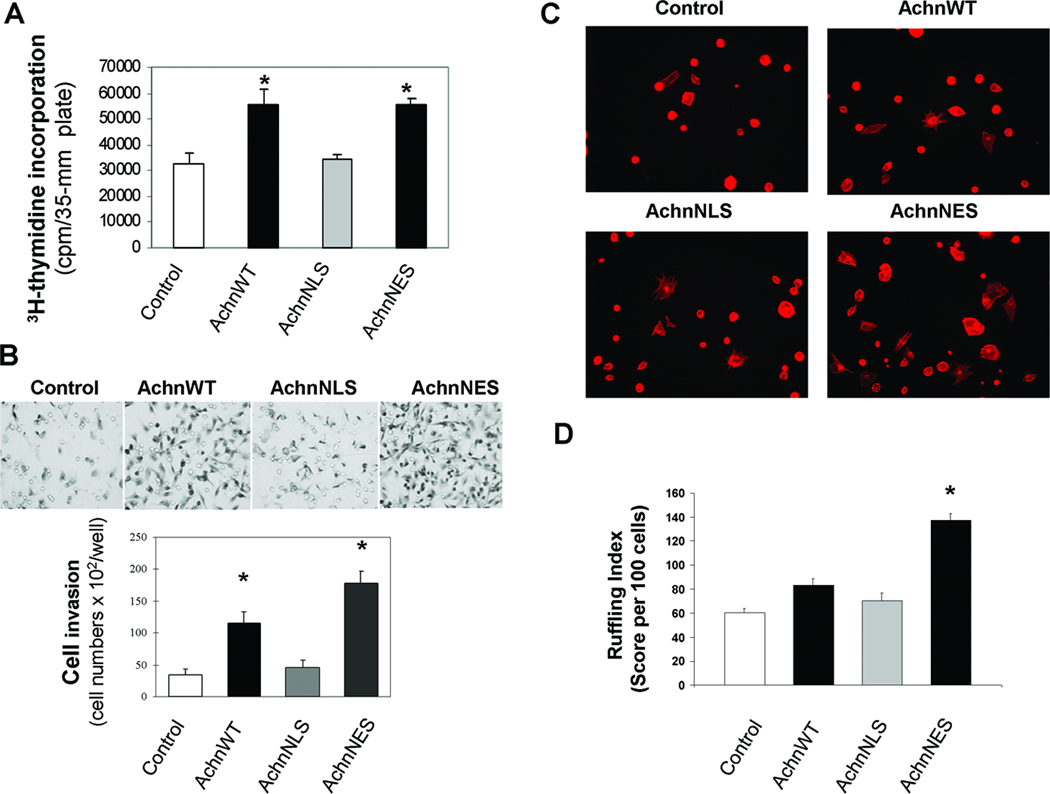
To determine if Achn could influence the behavior of human breast cancer cells in vitro, we measured a number of physiological activities in MDA-MB-231 cells expressing AchnWT, AchnNLS and AchnNES (Figure 4A). Expression of ectopic AchnWT led to a 1.7 fold increase in cell proliferation relative to controls. This effect was dependent on the subcellular compartment in which the ectopic Achn protein accumulated, since removal of the NLS but not the NES abrogated this effect. These data support the hypothesis that nuclear Achn can enhance cell division in human breast cancer cells.
Figure 4. Nuclear targeting of Achn is required for enhanced cell proliferation, invasion, and lamellipodia formation in vitro.
A. MDA-MB-231 cells expressing ectopic Achn-GFP fusion proteins were grown to approximately 80% confluency and then 3H-thymidine (1 µCi) was added for 6 hr in the serum-free medium. Subsequent DNA labeling was quantified (Mean ± SE, n=6). * = P<0.05 compared with control. B. MDA-MB-231 cells expressing ectopic Achn-GFP fusion proteins were deposited onto Matrigel in transwells to examine cell invasion. Cells were examined for invasion after 18 hours (top) and then quantified (bottom). (Mean ± SE, n=4). * = P<0.05 compared with control. C. MDA-MB-435 cell lines were processed to label lamellipodia and then viewed via fluorescence microscopy at 20X. D. Individual cells were scored according to the degree of ruffling (Mean ± SE, n=5) *=P<0.001.
