Abstract
Objective:
The present study was designed to evaluate acute and repeated dose toxicity of the methanol extract (ME) of the Gmelina arborea stem bark.
Materials and Methods:
For the acute toxicity study, ME of G. arborea was orally administered to Swiss albino mice at a dose range of 300–5000 mg/kg. For the repeated dose toxicity study, the Wistar rats of either sex were orally administered with ME of G. arborea at the doses of 300, 1000, and 2000 mg/kg/day for a period of 28 days. The effects on body weight, food and water consumption, organ weight, hematology, clinical chemistry as well as histology were studied.
Results:
The administration of ME from the G. arborea bark at 300–5000 mg/kg did not produce mortality or significant changes in the clinical signs. The no-observed adverse effect level (NOAEL) of ME was 5000 mg/kg. There were no significant differences in the general condition, growth, organ weights, hematological parameters, clinical chemistry values, or gross and microscopic appearance of the organs from the treatment groups as compared to the control group.
Conclusion:
ME of G. arborea was found safe in acute and repeated dose toxicity studies when tested in mice and rats.
Keywords: Acute toxicity, Gmelina arborea, methanol extract, repeated dose toxicity
INTRODUCTION
Since ancient times, plants have commonly been used in folk medicine for the treatment of various ailments. The rationale for the utilization of medicinal plants has rested largely on the long-term clinical experience with little or no scientific data on their efficacy and safety. However, in the recent past, pharmacological and toxicological effects of these plants have begun to receive attention from scientists for the verification of their claimed pharmacological and therapeutic properties.[1]
Gmelina arborea Roxb. (Verbenaceae), popularly known as Shivan, is an important medicinal plant in the Indian Ayurvedic system of medicine. The drupes, leaves, flowers, roots, and bark are used in traditional medicine. The plant is used in snake-bite and scorpion sting. The juice of tender leaves added to cow's milk is used in gonorrhea. Leaves ground into paste with water are applied to the forehead for headache in fevers.[2] The plant is anthelmintic and is useful in treatment of piles, abdominal pains, burning sensations, fever,[3] and diabetes.[4]
A large number of phytoconstituents have been identified in different parts of G. arborea including flavonoids, steroids, alkaloids, glycosides, and lignans. Luteolin,[5] indole alkaloids,[6] and iridoid glycosides[7] have been isolated from the leaves. The occurrence of hentriacontanol[8] and lignans like arboreol, isoarboreol, methyl arboreol, arborone, gmelanone, gummadiol, gmelanone, and 7-oxodihydrogmelinol[9–11] in the heartwood has also been reported. Crude extracts of G. arborea have also been investigated for different pharmacological activities. The extract form of leaves is reported to have wound-healing properties.[12] The aqueous methanol extract (ME) of the bark showed an antidiarrheal activity in castor oil-induced diarrhea in mice.[13] The aqueous extract of the bark and fruit of G. arborea have been studied for a hepatoprotectant and antioxidant activity using liver slice culture.[14]
In spite of the wide use of G. arborea in traditional medicine, data on the systematic evaluation of its toxic effects is lacking. Therefore, the aim of the present study was to investigate the acute and repeated dose toxic effects of ME of the G. arborea bark in rodents.
MATERIALS AND METHODS
Plant material
The bark of G. arborea was collected in the month of April from Jawhar (District Thane), Maharashtra, India. It was identified and authenticated by Dr. P. S. N. Rao of Botanical Survey of India, Pune, Maharashtra, India. A voucher specimen (b-03) of the bark is deposited in the department for future reference. The plant material was then shade dried at a temperature of 30°C±3°C for a period of 15 days and ground to get coarse powder having a particle size not more than 1700 µm.
Preparation of the methanol extract
ME of the bark of G. arborea was prepared by Soxhlet extraction technique. The powdered bark, 500 g, was packed in the Soxhlet extractor (BOROSIL®, India) and extracted with methanol. After complete extraction, ME was filtered and concentrated under reduced pressure by using a rotary vacuum evaporator. Then the extract was dried in a vacuum dryer and stored at −20°C until used. The yield of ME was found to be 28% w/w with respect to the powdered bark. The extract for administration was prepared with double distilled water to get a final concentration of 500 mg/mL. It was administered per orally to mice according to their body weights to attain the required dose. For example, if the weight of mice was 30 g, then 0.3 ml was orally administered to attain a final dose of 5000 mg/kg.
