Abstract
Male wistar rats (weighting 160–180 g) were divided in six groups of 6 animals per group. Group A and F served as control. Groups B, C, D and E received acrylamide at 20 mg/kg body weight for 28 days and groups C and E received additionally vitamin E (50 IU/kg body weight) for 1 to 28 days and 29 – 42nd days of experiment, respectively. The animals from groups A, B, and C were sacrificed on day 28th of experiment and from groups D, E, and F on 42nd day of experiment, respectively. There was significant decrease in the total sperm count and significant increase in the dead sperm count on day 28th of study due to acrylamide toxicity. At recovery period, there was significant increase in the total sperm count of vitamin-E-treated group of animals as compared to untreated toxicated rats. But, values were significantly lower than control animals. Microscopically, the lesions in the testes of acrylamide intoxicated rats at 28th day revealed destruction of seminiferous tubules at periphery. No spermatid and spermatocytes were seen in the seminiferous tubules. Detachment of spermatogonial cells started at periphery of seminiferous tubules. Atrophy of seminiferous tubules was a constant finding. Some tubules showed vacuolar degenerative changes in germinal epithelium. During the recovery period, destruction of seminiferous tubules, detachment of spermatogonial cells, and atrophy of seminiferous tubules were observed in group D and E. Few sections revealed only spermatogonial cells. At recovery period vitamin-E-treated rats revealed somewhat better architecture of the seminiferous tubules. Late spermatids were seen in few seminiferous tubules and other revealed starting of spermatogenesis. Thus, it appears that Vitamin E is not able to protect testes from acrylamide toxicity during active feeding, but after cessation of acrylamide feeding treatment with vitamin E revealed faster recovery as compare to not treated group.
Keywords: Acrylamide toxicity, protective effect of vitamin E, testicular toxicity
INTRODUCTION
Acrylamide (CH2=CHCONH2) is a widely used industrial chemical. Acrylamide is a white, odorless, crystalline solid at room temperature with a molecular formula of C3H5NO.[1] Acrylamide has been reported to be present in plant material such as potatoes, carrots, radish, lettuce, Chinese cabbage, parsley, onions, spinach, and rice paddy,[2] in sugar[3] and olives.[4] Acrylamide is found in carbohydrate-rich food prepared at high temperatures such as French fries and potato chips that are consumed by humans. Consumption of these foods may result in significant human exposure to acrylamide. The formation of acrylamide is associated with high temperature (higher than 200°C) cooking process of certain carbohydrate rich foods, especially when asparagines react with sugar.[5] Furthermore, acrylamide can undergo oxidative biotransformation by cytochrome P450 (CYP) 2E1.[6] The resulting metabolite is an epoxide derivative, that is, glycidamide, which is more reactive towards DNA and proteins than the parent compound, acrylamide.[7] The biological consequences of acrylamide exposure have chiefly centered on neurotoxicity ever since this effect was observed in humans occupationally exposed to this compound.[8] Subsequently, experimental exposure of rodents to acrylamide has also revealed a carcinogenic mode of action for this chemical.[4] Acrylamide also crosses placenta and passes in a significant concentration to developing fetus leading to direct prenatal and postnatal changes in rodent off-springs.[9] Vitamin E can protect critical cellular structures against damage from both free radicals such as peroxy radical, hydroxyl radical, and super oxide and from oxidation products such as malondialdehyde and hydroxynonenal.[10] Vitamin E, as an important antioxidant, plays a role in inhibition of mutagen formation, and repair of membranes and DNA. Therefore, it has been suggested that vitamin E may be useful in cancer prevention.[11] Significant improvement in the histological profiles of the cryptorchid testes of vitamin-E-treated group was observed as compared to the cryptorchid alone group.[12] The aim of this study was to determine the effect of acrylamide exposure on testes of rats and to assess whether these effects can be ameliorated by co-treatment with vitamin E for 4 weeks or during the recovery period.
MATERIALS AND METHODS
Chemicals
Acrylamide (99.50%) and vitamin E pure were purchased from Hi Media Laboratories Pvt. Limited, Mumbai. All other required chemicals were used of the extra pure grade.