We next sought to determine if Achn could influence the invasiveness of these cells. Achn-engineered MDA-MB-231 cells were evaluated for their ability to enter a layer of Matrigel in a standard invasion assay. Expression of ectopic Achn resulted in a 2.7-fold increase in invasion through the Matrigel during the 18 hr test period (Figure 4B). As with proliferation, this effect required nuclear targeting of the ectopic Achn, as removal of the NLS negated this enhancement. Removal of the NES, which traps Achn in the nucleus, was more effective in driving invasion than the AchnWT construct, although the reason for this enhancement is unknown (Figure 4B).
To determine if these effects were restricted to MDA-MB-231 cells, we repeated this experiment with Achn-infected MDA-MB-435, another invasive basal-like breast cancer line. In agreement with our data using MDA-MB-231 cells, invasion was facilitated by ectopic AchnWT and AchnNES expression, but not by infection with vectors encoding GFP alone or GFP-tagged AchnNLS (data not shown). These MDA-MB-435 cells were also used in a lamellipodia assay to further evaluate the aggressive behavior associated with expression of ectopic Achn. AchnNES expressing cells displayed a 5-fold increase in the number of cells with lamellipodia compared to the other cell types (Figure 4C). When lamellipodia formation was quantified, AchnNES was then shown to have twice the value observed in the control, AchnWT and AchnNLS cells (Figure 4D). These data suggest Achn alters the behavior of both MDA-MB-231 and MDA-MB-435 cells, and that these effects are dependent on nuclear localization.
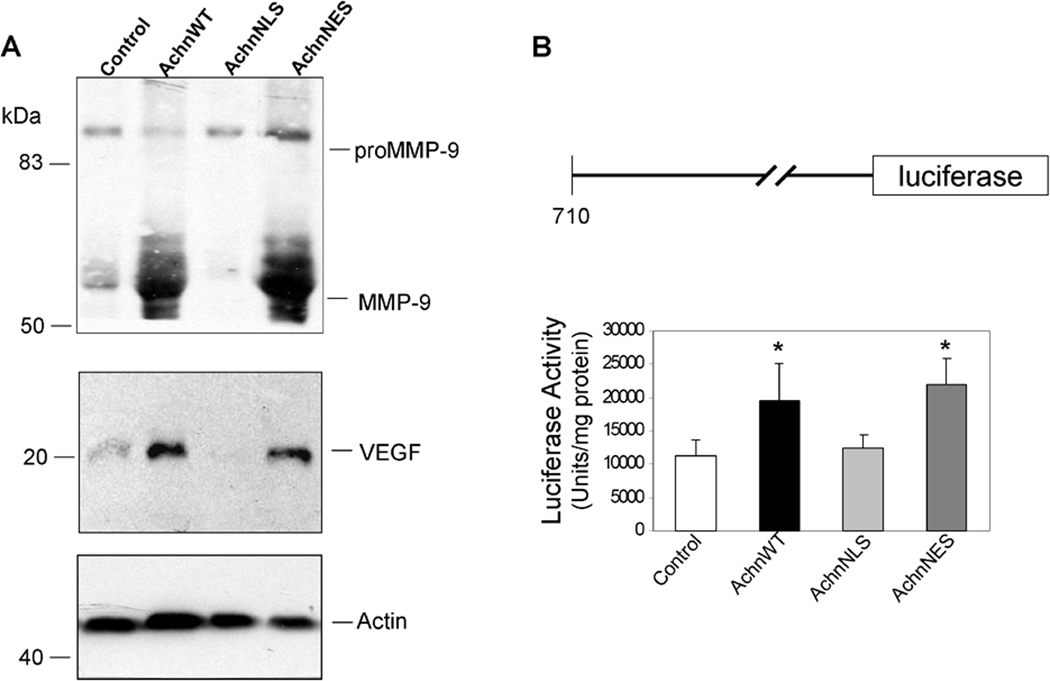
One of the key mediators of invasion for cancer cells is expression of MMP-937–39. To determine if ectopic Achn modulates MMP-9 expression, we collected conditioned media from Achn-engineered MDA-MB-231 cells and performed Western blot analysis (Figure 5A). Expression of ectopic AchnWT was a potent inducer of MMP-9 expression. This MMP-9 was presumably an active form of the protein because it was detected as the predominant signal below the proMMP-9. As with the other effects mediated by ectopic Achn, the increase in MMP-9 expression was dependent upon the ability of the protein to localize to the nucleus, since loss of the NLS blocked this effect.