Experimental animals
Swiss albino mice and Wistar albino rats were purchased from Haffkine Institute, Mumbai, Maharashtra, India. All animals were maintained in an air-conditioned room at 25°C±2°C, with a relative humidity of 75%±5%, and a 12-h light/dark cycle. A basal diet (Amrut Feeds, Maharashtra, India) and tap water were provided ad libitum. Male and female rats were assigned to each dose group by stratified random sampling based on body weight. The animals were kept under laboratory conditions for an acclimatization period of 7 days before carrying out the experiments. All the experiments were approved by the Institutional Animal Ethics Committee (approval nos. CPCSEA/SPTM/P-5/2008 and CPCSEA/SPTM/P-6/2008) constituted as per the norms of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and complied with the NIH guidelines on experimentation on animals.[15]
Acute toxicity study
The acute toxicity of G. arborea ME was evaluated in mice (25-35 g) using the OECD Guideline.[16] Four groups containing three female mice received ME at doses of 0, 300, 2000, and 5000 mg/kg body weight, orally after a short fasting period.
The general behavior of the animal was continuously monitored for 1 h after dosing, periodically during the first 24 h (with special attention given during the first 4 h), and daily thereafter for a total of 14 days. The detailed cage-side observations were conducted including changes in eyes and mucous membranes, skin and fur, respiratory, circulatory, autonomic, and central nervous systems, and also somatomotor activity and behavior pattern. Special attention was directed to observations of convulsions, tremors, diarrhea, salivation, lethargy, sleep, and coma. In addition, body weight and food and water intake were recorded at 2-day intervals. Surviving animals were fasted overnight, then weighed, and humanely killed on day 15 using anesthetic ether, and selected vital organs were excised and macroscopically examined.
Repeated dose toxicity study
A repeated toxicity study was conducted on Wistar rats of either sex (150–170 g). The animals were divided in four groups (0 mg/kg, 300 mg/kg low dose, 1000 mg/ kg medium dose, and 2000 mg/kg high dose), each containing five males and five females. While the extract was orally administered using gavage to test groups, distilled water was administered to the control group for 28 days. The maximum volume administered was not greater than 2 mL/100 g body weight. All animals were supplied with standard food and water ad libitum during the testing periods. All rats were observed daily for toxic manifestations and mortality. Body weight and water and food intake were measured once a week. For serum biochemistry determinations, blood samples were collected in nonheparinized tubes. To obtain the serum, samples were placed at room temperature for approximately 30 min and the tubes centrifuged at 3000 rpm for 10 min. The collected supernatants were used for analysis.[17] Biochemical parameters like cholesterol, high density lipoproteins (HDL), triglycerides (TGL), bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein, albumin, blood urea nitrogen (BUN), creatinine, and glucose were determined using an autoanalyzer (Erba Chem 7, Germany). The blood samples were also analyzed for important inorganic ions like sodium, potassium, calcium, phosphate, and chloride. Hematological analysis included the determination of parameters like hemoglobin, hematocrit, total red blood corpuscles (RBC), total white blood corpuscles (WBC), and platelets. Different red cell indices including mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were also determined using an automatic hematological analyzer (Sysmex, Japan).
Surviving animals were fasted overnight, then weighed, and humanely killed on day 29. Vital organs like liver, lungs, kidneys, adrenals, gonads, spleen, heart, and brain were excised, weighed, and macroscopically examined.
Histopathological evaluation
The organs of the ME-treated and control groups were stained with the hematoxylin–eosin (H and E) stain following fixation with 10% formalin and embedding in paraffin wax. A histopathologist performed a complete examination of the tissue samples.
Statistical analysis
The differences among treated and control groups for serum biochemistry parameters, serum electrolytes, hematological parameters, and organ weights were determined using the statistical software Sigmastat ver. 2.03 for Windows. Comparisons among different groups were performed by analysis of variance using the ANOVA test. A significant difference between control and experimental groups was assessed by Student's t-test. All data are expressed as mean±standard error of mean (SEM); P values less than 0.05 were considered to be significant.