Maintenance of animals
Male wistar rats weighing approximately 160-180 g were procured. The animals were acclimatized for 7 days prior to experiment. The institutional ethics committee approved the experimental protocols. All the animals used in this study were placed in cages in an air conditioned room maintained at temperature of 25°C±30°C and 12 h light and dark schedule. Throughout the experiment, the animals were provided standard food pellet (Ashirwad industries, Nagpur) and water ad libitum. Essential cleanliness conditions were also maintained.
Experimental protocol
Experiment was designed for the duration of 28 days to study sub acute toxicity of acrylamide as per OECD guidelines.
Wistar rats were divided into six groups of six animals per group and the groups were as follows.
Group A – control animals (received vehicle, no treatment 28 days).
Group B – acrylamide (20 mg/kg body weight for 28 days).
Group C – acrylamide (20 mg/kg body weight)+vitamin E (50 IU/kg body weight) for 28 days.
Group D – acrylamide (20 mg/kg body weight for 28 days, 29 days onward stop treatment).
Group E – acrylamide (20 mg/kg body weight for 28 days) and 29 days onward vitamin E (50 IU/kg body weight) up to 42 days.
Group F – control animals (received vehicle, no treatment 42 days).
All the above groups were treated once every day. All the animals from group A, B, C and D, E, F were sacrificed on 28th and 42thday, respectively. The total sperm count and dead sperm count from caput, corpus, cauda, and testes was done as per standard procedure.[13] Testes were collected and fixed in neutral formal saline for histopathological examinations.[14]
Statistical analysis
Data generated are expressed as the mean±SE. Statistical analysis was done using one way ANOVA.[15] The level of significance was set at P≤0.05.
RESULTS AND DISCUSSION
Total sperm count and dead sperm count (%)
Observations of the total sperm count and dead sperm count from all the treatment groups at both periods of observations are presented [Figures 1 and 2]. In this study, the total sperm was decreased and dead sperm count was increased significantly due to feeding of acrylamide in rats the finding is in accordance with Yang et al.[16] The observation of the recovery period, the total sperm count of toxicated group treated vitamin E showed quality and number improvement of sperms as compared to toxicated but untreated. Protective effect of vitamin E during malathion-induced reduction on the total sperm count and quality of sperms was reported earlier.[17] Restoration of the total sperm count might be due to prevention of degeneration of seminiferous tubules, sloughing of seminiferous epithelium due to vitamin E as revealed in this study.
Figure 1.
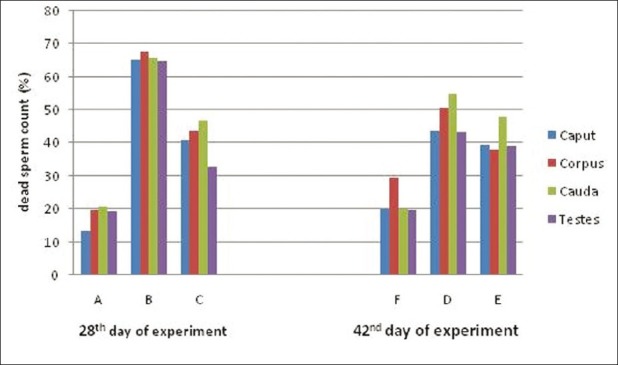
Mean value of dead sperm count (%) of rats exposed to acrylamide and vitamin E for 28 and 42 days
Figure 2.
