Abstract
The safety profile of alcoholic extract of stem-bark of B. ovalifoliolata was investigated in male Wistar albino rats as per OECD guidelines 407. A total of 24 male Wistar rats were divided into four groups of six rats each. Group 1 served as control and was given 0.3% carboxymethylcellulose, groups 2, 3 and 4 were given alcoholic extract of B. ovalifoliolata @ 100, 500 and 1000 mg/kg respectively in 0.3% carboxymethylcellulose orally for 28 days. The animals were observed daily for clinical signs, mortality, physiological and behavioral changes. Body weights were measured at weekly intervals and various hematological parameters like Hb, PCV, TEC, TLC and serum biochemical profile which included AST, ALT, creatine phosphokinase, creatinine, total protein and antioxidant parameters like TBARS and GSH in liver were estimated at the end of experimental period. There were no clinical signs of abnormality. The weekly body weights, organ weights and hematological parameters did not vary significantly amongst the groups. The mean activity of AST, ALT and CPK, and the concentration of serum creatinine, total protein, TBARS and GSH did not differ significantly among the groups. Histological abnormalities of toxicological significance were not detected in groups 2 and 3. However, mild histopathological alterations were observed in higher dose group 4. In conclusion, the present study revealed that the alcoholic extract of stem-bark of B. ovalifoliolata is safe at lower doses of 100 and 500 mg/kg. Hence, alcoholic extract of stem bark of B. ovalifoliolata is safe and no observed adverse effect level (NOAEL) is found to be 500 mg/kg following repeated oral administration for 28 days in rats.
Keywords: B. ovalifoliolata, hematology, oxidative stress, safety evaluation, serum biochemistry
INTRODUCTION
Herbal remedies have been used in the management of various diseases from ages and herbs are considered to be safe (Oduola et al.).[1] These remedies are commonly self-medicated, more often with no proper dose regimen. Such indiscriminate use without recourse to safety and adverse effects on biological system is considered as dangerous (Aboyade et al.).[2] Although, the literature has documented several toxicities resulting from the use of herbs on many occasions, still the potential toxicity of herbs has not been recognized by the general public or by professional groups of traditional medicine (Jou-fang).[3] The use of medicinal plants as raw materials in the production of drugs is gaining popularity (Olaleye)[4] and hence, it has become imperative to assess the safety or toxicity of plants used in folklore medicine.
Boswellia ovalifoliolata (Family: Burseraceae), an endangered plant vernacularly known as Konda guggilam, is a deciduous tree endemic to Seshachalam hills of Chittoor and Kadapa districts of A.P. In folklore, the stem and bark are used to cure hydrocoel, inflammation and swellings and stomach ulcers (Pullaiah).[5] Two macrocyclic diaryl ether heptanoids were identified from the stems of B. ovalifoliolata viz., ovalifoliolatin A and B together with three compounds acerogenin c, 3 α-hydroxyurs-12-ene and sitost-4-ene-3-one. This class of diaryl heptanoids exhibit a broad range of potent biological activities that include anti-inflammatory, anti-hepatotoxic, antifungal, antibacterial and related effects (Niranjan Reddy et al.).[6] The alcoholic extract of B.ovalifoliolata stem bark extract is reported to exhibit anti-inflammatory activity in rats (Sakunthala Devi et al.).[7] The present effort is, therefore, aimed to investigate the safety profile of the stem bark alcoholic extract of the plant following oral administration for 28 days in adult male Wistar rats as per OECD guidelines 407(OECD).[8]
MATERIALS AND METHODS
Plant extract
The stem-bark of B.ovalifoliolata was collected, cleaned, shade dried and ground into a coarse powder. About 100 g of stem-bark powder was macerated in 500 ml of 95% ethanol v/v with intermittent mixing using a glass rod for 72 h and filtered. The filtrate was evaporated at 55°C on a hot plate to one fourth of the volume. The yield obtained was 10% w/w.
Animals
Male Wistar albino rats weighing 270-320 g were used in the present study. The animals were housed in a well cross ventilated room at 27±2°C, relative humidity 44-56%, light and dark cycles of 12 h and 12 h, respectively throughout the experiment. The animals were provided with standard pellet diet supplied by Hindustan Lever Ltd., Bangalore and water ad libitum. The study was conducted with prior permission of Institutional animal ethics committee and in accordance to OECD guidelines 407 (OECD).[8]
Experimental design
A total of 24 rats were randomly allotted to control and three treatment groups consisting of six rats each. The control group received 0.3% carboxymethylcellulose. The extract was made into suspension with 0.3% carboxymethylcellulose and administered orally in three doses of 100, 500, 1000 mg/kg to groups 2, 3 and 4 respectively on a daily basis for a period of 28 days.