RESULTS
Acute toxicity study
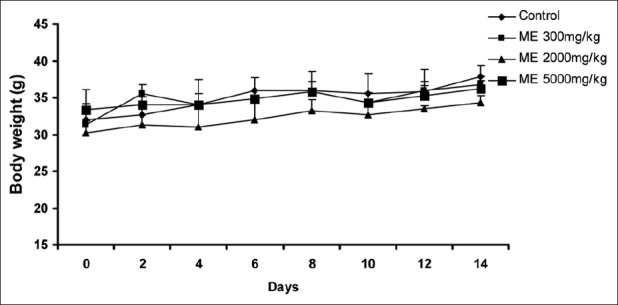
No lethal effects or mortality was observed in animals throughout the test period following single oral administration at all selected dose levels of ME [Table 1]. The animals did not show any changes in the general appearance during the observation period. Morphological characteristics (fur, skin, eyes, and nose) were unchanged. The treated animals did not show any tremors, convulsion, salivation, diarrhea, lethargy, or unusual behaviors such as self-mutilation, walking backward etc. There was no significant difference in body weights [Figure 1], and food and water intake in the treatment groups when compared with control animals [Figure 2]. The LD50 value for the oral administration of the G. arborea extract is more than 5000 mg/kg body weight.
Table 1.
Sign of toxicity and mortality results of the acute toxicity study of the methanol extract of Gmelina arborea in mice
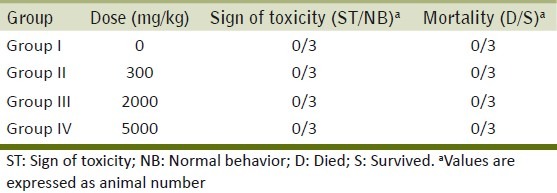
Figure 1.
Effect of ME on body weight changes in the acute toxicity study. Each point represents mean±SEM (n=3)
Figure 2.
Effect of ME on the food (a) and water (b) intake in the acute toxicity study. Each point represents mean±SEM (n=3)
Repeated dose toxicity study
The administration of ME for 28 days did not show any adverse symptoms of toxicity and mortality at all selected doses.
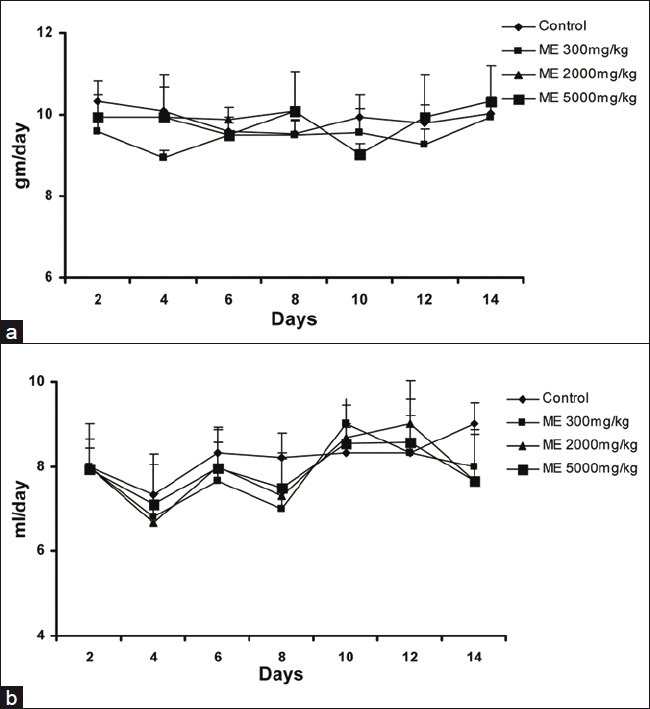
In all groups, body weight gradually increased for 28 days, and changes in the body weight in ME-treated groups relative to the control group were not significant during the experimental period [Figure 3]. The food and water consumption of male and female rats in treatment groups did not show any significant changes when compared with animals in the control group [Figures 4 and 5].
Figure 3.
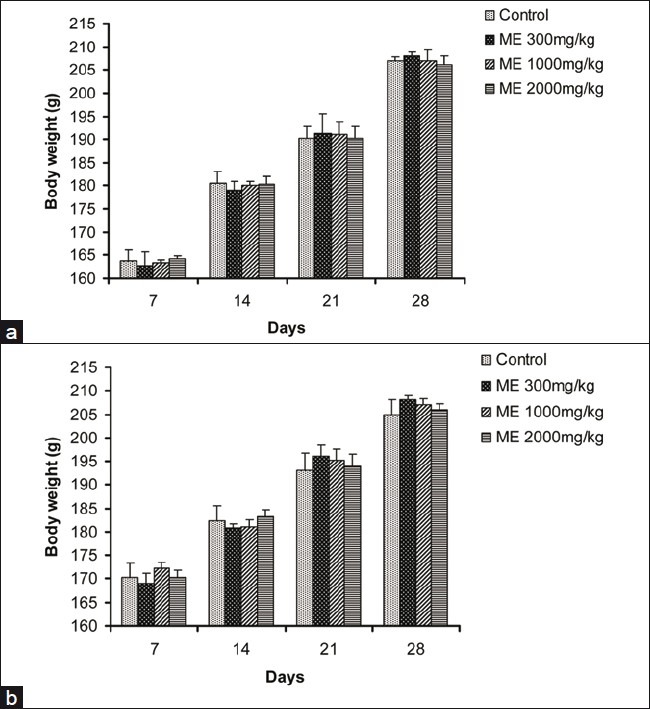
Effect of ME on body weight changes in male rats (a) and female rats (b) in the 28-day repeated dose toxicity study. Data are expressed as mean±SEM (n=5)
Figure 4.