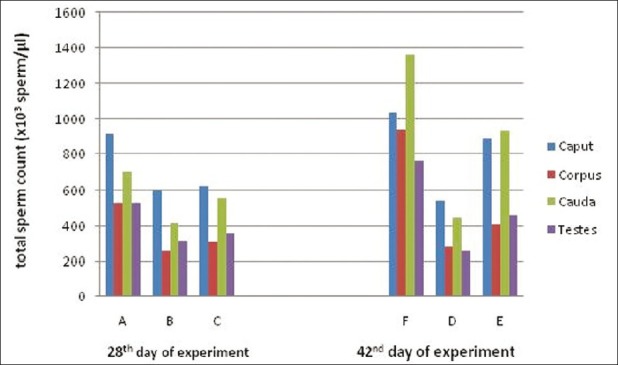
Mean value of total sperm count (×103 sperm/μL) of rats exposed to acrylamide and vitamin E for 28th and 42nd days
Histopathological findings
Microscopically, the lesions in the testes from acrylamide toxicated group were destruction of seminiferous tubules at periphery. No spermatid and spermatocytes seen in the seminiferous tubules [Figure 3]. Detachment of spermatogonial cells started at periphery of seminiferous tubules. Atrophy of seminiferous tubules was a constant finding. Tubules of testes showed vacuolar degenerative changes in germinal epithelium. Similar lesions were observed in groups C [Figure 4]. At the end of recovery period, that is, 42th day changes in testes of group D were destruction of seminiferous tubules, detachment of spermatogonial cells and atrophy of seminiferous tubules similar to group B. Few sections revealed only spermatogonial cells [Figure 5]. Group E that received vitamin E additional at 28th day revealed somewhat better architecture of the seminiferous tubules as compared to group D. Late spermatids were seen in few seminiferous tubules and other revealed starting of spermatogenesis [Figure 6]. Atrophy of seminiferous tubules observed in group D was similar to the findings made earlier.[16,18] Acrylamide-induced degeneration of seminiferous tubules as observed in present study was also reported earlier.[8] Acrylamide treatment lowers testosterone level leading to degeneration of seminiferous tubules with sloughing of seminiferous epithelium and spermatogenic cells.[16] The observation of recovery period suggests that group treated with vitamin E showed spermatogonial cells in seminiferous tubules structurally normal and is comparable to the control group. The protection was better than the group in which vitamin E was not fed additionally. The insufficient protective effect of vitamin E during active acrylamide feeding can be explained considering several factors. Vitamin E @ 100 mg/kg b. wt was found to be ineffective to protect testicular tissues against radiation-induced damage.[19] Partial protective role of vitamin E was recorded earlier during ethane dimethane sulfonate-induced[20] testicular toxicity in rats. Vitamin E has been proven to protect testicular tissues against experimental cryptorchidism in rats.[12] Our earlier report also suggest beneficial role of vitamin E during recovery period than active feeding of acrylamide in rats.[21] Vitamin E can protect critical cellular structures against damage from both free radicals and from oxidation products.[10] Thus, the increased free radicals generated by acrylamide exposure in testes might have been scavenged from the testes during recovery period.
Figure 3.
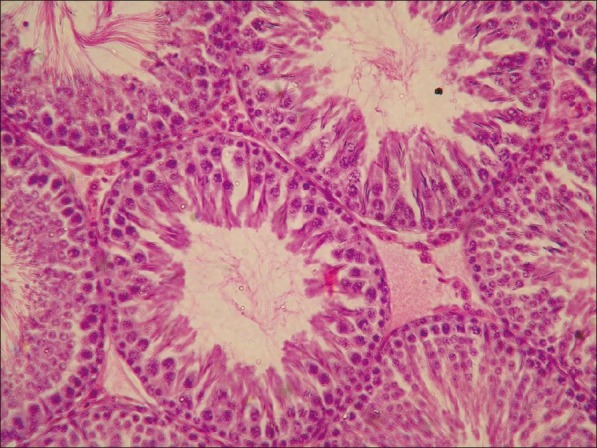
Testes from group B showing vacuolar degeneration of germinal epithelium and loss of spermatids. (H and E, ×200)
Figure 4.
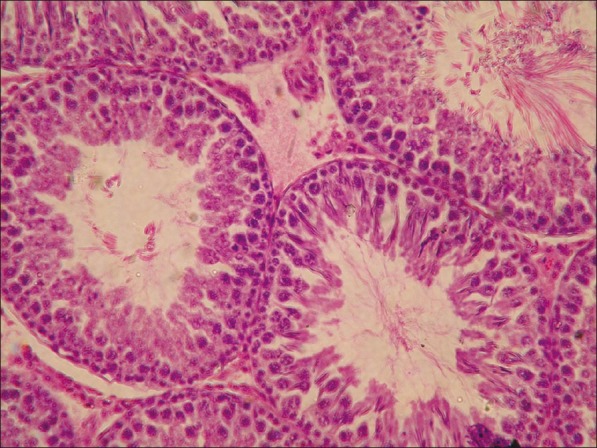
Testes from group C showing arrest in the development of spermatogonial cells (H and E, ×200)
Figure 5.