Clinical signs
During the period of administration of the extract, the rats were observed daily for signs of toxicity, physiological and behavioral changes, if any.
Body weight changes
The body weights of all the animals were monitored at the onset of the study and at weekly intervals till the end of the experimental period.
Blood collection
On day 29, rats were anesthetized with ether and blood was collected by orbital puncture. Whole blood collected in EDTA was utilized for hematological studies and serum obtained after clotting and centrifugation of blood was utilized for biochemical analysis.
Serum biochemical parameters
Serum was separated from clotted blood samples by centrifugation at 3000 rpm for 10 min and was utilized for biochemical analysis. The activities of aspartate transaminase (AST), alanine transaminase (ALT), creatinine phosphokinase (CPK), creatinine, and total protein were estimated using standard kits supplied by Qualigens, Pvt. Ltd, Mumbai.
Hematology
Hematological parameters viz., hemoglobin, packed cell volume (PCV), total erythrocyte count (TEC), total leukocyte count (TLC) were estimated using standard laboratory procedures.
Antioxidant profile
Liver samples were collected and homogenized in ice-cold phosphate buffer (pH 7.4) for the estimation of lipid peroxidation (TBARS) (Subramanian et al.)[9] and reduced glutathione (GSH) content (Moron et al.).[10]
Gross and histopathology
A detailed post-mortem examination was conducted on the rats sacrificed from each group. Vital organs such as liver, heart, kidney, spleen and testes were weighed immediately after dissection to avoid drying and examined macroscopically for gross lesions, if any. Tissue portions were then fixed in 10% neutral buffered formalin and were processed for routine histopathological processing. Sections of 5-6 μm thickness were prepared and stained by hematoxylin and eosin (Singh and Sulochana).[11] The stomachs were cut open along the greater curvature and gently washed with normal saline and gastric mucosa was examined for the presence of erosions, hemorrhagic spots, ulcers and perforations, if any, with the help of magnifying lens.
Statistical analysis
The data was expressed as mean±S.E. Statistical differences between groups were tested using one way ANOVA followed by Duncan's multiple comparison test using SPSS 15.0 V software and the level of significance was set at P<0.05.
RESULTS
The results of weekly body weights, organ weights, hematology, sero-biochemical profile, and oxidative profile are presented in Tables 1–5 respectively.
Table 1.
Effect of alcoholic extract of B. ovalifoliolata on weekly body weights (g)
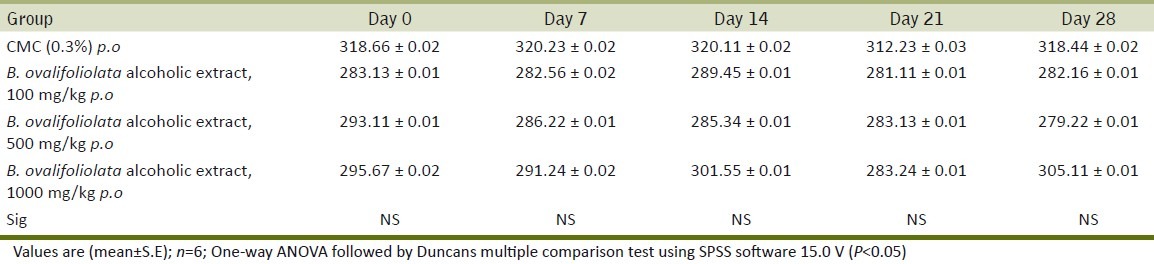
Table 5.
Effect of alcoholic extract of B.ovalifoliolata on oxidative stress in liver
Clinical signs
The clinical signs in the treatment groups showed no abnormalities in terms of changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions and autonomic activity (e.g. lacrimation, piloerection, pupil size, unusual respiratory pattern) gait, posture and response to handling as well as the presence of clonic or tonic movements, stereotypes (e.g. excessive grooming, repetitive circling) or bizarre behavior (e.g. selfmutilation, walking backwards). Further, there were no signs of toxicity in all the groups.