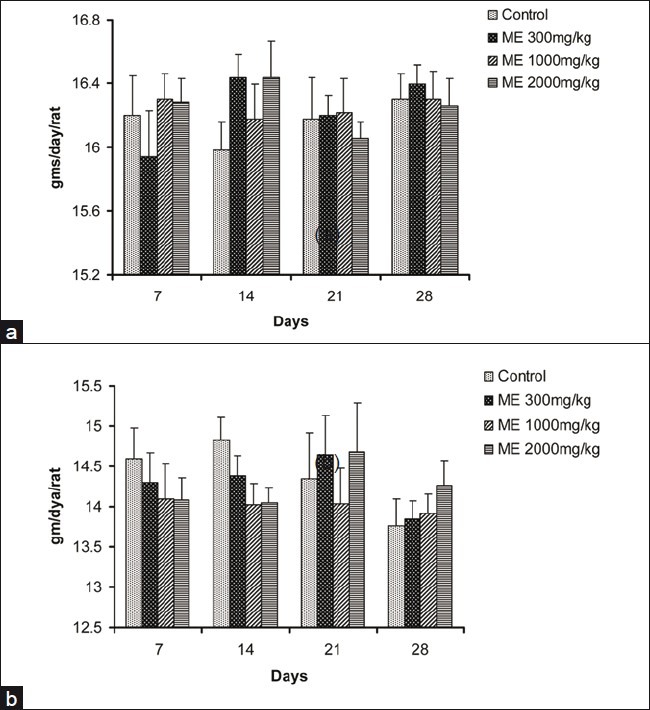
Effect of ME on the food intake in male (a) and female (b) rats in the 28-day repeated dose toxicity study. Data are expressed as mean±SEM (n=5)
Figure 5.
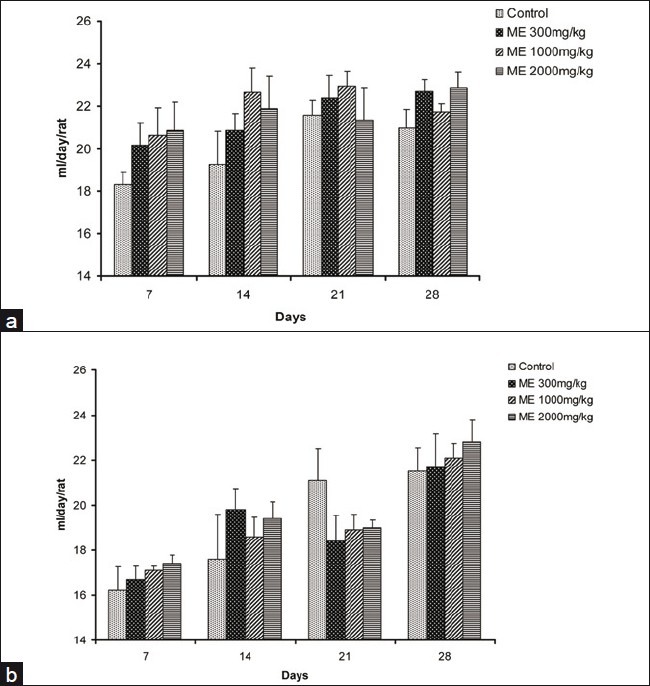
Effect of ME on the water intake in male (a) and female (b) rats in the 28-day repeated dose toxicity study. Data are expressed as mean±SEM (n=5)
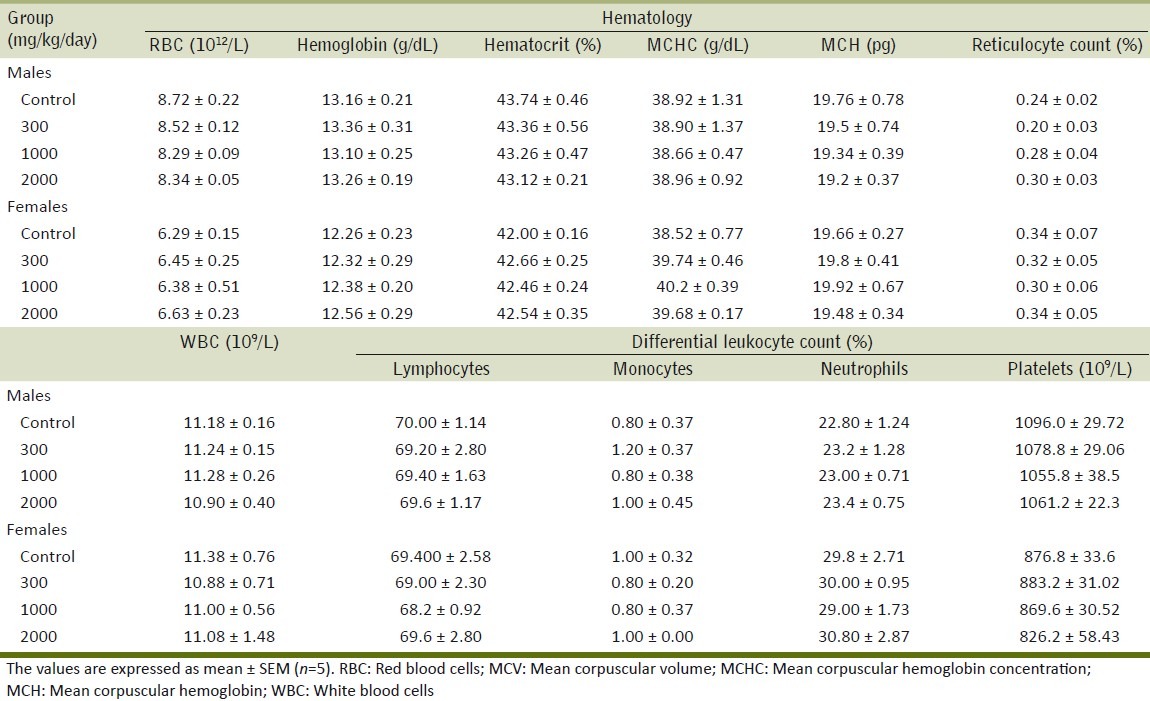
Table 2 summarizes the results of the effect of ME on different biochemical parameters. No significant differences were observed in any of the biochemical parameters examined in