Figure 5. Nuclear Achn enhances MMP-9 and VEGF expression and induces luciferase activity of MMP-9 promoter.
A. MDA-MB-231 cells expressing ectopic Achn-GFP fusion proteins were incubated in serum-free medium for 24 hr and then the conditioned medium was collected to evaluate expression levels of MMP-9 and VEGF, where cell lysates were subjected for actin expression by immunoblotting. Arrow indicates proteolytically processed MMP-9. B. The first 710 nucleotides 5’ to the translation initiation site of human MMP-9 were introduced upstream of a luciferase reporter gene and the resulting plasmid was transfected into MDA-MB-231 cells ectopically expressing different versions of Achn. After 48 hr, cells were lysed and luciferase activity measured and normalized to the levels of lysate protein. (Mean ± SE, n=3). * = P<0.05. Vector control activities in any group were below 2000 units/mg protein (data not shown).
Unlike the MDA-MB-231 cells, which normally express low levels of MMP-9 (Figure 5), the non-tumorigenic HBL-100 human mammary epithelial cells do not express detectable MMP-9 protein40. To determine if the expression of ectopic Achn can induce MMP-9 expression de novo, we infected HBL-100 cells with the Achn retroviral construct and measured MMP-9 expression by Western blot analysis. No MMP-9 expression could be detected in either the vector control or the Achn-engineered cells (data not shown).
Increased MMP-9 expression could result in the enhanced production and/or activity of VEGF, a potent angiogenic factor15, 41. We therefore examined the expression of VEGF in Achn engineered MDA-MB-231 cells. Western blot analysis demonstrated that VEGF expression was significantly elevated in AchnWT and AchnNES expressing cells, but not in those with AchnNLS (Figure 5A).
To determine if the effects of ectopic Achn to increase the accumulation of MMP-9 protein might occur at the transcriptional level, Achn-engineered cells were transiently transfected with an MMP-9 promoter/luciferase reporter construct (Figure 5B). Ectopic AchnWT was able to induce a two-fold increase in reporter activity relative to the empty vector control (Figure 5B). As with all the other physiological parameters that we have found to be influenced by Achn, enhanced MMP-9 reporter activity was dependent on the ability of Achn to enter the nucleus.