Body weights and organ weights
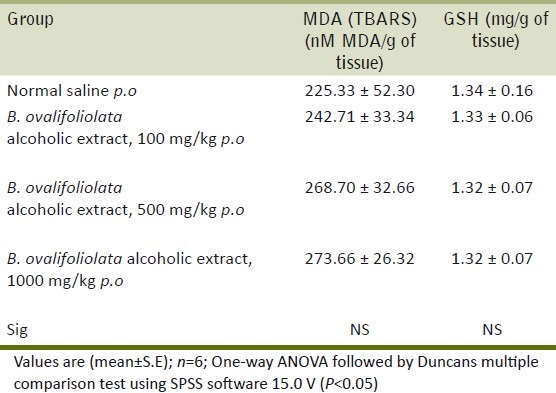
The body weights [Table 1] and organ weights [Table 2] of liver, kidney, heart, spleen, lungs and testes showed no significant difference between the treated groups and control group and further, at the doses studied, there was no swelling, atrophy or hypertrophy of various organs.
Table 2.
Effect of alcoholic extract of B. ovalifoliolata on organ weights (g)
Biochemical parameters
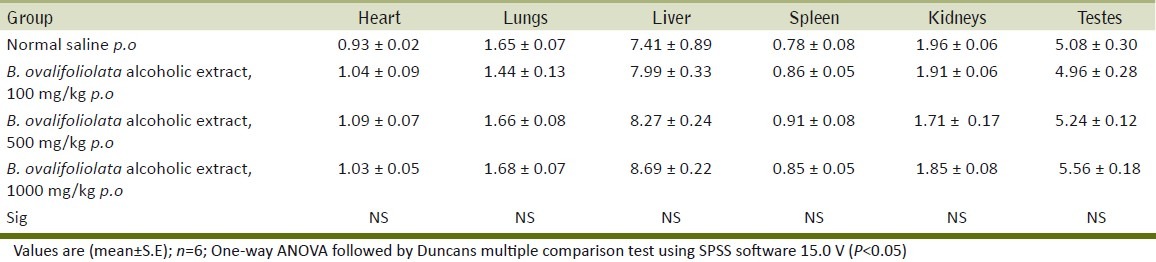
The biochemical parameters like AST, ALT, CPK, creatinine, total protein showed no significant difference between treatment groups and control group indicating that the extract is not toxic to vital organs like liver, kidney and heart [Table 3].
Table 3.
Effect of alcoholic extract of B. ovalifoliolata on serum biochemical parameters
Hematological parameters
The hematological parameters like PCV, Hb, TEC and TLC showed no significant difference between treatment groups and control group, which shows that the extract is not toxic to the hematopoietic system [Table 4].
Table 4.
Effect of alcoholic extract of B. ovalifoliolata on hematological profile
Antioxidant parameters
The antioxidant status of liver assessed in terms of TBARS and GSH levels was not altered indicating that there are no potential toxic compounds in the extract that can cause oxidative damage [Table 5].
Gross and histopathology
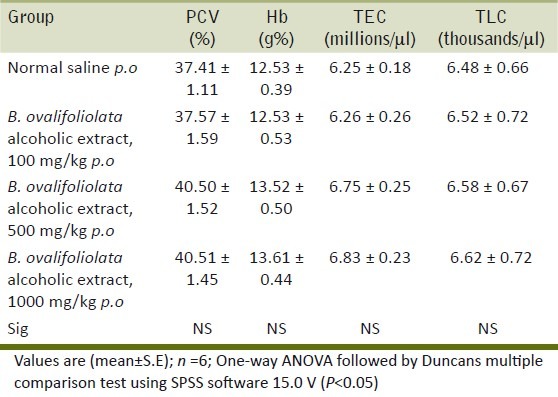
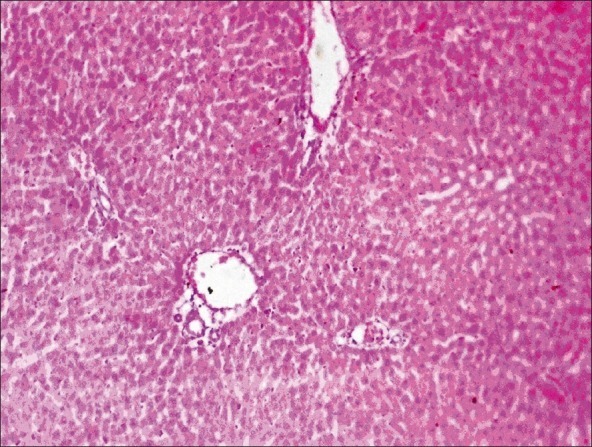
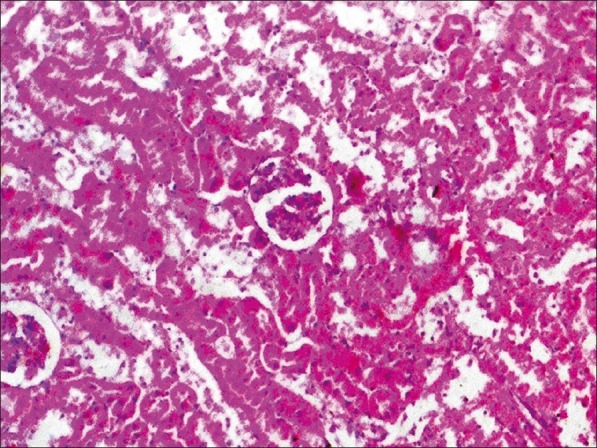