treated groups as compared with the control group. The results of the effect of the repeated administration of ME on serum electrolytes are summarized in Table 3. The extract did not exhibit any effect on levels of serum electrolytes. Table 4 shows the effect of ME on different hematological parameters. The different hematological parameters were not significantly different in male and female treatment groups from control rats.
Table 2.
Effect of the methanol extract of Gmelina arborea (ME) on biochemical parameters in the repeated dose toxicity study
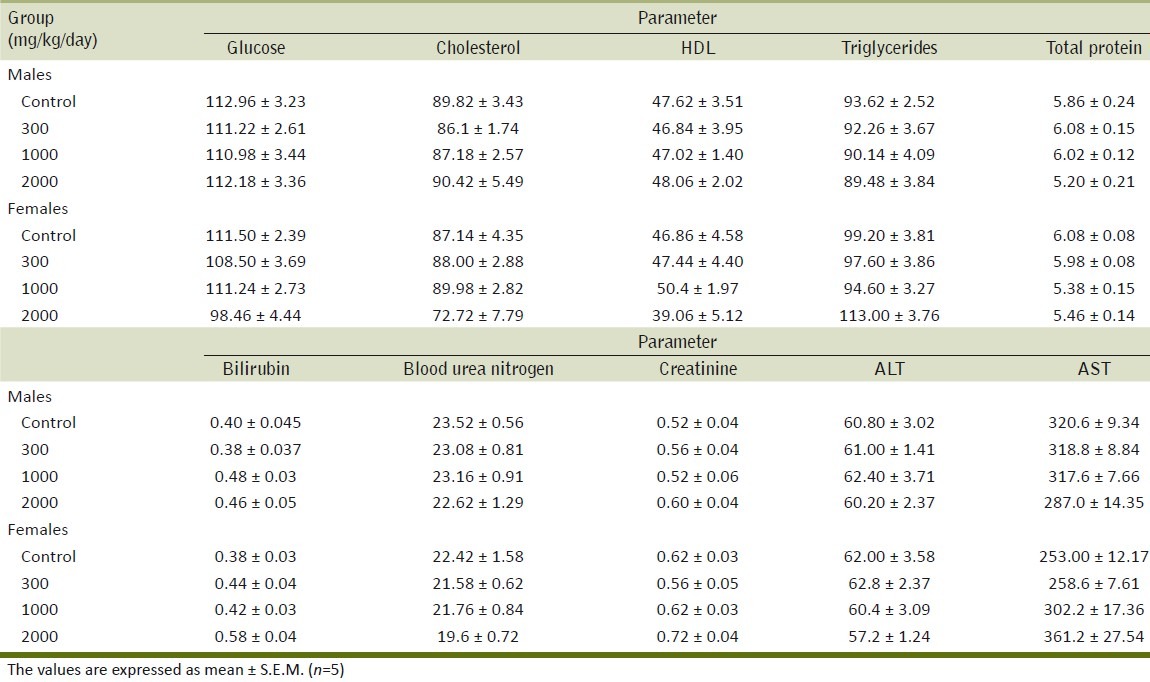
Table 3.