Achn enhances tumor growth and angiogenesis of human breast cancers in a mouse xenograft model
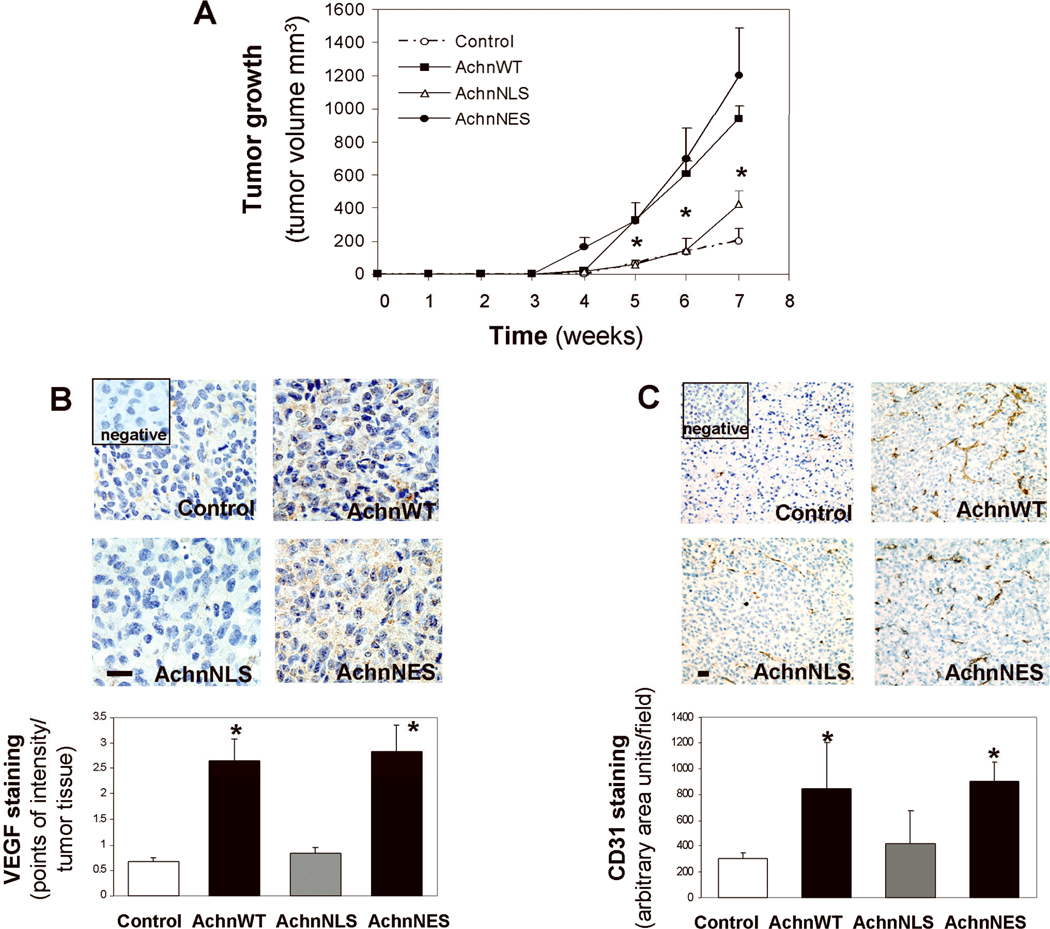
All of these in vitro analyses support the hypothesis that Achn can facilitate cellular responses associated with enhanced tumor growth. To extend this study in vivo, we introduced Achn-engineered MDA-MB-231 cells subcutaneously into 4-week old female SCID/Beige nude mice and measured the gross size of the tumors on a weekly basis (Figure 6A). No palpable tumors were discernable until week 4 after injection. However, by the fifth week, the AchnWT and AchnNES expressing tumors were substantially larger than those produced by cells expressing either the empty vector or AchnNLS. By seven weeks this difference was quite dramatic and the experiment was terminated and the tumors were excised for histological examination. The volume of the AchnWT and AchnNES tumors were approximately five times greater than those isolated from the other experimental groups (Figure 6A).
Figure 6. Nuclear Achn exacerbates tumor growth in vivo.
MDA-MB-231 cells expressing ectopic Achn-GFP fusion proteins were injected into immunodeficient SCID mice as described in the Methods. Each group contained six mice. A.. Tumor sizes were measured weekly for 7 weeks and growth curves plotted. (Mean ± SE, n=6). * = P<0.05 compared with the control group at the same time point. B. Paraffin-embedded tumor sections were processed for VEGF staining with IHC in which brown staining indicated positive signal. The images represented one of six tumor samples in each group and the data were analyzed using intensity quantification as described in the Methods. C. Frozen tumor samples were analyzed for vessel density using IHC staining of CD31. A vessel area with positive CD31 staining in 6–8 fields of each section was calculated using the NIH Image J analysis program. Mean ± SE, n=5–6. *=P<0.05 compared with control or AchnNLS. Bars=10 µm.