Histopathological examination of liver, kidney, heart and testes did not reveal any lesions of toxicological significance in lower dose groups 2 and 3; however, in higher dose group 4 (1000 mg/kg) mild proliferation of bile ducts in liver [Figure 1], mild necrotic changes in tubular epithelial cells of kidney [Figure 2], mild congestion in heart [Figure 3] and inter tubular edema and severe necrotic degeneration of seminiferous tubules in testes were observed [Figure 4]. Further, at higher doses (1000 mg/kg), rats showed desquamation of epithelium lining of gastric mucosa [Figure 5].
Figure 1.
Group 4 liver showing mild proliferation of bile duct (H and E, ×70)
Figure 2.
Group 4 kidney section showing mild necrotic changes in tubular epithelial cells (H and E, ×70)
Figure 3.
Group 4 heart section showing mild congestion (H and E, ×70)
Figure 4.
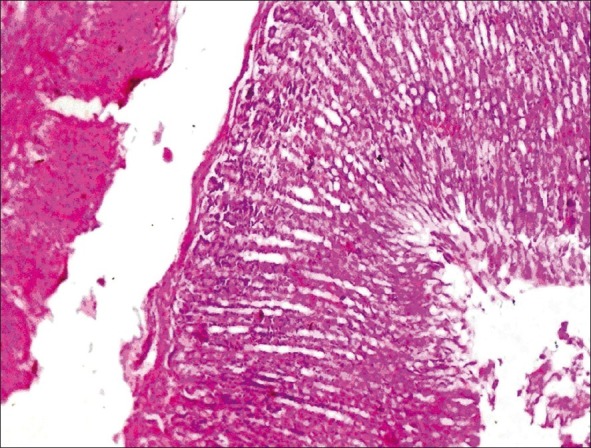
Group 4 testis showing interlobular edema and severe necrotic changes in seminiferous tubular epithelial cells (H and E, ×70)
Figure 5.
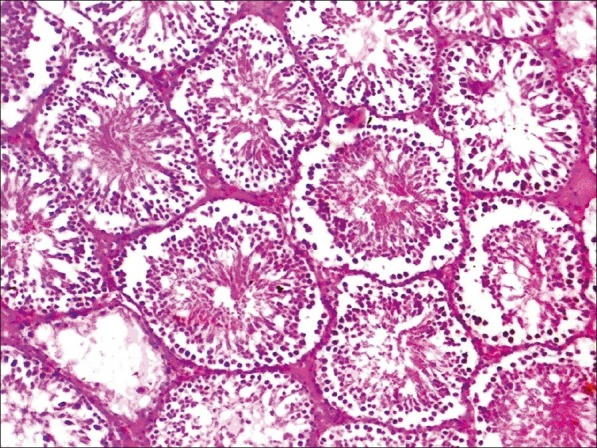
Group 4 stomach showing the desquamation of lining epithelial cells
DISCUSSION
The use of herbal remedies is wide spread with about 80% of the population in developing countries depending on them. Despite this widespread use, few scientific studies are available that ascertained the safety of traditional remedies (Saggu et al.).[12] Therefore, the need to provide information on the toxic implications of these remedies cannot be over emphasized. In this scenario, in the present study, the repeated 28 day oral toxicity of alcoholic extract of stem bark of B.ovalifoliolata was investigated for its safety in male rats by assessing clinical signs, hematological parameters, biochemical parameters, antioxidant parameters and histopathological studies of liver, kidney, heart, testes and gastric mucosa.