Effect of the methanol extract of Gmelina arborea (ME) on serum electrolytes in the repeated dose toxicity study
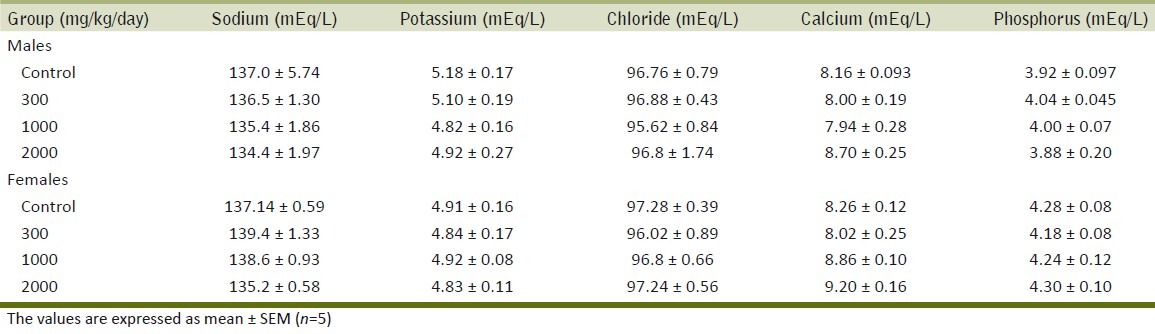
Table 4.
Effect of the methanol extract of Gmelina arborea (ME) on hematological values in the repeated dose toxicity study
The organ weights of male and female rats did not show significant differences between the control and treated groups [Tables 5 and 6] of either sex.
Table 5.
Effect of the methanol extract of Gmelina arborea (ME) on organ weights (g) in rats in repeated dose toxicity study
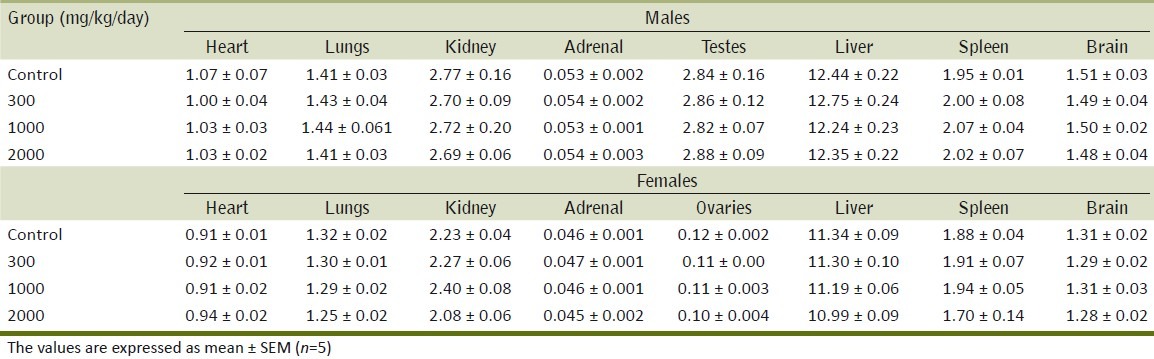
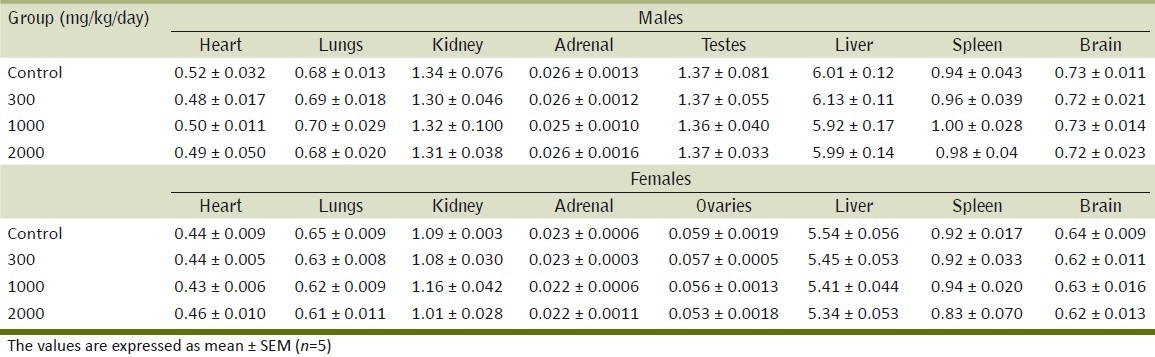
Table 6.