In order to determine if this Achn-dependent enhancement of tumor growth is correlated with the increased expression of VEGF as seen in vitro (Figure 5A), we performed VEGF immunohistochemical staining of the tumors (Figure 6B). In agreement with in vitro studies above,, we observed that VEGF expression in AchnWT and AchnNES tumors was approximately 5-fold greater than the expression observed in the control and AchnNLS tumors. Based on the increased expression of VEGF, and the enhanced size of the tumors derived from cells expressing AchnWT or AchnNES, we predicted that these tumors would contain a greater level of microvascularization. To test this hypothesis, we stained tumor sections with an antiserum against CD31, an angiogenic marker (Figure 6C). As predicted, expression of either AchnWT or AchnNES, but not AchnNLS resulted in a dramatic enhancement of angiogenesis.
DISCUSSION
Acheron is a newly discovered protein that defines a new subfamily of La proteins24. Initial knock-down experiments in zebrafish embryos suggest that Achn plays a critical role in early developmental decisions in several lineages, including neurons and muscles29. Complementary studies in C2C12 myoblasts have demonstrated that Achn serves a regulatory role in the control of both cell death and differentiation, since loss of Achn expression (antisense or siRNA) or function (dominant-negative Achn) blocks both myotube formation and apoptosis following the loss of growth factors. Achn also regulates integrin expression in C2C12 myoblasts and alters the ability of cells to both adhere to and migrate on extracellular matrix proteins30.
As part of our analysis of Achn expression in mouse and human tissues, we observed that Achn was specifically expressed within the myoepithelial cells of the mammary gland (Figure 1A). No expression was detected in other cell types within the tissue. Given the ability of Achn to alter developmental decisions and physiological responses prompted us to ask if Achn might also play a role in human pathogenesis. An in silico analysis of human breast cancers suggested that Achn is upregulated in some basal-like carcinomas of the breast (see Results). In agreement with these data, preliminary immunohistochemical analysis of human breast cancers demonstrated that Achn is expressed at much higher levels within the carcinoma than in the adjacent non-diseased mammary epithelium (Figure 1B). A preliminary microarray analysis in mammary tumors suggests that Achn expression is significantly lower in non-basal tumors relative to normal controls and significantly elevated in the basal-like tumors (Figure 2). Basal-like breast cancers are more aggress than the non-basal tumors and have a poorer prognosis42. While these results are preliminary, they do suggest that Achn expression may be positively correlated with disease severity, and a larger epidemiological study may be warranted.
Expression of ectopic Achn in human MDA-MB-231 breast cancer cells was sufficient to drive a number of physiological responses associated with elevated aggressive behavior both in vitro and in an in vivo xenograft model, including: proliferation (Figure 4A), invasion (Figure 4B), lamellipodia formation (Figures 4C and D), MMP-9 and VEGF expression (Figure 5A), tumor growth and angiogenesis (Figure 6). All of these responses are inter-related and all would be expected to contribute to the observed Achn-driven growth of tumors in vivo.
MMP-9 functions as a protease to degrade extracellular matrix proteins, most notably the basement membrane protein type IV collagen43. Degradation of type IV collagen facilitates tumor invasiveness for a variety of carcinomas9, 44, 45. Transgenic animals that drive expression of ectopic MMP-9 in the mammary gland display a significant increase in both primary mammary tumors and ectopic tumors that developed in the skin, supporting the hypothesis that MMP-9 enhances tumor growth and metastasis36, 46. In agreement with this hypothesis, ectopic expression of MMP-9 in non-metastatic rat embryo cells conferred a metastatic phenotype in vitro43. In contrast, blockade of either MMP-9 expression or pro-MMP-9 processing inhibited the invasive behavior in vitro and cancer growth and metastasis in vivo45, 47. Several studies have shown that MMP-9 promotes the activation and/or release of VEGF in vivo, which in turn augments tumor angiogenesis and expansion15, 41.