The clinical signs were normal in all the treatment groups and there was no significant difference in terms of body weight and organ weights in the treatment groups indicating the absence of potential toxic principles in the extract. In general, reduction in body weights and internal organ weights are a simple and sensitive index of toxicity after exposure to potentially toxic substances (Shrinath et al.).[13]
Liver is the major organ responsible for metabolism of toxic substances that enter the body (Alisi et al.)[14] and the functions of the liver can be detrimentally altered by liver injury resulting from acute or chronic exposure to toxicants or by situations affecting both ß-oxidation and the respiratory chain enzymes (Alisi et al.).[14] Further, hepatotoxicity has been viewed as liver injury associated with impaired liver function caused by exposure to drug or other noninfectious agents or herbs (Navarro;[15] Oduola et al.[1] ) Serum enzyme activities are used as indicators of chemical-induced liver damage (Drotman and Lawhorn).[16] The study on the activities of different biomarker enzymes such as AST and ALT, and the concentration of total protein have been found to be of great value in the assessment of clinical and experimental liver damage. Membrane damage releases the enzyme into circulation and therefore they can be measured in serum (Teo et al.).[17] In the present study, the plant extract did not alter the activities of AST, ALT and total protein levels indicating no potential liver damage [Table 3].
Non-protein nitrogenous (NPN) substances such as urea and serum creatinine are increased only when renal function is below 30% of its original capacity in animals (Kaneko et al.).[18] In the present study, there was no significant difference in the creatinine levels in all of the alcoholic extract treated groups.
The activity of creatine phosphokinase (CPK) has been used to assess cardiac damage as it is a biomarker enzyme (Adeneye et al.).[19] The CPK levels were not altered in the present study using alcoholic extract of the herb indicating non-toxic implications to the heart.
The hematopoietic system is very sensitive to the toxic effects of chemicals and serves as an important indicator of the physiological and pathophysiological status of both animals and humans (Hashemi et al.).[20] After 28 days of treatment using the alcoholic extract of B. ovalifoliolata, there were no treatment-related changes in hematological parameters. The study revealed that the orally administered doses are non-toxic and did not interfere with erythropoiesis and leucopoiesis in rats. This shows that the plant is safe for use because some plants are known to cause destruction of red blood cells leading to anemia (Blood and Radostits;[21] Adedapo[22,23]).
Increased levels of lipid peroxides and reduced glutathione activity reflect the hepatic toxicity of the compound (Adeneye et al.).[19] In the present study, the levels were not altered indicating that there are no potential toxic compounds in the extract that can cause oxidative damage.
The gross and histopathological changes in liver, kidney, heart and gastric mucosa revealed no abnormalities in the groups 2 and 3 which were administered 100 mg and 500 mg/kg of the extract. However, in group 4 which was given higher dose of the extract (1000 mg/kg) showed bile duct proliferation, degeneration of tubular epithelial cells in kidney, congestion of heart and desquamation of gastric epithelial lining indicating that the extract is not safe beyond 500 mg/kg upon repeated oral administration for 28 days.