Effect of the methanol extract of Gmelina arborea (ME) on relative organ weights in rats in the repeated dose toxicity study
No treatment-related gross or microscopic changes were noticed in internal organs during the pathological examination.
DISCUSSION
The popularity of herbal medicine is increasing in developing countries. It is often believed that such remedies don not have adverse effects, since these treatments are “natural” and commonly used for self-medication without supervision. These medicinal plants possess several biological activities in humans but very little is known regarding their potential toxicity.[18] The same is also applicable to G. arborea.
In the acute oral toxicity study, a product is considered safe if no death occurs and no clinical signs are observed at doses below 5 g/kg.[19] ME of G. arborea did not show any toxic reactions at a dose of 5 g/kg. Thus, the no-observed adverse effect level (NOAEL) of ME was 5000 mg/kg.
Generally, the decrease in the body weight gain is a simple and sensitive index of toxicity after exposure to potentially toxic substances.[20–22] In the present study, ME at all selected doses did not show significant changes in body weights as compared to the control group. This suggests that ME did not obstruct the growth of experimental animals. The necropsy performed after 14 days showed no significant changes in organ gross anatomy in all treatment groups when compared with control. Therefore, this plant can be considered as safe when tested for acute toxicity.
In the 28-day repeated dose toxicity study, G. arborea did not appear to affect the behavior of the rats at the administrated oral doses of 300, 1000, and 2000 mg/kg.
There were no significant differences in biochemical parameters of the groups treated with ME of G. arborea compared to the control. The lack of significant alterations in the levels of ALT, AST, creatinine, and BUN are good indicators of liver and kidney functions,[23] which suggests that the repeated administration of ME of Gmelina arborea do not have toxic effects on liver and kidney.
The plant has been reported for the presence of phenolics as one of the important phytoconstituents. Plant phenolics are well known for their antioxidant activity. The plant has shown a significant antioxidant activity in in vitro models like DPPH, FRAP, and ABTS assay. The phenolics in G. arborea may play a protective role against the oxidative damage to the liver cells by scavenging the free radicals.
The hematopoietic system is very sensitive to toxic compounds and serves as an important index of the physiological and pathological status for both animals and humans.[24] There were no treatment-related changes in the different hematological parameters between the control and treatment group after 28 days of treatment, with G. Arborea ME. It indicates that ME does not affect hematopoiesis and leucopoiesis in experimental animals. Thus, the orally administrated doses of the extract (300, 1000, and 2000 mg/kg) were nontoxic and did not interfere with the production of circulating red blood cells, white blood cells, and platelets.
The histopathological studies of important organs after the administration of ME indicated no alterations in tissue structures. This supports the results from biochemical analysis, and the oral administration of ME at a high dose of 2000 mg/kg/day for 28 days was well tolerated by the treated rats.