Ectopic expression of Achn resulted in enhanced expression of pro-MMP-9 and the subsequent accumulation of high levels of processed MMP-9 in MDA-MB-231 cells (Figure 5A). Interestingly, this was not observed with the HBL-100 cells, a non-transformed human breast epithelial cell line (data not shown) suggesting that Achn does not induce the de novo expression of MMP-9, but instead may act to enhance its basal level of expression. It is not known if Achn can function as a transcriptional regulator. It does not have an identifiable acid activation domain or DNA binding motifs24 that would be anticipated in transcription factors. Nevertheless, the data presented here suggests that Achn-dependent MMP-9 expression is regulated directly or indirectly at the level of transcription since ectopic Achn could drive luciferase activity from the presumptive MMP-9 promoter (Figure 5B). The first 710 bp upstream of the MMP-9 promoter harbors multiple consensus motifs for the binding of transcription factors such as Egr-1, Sp1, and NF-kB. Substantial evidence from previous studies has indicated that each of these transcription factors has the ability to transactivate the MMP-9 gene48–50. It has been shown that Achn is an RNA binding protein that binds to stem lop sequences in the 5’ UTR of collagen I27. It is possible that the effects of Achn on MMP-9 expression are indirect and mediated by alterations in the abundance of transcription factors that regulate the MMP-9 promoter.
Achn has putative nuclear localization (NLS) and export signals (NES)24. Using eGFP-tagging and mutagenesis we have demonstrated that the NLS and NES signals are both functional and necessary to shuttle Achn in and out of the nucleus respectively (Figure 2). The ability of Achn to enter the nucleus is essential for all of the biological functions we have examined in this study, since loss of the NLS abrogates the effects of ectopic Achn on: proliferation, lamellipodia, invasion, MMP-9 expression, VEGF expression and enhanced tumor growth in vivo. Immunohistochemical analysis of Achn expression in a variety of normal mouse and human tissues reveals that it predominantly localizes to the cytoplasm (Schwartz et al., in preparation). IHC analysis of normal and malignant mammary tissue has also demonstrated that Achn is found primarily in the cytoplasm (Figure 1), although we do observe some nuclear staining in some mesodermal tumors (H. Glenn, J. Mueller and L.M. Schwartz, unpublished). The failure to observe significant nuclear accumulation of Achn in the mammary tumors in vivo could suggest that: 1) endogenous Achn does not play a role in the etiology of tumor behavior; 2) only low levels of nuclear Achn are required to enhance tumor growth; or 3) Achn needs only localize to the nucleus transiently to drive metastasis. In preliminary studies we have found that Achn can be driven into the nucleus of some cells transiently following growth factor stimulation (H. Glenn and L.M. Schwartz, unpublished). This would tend to support the third hypothesis, although more direct testing is required.
In summary, this is the first report suggesting a role for Achn in any human disease. It is differentially expressed in breast cancer and is preferentially expressed in basal-like tumors. It can increase the growth and invasive properties of human breast cancer cells in vitro and tumor cell growth in vivo. A more detailed epidemiological analysis of Achn expression in human breast cancer will be important for determining if Achn has utility as either a diagnostic biomarker or a target for therapeutic intervention.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jeff Kane for help with the development of the anti-Acheron antiserum. This work was supported by grants from the Rays of Hope Foundation, the Department of Defense and the National Institutes of Health.