In conclusion, the safety study on stem-bark extract of B. ovalifoliolata revealed no adverse effects of toxicological importance at doses of 100 and 500 mg/kg b.wt whereas at the dose level of 1000 mg/kg revealed mild histopathological changes on repeated 28 day oral toxicity study in rats. Hence, it is concluded that the drug is safe for use with a no observed adverse effect level (NOAEL) of 500 mg/kg/day when administered orally for 28 consecutive days. However, as the higher dose (1000 mg/ kg) of the extract revealed mild histopathological changes, it is recommended that chronic toxicity, mutagenicity and carcinogenicity studies are further warranted to support the safe and sound use of the herb.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Oduola T, Popoola GB, Avwioro OG, Oduola TA, Ademosun AA, Lawal MO. Use of Jatropha gossypifolia stem latex as a haemostatic agent: How safe is it? J Med Plants Res. 2007;1:014–17. [Google Scholar]
- 2.Aboyade O, Yakubu M, Grierson D, Afolayan A. Studies on the toxicological effect of the aqueous extract of the fresh, dried and boiled berries of Solanum aculeastrum Dunal in male Wistar rats. Hum Exp Toxicol. 2009;28:765–75. doi: 10.1177/0960327109354545. [DOI] [PubMed] [Google Scholar]
- 3.Jou-fang D. Clinical toxicity of Herbal medicine in Taiwan. 7th International Conference on Health problems related to the Chinese. 1994 [Google Scholar]
- 4.Olaleye MT. Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. J Med Plants Res. 2007;1:9–13. [Google Scholar]
- 5.Pullaiah T. Medicinal plants in A.P (India) New Delhi: Regency publications; 2002. p. 51. [Google Scholar]
- 6.Lakshmi Niranjan Reddy V, Ravinder K, Srinivasulu M, Venkateshwar Goud T, Malla Reddy S, Srujankumar D, et al. Two new macrocyclic diaryl ether heptanoids from Boswellia ovalifoliolata. Chem Pharm Bull (Tokyo) 2003;51:1081–4. doi: 10.1248/cpb.51.1081. [DOI] [PubMed] [Google Scholar]
- 7.Sakunthala Devi PR, Adilaxmamma K, Srinivasa Rao G, Srilatha Ch, Alpha Raj M. Evaluation of anti-inflammatory activity of stem-bark of Boswelia ovalifoliata in rats. [Last accessed on 2010 Sept 28];Inventi Rapid: Ethanopharmacology. 2010 1:ep159. Available from: http://www.inventi.in . [Google Scholar]
- 8.The OECD guideline for testing of chemical: 407 repeated dose oral toxicity-Rodent:28-day or 14-day study. Paris: OECD; 2001. OECD (The Organization of Economic Co-operation Development) pp. 1–7. [Google Scholar]
- 9.Balasubramanian KA, Manohar M, Mathan VI. An unidentified inhibitor of lipid peroxidation in intestinal mucosa. Biochim Biophys Acta. 1988;962:51–8. doi: 10.1016/0005-2760(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 10.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–8. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 11.Singh UB, Sulochana S. Handbook of Histological and Histochemical Techniques. 2nd ed. Hyderabad: Premier Publishing House; 2007. [Google Scholar]
- 12.Saggu S, Divekar HM, Gupta V, Sawhney RC, Barnerjee PK, Kumar R. Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides) leaf extract: A dose dependent study. Food Chem Toxicol. 2007;45:609–17. doi: 10.1016/j.fct.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Baliga MS, Jagetia GC, Ulloor JN, Baliga MP, Venkatesh P, Reddy R, et al. The evaluation of the acute toxicity and long term safety of hydroalcoholic extract of Sapthaparna (Alstonia scholaris) in mice and rats. Toxicol Lett. 2004;151:317–26. doi: 10.1016/j.toxlet.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Alisi CS, Onyeze GO. Nitric oxide scavenging ability of ethyl acetate fraction of methanolic leaf extracts of Chromolaena odorata. Afr J Biochem Res. 2008;2:145–50. [Google Scholar]
- 15.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–9. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 16.Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 17.Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V. A 90-day oral gavage toxicity study of D-methylphenidate and D,L-methylphenidate in Sprague-Dawley rats. Toxicology. 2002;179:183–96. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko JJ, Harvey JW, Michael LB. Clinical biochemistry of Domestic animals. 5th ed. New York: Academic Press; 1997. pp. 857–79. [Google Scholar]
- 19.Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol. 2006;105:374–9. doi: 10.1016/j.jep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Hashemi SR, Zulkilfi I, Bejo MH, Farida A, Somchit MN. Acute toxicity study and phytochemical screening of selected herbal aqueous extract in broiler chickens. Int J Pharma. 2008;4:352–60. [Google Scholar]
- 21.Blood DC, Radostits OM. Veterinary Medicine. London: Balliere Tindall; 1989. p. 46. [Google Scholar]
- 22.Adedapo AA, Abatan MO, Olorunsogo OO. Effects of some plants of the spurge family on the haematological and biochemical parameters of rats. Vet Arhiv. 2007;77:29–38. [Google Scholar]
- 23.Adedapo AA, Abatan MO, Olorunsogo OO. Toxic effects of some plants in the genus Euphorbia on haematological and biochemical parameters of rats. Vet Arhiv. 2004;74:53–62. [Google Scholar]