In conclusion, the methanol extract of G. arborea was found to be safe in acute and repeated dose toxicities in mice and rats.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rebecca MA, Ishii-Iwamoto EL, Grespan R, Cuman RK, Caparroz-Assef SM, Mello JC, et al. Toxicological studies on Stryphnodendron adstringens. J Ethnopharmacol. 2002;83:101–4. doi: 10.1016/s0378-8741(02)00219-2. [DOI] [PubMed] [Google Scholar]
- 2.Nadkarni KM. Indian Materia Medica. Mumbai: Popular Prakashan; 2000. pp. 584–5. [Google Scholar]
- 3.Kirtikar KR, Basu BD. Indian Medicinal Plants. Dehradun: International Book Distributors; 1999. pp. 1932–3. [Google Scholar]
- 4.Khan IA, Khanum A. Herbal Therapy for Diabetes. Hyderabad: Ukaaz Publications; 2005. pp. 34–5. [Google Scholar]
- 5.Rao DV, Rao EV, Viswanathan N. Occurrence of luteolin in the leaves of Gmelina arborea Linn. Curr Sci. 1967;3:71–4. [Google Scholar]
- 6.Bhattacharjee AK, Das AK. Phytochemical survey of few mysore Plants. Econ Bot. 1969;23:274–6. [Google Scholar]
- 7.Hosny M, Rosaazza JP. Gmelinosides A-L, Twelve acylated iridoid glycosides from Gmelina arborea. J Nat Prod. 1998;61:734–42. doi: 10.1021/np970447u. [DOI] [PubMed] [Google Scholar]
- 8.Joshi KC, Lalit Prakash, Singh LB. Extractives from heartwood. J Indian Chem Soc. 1971;48:1175–6. [Google Scholar]
- 9.Govindachari TR, Parthasarathy PC, Dasai HK. Arboreol, A new lignan from Gmelina arborea. Indian J Chem. 1972;10:1120–2. [Google Scholar]
- 10.Anjaneyulu AS, Jaganmohan RK, Kameswara RV, Ramachandra RL, Subrahmanyam C, Pelter A, et al. The structures of lignans from Gmelina arborea. Tetrahedron. 1975;31:1277–85. [Google Scholar]
- 11.Satyanarayana P, Koteswara R, Ward RS, Pelter A. Arborone and 7-Oxo-dihydrogmelinol: Two New Keto-Lignans from Gmelina arborea. J Nat Prod. 1986;49:1061–4. [Google Scholar]
- 12.Shirwaikar A, Ghosh S, Padma GM Rao. Effects of Gmelina arborea Roxb. leaves on wound healing in rats. J Nat Remedies. 2003;3:45–8. [Google Scholar]
- 13.Agunua A, Yusuf S, Andrew GO, Zezi AU, Abdurahman EM. Evaluation of five medicinal plants used in diarrhoea treatment in Nigeria. J Ethnopharmacol. 2005;101:27–30. doi: 10.1016/j.jep.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Sinha S, Dixit P, Bhargava S, Devasagayam TP, Ghaskadbi S. Bark and fruit extracts of Gmelina arborea protect liver cells from oxidative stress. Pharm Biol. 2006;44:237–43. [Google Scholar]
- 15. [Last accessed on 2010 Dec 30]. Available from: http://bioethics.od.nih.gov/animals.html .
- 16.OECD Guidelines for testing of Chemicals. No. 423. Paris, France: Organization for Economic Cooperation and Development; 2001. OECD: Acute oral toxicity test method. [Google Scholar]
- 17.Gutiérrez A, Gámez R, Mas R, Noa M, Pardo B, Marrero G, et al. Oral subchronic toxicity of a lipid extract from Roystonea regia fruits in mice. Drug Chem Toxicol. 2008;31:217–28. doi: 10.1080/01480540701873152. [DOI] [PubMed] [Google Scholar]
- 18.Rosidah, Yam MF, Sadikun A, Ahmad M, Akowuah GA, Asmawi MZ. Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol. 2009;123:244–9. doi: 10.1016/j.jep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Brock WJ, Trochimowicz HJ, Millischer RJ, Farr C, Kawano T, Rusch GM. Acute and subchronic toxicity of 1,1-dichloro-1- fluoroethane (HCFC-141b) Food Chem Toxicol. 1995;33:483–90. doi: 10.1016/0278-6915(95)00008-p. [DOI] [PubMed] [Google Scholar]
- 20.Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V. A 90-day oral gavage toxicity study of D-methylphenidate and D, L methylphenidate in Sprague-Dawley rats. Toxicology. 2002;179:183–96. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 21.Tofovic SP, Jackson EK. Effect of long-term caffeine consumption on renal function in spontaneously hypertensive heart failure prone rats. J Cardiovasc Pharmacol. 1999;33:360–6. doi: 10.1097/00005344-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA. Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm. 2002;70:135–45. [Google Scholar]
- 23.El Hilaly J, Israili Z, Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol. 2004;91:43–50. doi: 10.1016/j.jep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol. 2006;105:374–9. doi: 10.1016/j.jep.2005.11.027. [DOI] [PubMed] [Google Scholar]