Abbreviations
- Achn
Acheron
- AchnWT
wild-type Achn
- AchnNLS
Achn lacking its nuclear localization signal
- AchnNES
Achn lacking its nuclear export signal
- ECM
extracellular matrix
- MMPs
matrix metalloproteinases
- VEGF
vascular endothelial growth factor
- FGF-2 or bFGF
fibroblast growth factor-2
- PDGF
platelet derived growth factor
- LARP6
La Related Protein 6
- La
Lupus Antigen
- NLS
nuclear localization signal
- NES
nuclear export signal
- eGFP
enhanced green fluorescent protein
- DMEM
Dulbecco’s modified Eagle medium
- IHC
immunohistochemical
- SMA
smooth muscle actin
- ERα
estrogen receptor alpha
- DCIS
ductal carcinoma in situ
Footnotes
Impact: This study is the first to suggest a role for the newly described Acheron protein in human pathogenesis. Multiple lines of evidence are presented to support the hypothesis that Acheron is differentially expressed in some human breast cancers and that Acheron expression enhances tumor formation and angiogenesis.
REFERENCES
- 1.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 4.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 5.Bogenrieder T, Herlyn M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- 7.Pulyaeva H, Bueno J, Polette M, Birembaut P, Sato H, Seiki M, Thompson EW. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells. Clin Exp Metastasis. 1997;15:111–120. doi: 10.1023/a:1018444609098. [DOI] [PubMed] [Google Scholar]
- 8.Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997;76:651–660. [PubMed] [Google Scholar]
- 9.Kupferman ME, Fini ME, Muller WJ, Weber R, Cheng Y, Muschel RJ. Matrix metalloproteinase 9 promoter activity is induced coincident with invasion during tumor progression. Am J Pathol. 2000;157:1777–1783. doi: 10.1016/S0002-9440(10)64815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- 12.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 14.Freudenberg JA, Chen WT. Induction of Smad1 by MT1-MMP contributes to tumor growth. Int J Cancer. 2007;121:966–977. doi: 10.1002/ijc.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: Implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 16.Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jimenez-Baranda S, Gomez-Mouton C, Martinez-A C, Manes S. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004;117:1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- 17.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 18.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 19.Klagsbrun M, D'Amore PA. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev. 1996;7:259–270. doi: 10.1016/s1359-6101(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 23.Langer R, Conn H, Vacanti J, Haudenschild C, Folkman J. Control of tumor growth in animals by infusion of an angiogenesis inhibitor. Proc Natl Acad Sci U S A. 1980;77:4331–4335. doi: 10.1073/pnas.77.7.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valavanis C, Wang Z, Sun D, Vaine M, Schwartz LM. Acheron, a novel member of the lupus antigen family, is induced during the programmed cell death of skeletal muscles in the moth manduca sexta. Gene. 2007;393:101–109. doi: 10.1016/j.gene.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun R, Su Y, Zhao X, Qi J, Luo X, Yang Z, Yao Y, Luo X, Xia Z. Human calcium/calmodulin-dependent serine protein kinase regulates the expression of p21 via the E2A transcription factor. Biochem J. 2009;419:457–466. doi: 10.1042/BJ20080515. [DOI] [PubMed] [Google Scholar]