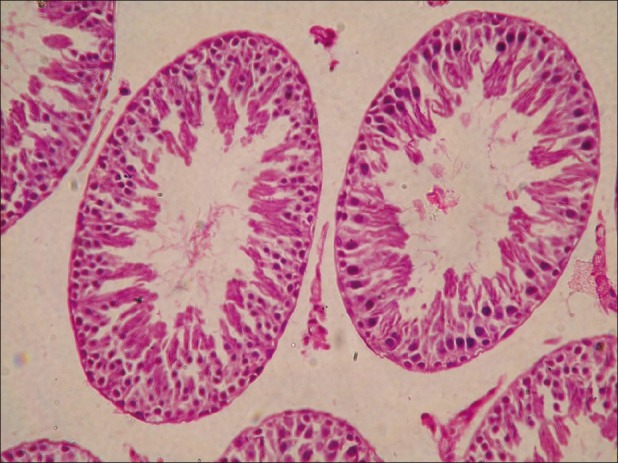
Testes from group D showing detachment of spermatogonial cells and no development of sperm in seminiferous tubules (H and E, ×200)
Figure 6.
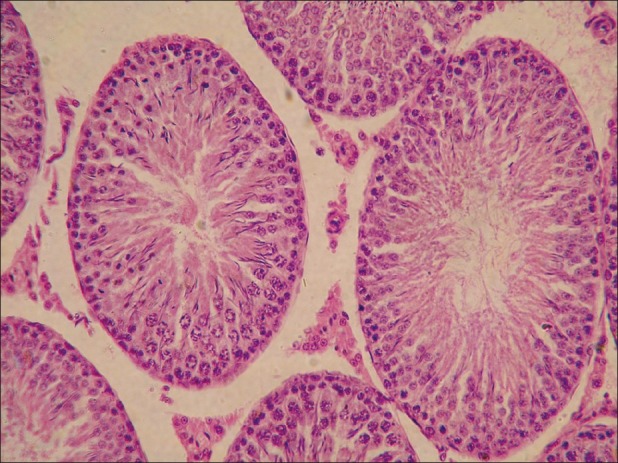
Testes from group E showing late spermatids in seminiferous tubules (H and E, ×200)
From this study, we record that, acrylamide induces degeneration of seminiferous tubules ultimately leads to decrease total sperm count and increase dead sperm count. Vitamin E is not able to protect testes from acrylamide toxicity during active feeding, but after cessation of acrylamide intoxication, treatment with vitamin E revealed faster recovery as compared to not treated group.
ACKNOWLEDGMENTS
Research presented in this manuscript was supported by grant from Department of Veterinary Pathology, Nagpur Veterinary College, Nagpur, India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Grivas S, Jagerstad M, Lingnert H, Skog K, Tornqvist M, Aman P. Acrylamide in food mechanisms of formation and influencing factors during heating of foods. Sweden: Swedish National Food Administration; 2002. [Google Scholar]
- 2.Arikawa A, Shiga M. Determination of trace acrylamide in the crops by gas chromatography. Bunseki Kagaku. 1980;29:33–39. [Google Scholar]
- 3.Schultzova J, Tekel J. Acrylamide monomer occurrence in sugar. Dtsch Lebensm Rundsch. 1996;92:281–2. [Google Scholar]
- 4.Friedman M. Chemistry, biochemistry, and safety of acrylamide: A review. J Agric Food Chem. 2003;51:4504–26. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 5.Rydberg P, Eriksson S, Tareke E, Karlsson P, Ehrenberg L, Törnqvist M. Investigation of factors that influnce the acrylamide content of heated foodstuffs. J Agric Food Chem. 2003;51:7012–8. doi: 10.1021/jf034649+. [DOI] [PubMed] [Google Scholar]
- 6.Sumner SC, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem Res Toxicol. 1999;12:1110–6. doi: 10.1021/tx990040k. [DOI] [PubMed] [Google Scholar]
- 7.Dearfield KL, Douglas GR, Ehling UH, Moore MM, Sega GA, Brusick DJ. Acrylamide: A view of its genotoxicity and assessment of heritable genetic risk. Mutat Res. 1995;330:71–99. doi: 10.1016/0027-5107(95)00037-j. [DOI] [PubMed] [Google Scholar]
- 8.McCollister DD, Oyen F, Rowe VK. Toxicology of acrylamide. Toxicol Appl Pharmacol. 1964;6:172–81. doi: 10.1016/0041-008x(64)90103-6. [DOI] [PubMed] [Google Scholar]
- 9.Edwards PM. The insensitivity of the developing rat foetus to the toxic effects of acrylamide. Chem Biol Interact. 1976;12:13–8. doi: 10.1016/0009-2797(76)90062-4. [DOI] [PubMed] [Google Scholar]