- 27.Cai L, Fritz D, Stefanovic L, Stefanovic B. Binding of LARP6 to the conserved 5' stem-loop regulates translation of mRNAs encoding type I collagen. J Mol Biol. 2010;395:309–326. doi: 10.1016/j.jmb.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai L, Fritz D, Stefanovic L, Stefanovic B. Nonmuscle myosin-dependent synthesis of type I collagen. J Mol Biol. 2010;401:564–578. doi: 10.1016/j.jmb.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Glenn H, Brown C, Valavanis C, Liu JX, Seth A, Thomas JE, Karlstrom RO, Schwartz LM. Regulation of muscle differentiation and survival by acheron. Mech Dev. 2009;126:700–709. doi: 10.1016/j.mod.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Glenn HL, Wang Z, Schwartz LM. Acheron, a lupus antigen family member, regulates integrin expression, adhesion, and motility in differentiating myoblasts. Am J Physiol Cell Physiol. 2010;298:C46–C55. doi: 10.1152/ajpcell.00387.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Stasinopoulos I, O'Brien DR, Wildes F, Glunde K, Bhujwalla ZM. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol Cancer Res. 2007;5:435–442. doi: 10.1158/1541-7786.MCR-07-0010. [DOI] [PubMed] [Google Scholar]
- 34.Miller ME, Holloway AC, Foster WG. Benzo-[a]-pyrene increases invasion in MDA-MB-231 breast cancer cells via increased COX-II expression and prostaglandin E2 (PGE2) output. Clin Exp Metastasis. 2005;22:149–156. doi: 10.1007/s10585-005-6536-x. [DOI] [PubMed] [Google Scholar]
- 35.Farina AR, Coppa A, Tiberio A, Tacconelli A, Turco A, Colletta G, Gulino A, Mackay AR. Transforming growth factor-beta1 enhances the invasiveness of human MDA-MB-231 breast cancer cells by up-regulating urokinase activity. Int J Cancer. 1998;75:721–730. doi: 10.1002/(sici)1097-0215(19980302)75:5<721::aid-ijc10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safina A, Vandette E, Bakin AV. ALK5 promotes tumor angiogenesis by upregulating matrix metalloproteinase-9 in tumor cells. Oncogene. 2007;26:2407–2422. doi: 10.1038/sj.onc.1210046. [DOI] [PubMed] [Google Scholar]
- 38.Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–4857. [PubMed] [Google Scholar]
- 39.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 40.Jezierska A, Matysiak W, Motyl T. ALCAM/CD166 protects breast cancer cells against apoptosis and autophagy. Med Sci Monit. 2006;12:BR263–BR273. [PubMed] [Google Scholar]
- 41.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawood S, Hu R, Homes MD, Collins LC, Schnitt SJ, Connolly J, Colditz GA, Tamimi RM. Defining breast cancer prognosis based on molecular phenotypes: Results from a large cohort study. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gum R, Wang H, Lengyel E, Juarez J, Boyd D. Regulation of 92 kDa type IV collagenase expression by the jun aminoterminal kinase- and the extracellular signal-regulated kinase-dependent signaling cascades. Oncogene. 1997;14:1481–1493. doi: 10.1038/sj.onc.1200973. [DOI] [PubMed] [Google Scholar]
- 44.Mehta PB, Jenkins BL, McCarthy L, Thilak L, Robson CN, Neal DE, Leung HY. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene. 2003;22:1381–1389. doi: 10.1038/sj.onc.1206154. [DOI] [PubMed] [Google Scholar]
- 45.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 46.Cheung LW, Leung PC, Wong AS. Gonadotropin-releasing hormone promotes ovarian cancer cell invasiveness through c-jun NH2-terminal kinase-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res. 2006;66:10902–10910. doi: 10.1158/0008-5472.CAN-06-2217. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Ou ZL, Hou YF, Luo JM, Shen ZZ, Ding J, Shao ZM. Enhanced expression of duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene. 2006;25:7201–7211. doi: 10.1038/sj.onc.1209703. [DOI] [PubMed] [Google Scholar]
- 48.Biesiada E, Razandi M, Levin ER. Egr-1 activates basic fibroblast growth factor transcription. mechanistic implications for astrocyte proliferation. J Biol Chem. 1996;271:18576–18581. doi: 10.1074/jbc.271.31.18576. [DOI] [PubMed] [Google Scholar]
- 49.Woo JH, Park JW, Lee SH, Kim YH, Lee IK, Gabrielson E, Lee SH, Lee HJ, Kho YH, Kwon TK. Dykellic acid inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting nuclear factor kappa B transcriptional activity. Cancer Res. 2003;63:3430–3434. [PubMed] [Google Scholar]
- 50.Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD, Dedhar S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9) Oncogene. 2000;19:5444–5452. doi: 10.1038/sj.onc.1203928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.