- 10.Erin AN, Spirin MM, Tabidze LV, Kagan VE. Formation of alpha-tocopherol complexes with fatty acids.A hypothetical mechanism of stabilization of biomembranes by vitamin E. Biochem Biophys Acta. 1984;774:96–102. doi: 10.1016/0005-2736(84)90279-7. [DOI] [PubMed] [Google Scholar]
- 11.London RS, Murphy L, Kitlowski KE. Breast cancer prevention by supplemental vitamin E. J Am Coll Nutr. 1985;4:559–64. doi: 10.1080/07315724.1985.10720098. [DOI] [PubMed] [Google Scholar]
- 12.Saalu LC, Oluyemi KA, Omotuyi IO. α- Tocopherol (vitamin E) attenuates the testicular toxicity with experimental cryptorchidism in rats. Afr J Biotechnol. 2007;6:1373–7. [Google Scholar]
- 13.Blom E. A one-minute live-dead sperm stain by means of eosin-nigrosin. Fertil Steril. 1950;1:176–7. [Google Scholar]
- 14.Luna AG. Manaul of histological staining methods of the Armed Force Institute of Pathology. 3rd ed. London: Mc Graw Hill Book Co; 1968. pp. 124–5. [Google Scholar]
- 15.Snecdecor GW, Cochron WG. Statistical methods. 6th ed. New Delhi: Oxford and IBH; 1994. [Google Scholar]
- 16.Yang HJ, Lee SH, Jin Y, Choi JH, Han CH, Lee MH. Gentoxicity and toxicological effects of acrylamide on reproductive system in male rats. J Vet Sci. 2005;6:103–9. [PubMed] [Google Scholar]
- 17.Uzun FG, Kalender S, Durak D, Demir F, Kalender Y. Malathion-induced testicular toxicity in male rats and the protective effect of vitamin C and E. Food Chem Toxicol. 2009;47:1903–8. doi: 10.1016/j.fct.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Burek JD, Albee RR, Beyer JE, Bell TJ, Carreon RM, Morden DC, et al. Subchronic toxicity of acrylamide administered to rats in the drinking water followed by up to 144 days of recovery. J Environ Pathol Toxicol. 1980;4:157–82. [PubMed] [Google Scholar]
- 19.Yalcinkaya FR, Gokce A, Guven EO, Davarci M, Cikim G H, Yelekar H, Balbay MD. Protective Effect of Vitamin E and Melatonin Against Radiation Induced Damage in testes of rats. J Anim Vet Adv. 2009;8:2335–40. [Google Scholar]
- 20.Sahinturk V, Guclu C, Baycu C. Protective effect of vitamin E on ethane dimethane sulfonate-induced testicular toxicity in rats. Asian J Androl. 2007;9:117–24. doi: 10.1111/j.1745-7262.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 21.Rahangadale SN, Kurkure B, Prajapati V, Hedaoo V, Bhandarkar AG. Neuroprotective effect of Vitamin E supplementation in wistar rat treated with acrylamide. Toxicol Int. 2012;19:1–18. doi: 10.4103/0971-6580.94505. [DOI] [PMC free article] [PubMed] [Google Scholar]


