Abstract
To evaluate immunotoxicological effects of environmental chemical, subacute toxicity of repeated (28 day) oral administration of acephate (Ace) in BALB/c mice was assessed. Thirty two (sixteen male and sixteen female) mice were divided into four different groups with each group containing eight (four male and four female) mice. Mice of Group C1 were administered normal saline only and served as control. Group T1 was given 1/40th of apparent LD50 (ALD50) (8.78 mg/kg), and group T2 was put on 1/30th of ALD50 [11.7 mg/kg], while group T3 received 1/20th of ALD50 [17.55 mg/kg] of Ace suspended in normal saline. The blood samples were collected from mice after 28 days of oral administration and analyzed for hematological, biochemical, and immunological parameters. The study showed that hematological parameters (monocytes and granulocytes) remained unaffected except total leukocyte count and lymphocyte which were decreased highly significantly [P≤0.01] in mice of group T3 on the 28th day of experiment. Serum total protein (TP) and serum globulin decreased significantly in mice of treatment groups dose dependently; however, no significant change was seen in serum albumin. Progressive increase in live body weight of mice decreased significantly in extremely toxic group only while spleen:body weight ratio decreased significantly in dose-dependent manner. Furthermore, Ace produced suppressed humoral immune response and the delayed-type hypersensitivity response to Sheep red blood cells (SRBCs) was altered nonsignificantly. The results of this study describe the suppression of immune responses following exposure to Ace at low concentrations in experimental mice.
Keywords: Acephate, biochemical, hematological, immunity, organ:body weight
INTRODUCTION
Acephate (O, S-Dimethyl acetylphosphoramidothioate) is among the top ten organophosphate insecticides sold throughout the world and currently registered for use on a variety of field, fruit, and vegetable crops; in food handling establishments; on ornamental plants both in greenhouses and outdoors (including lawns, turf, and cut flowers); and in and around the home. Acephate (Ace) was first registered in 1973 for ornamental uses and in 1974 for food uses (agricultural crops). Target pests include: Armyworms, aphids, beetles, bollworms, borers, budworms, cankerworms, crickets, cutworms, fire ants, fleas, grasshoppers, leafhoppers, loopers, mealybugs, mites, moths, roaches, spiders, thrips, wasps, weevils, and whiteflies. The toxicity of Ace is attributed to its bioactivation to methamidophos and has weak cholinesterase inhibitor action in non-target animals.[1]
Most of the studies on toxicity of pesticides have been focused on enzyme alterations, gross pathological effects, mutagenic, and carcinogenic potential of these agents. In recent years, the effect of pesticides on immune response has received attention. Many pesticide chemicals such as pyrethroids, organophosphate compounds [OPC], and organochlorines are known to cause suppression of the immune system. Immunosuppression leads to increased susceptibility to infectious diseases and decreased immune response to vaccination. Therefore, this study was conducted to investigate the effect of repeated (28 day) oral administration of Ace on hemato-immunological profiles in BALB/c mice.
MATERIALS AND METHODS
Experimental animals
This study was conducted on 6–8-week-old healthy BALB/c mice. The mice were procured from Cadila Pharmaceuticals, Dholka and kept in cages at Laboratory animal house, Veterinary College, Anand Agricultural University, Anand, Gujarat. The mice were provided with standard pelleted feed and water ad libitum.
Chemicals
Technical grade Ace [97.3% purity] used in this study was procured from Meghmani Industries Agro chemical, R and D Chemist Ahmedabad, India.
Experimental design
Mice were acclimatized for a period of 1 week before the start of oral dosing with Ace. All the mice were randomly divided into four groups [C1, T1, T2, and T3], with each group containing eight mice-four male and four female. Apparent oral LD50 [ALD50] of Ace 351 mg/kg was taken into consideration for calculation of different dose in the groups.[2] Mice were treated with Ace at dose rate of 1/40th LD50/40, 1/30th of LD50/30, and 1/20th of LD50/20 in three treatment groups for a period of 28 days. The group C1 was administered normal saline and served as control. Group T1 was given as 1/40th of LD50 [8.78 mg/kg], group T2 was put on 1/30th of LD50 [11.70 mg/kg] and group T3 received 1/20th of LD50 [17.55 mg/kg] of Ace suspended in normal saline. Acephate in normal saline were administered directly in esophagus by using mice oral feeding needle with 1 mL BD syringe.
The daily oral administration was continued for 28 days and the live weight was recorded prior to treatment and at weekly intervals and mice were observed for any toxicity symptoms during the entire period of experiment.
Hematobiochemical estimation
After 28 days of oral administration, the mice were weighed and blood samples were collected from retrorbital plexus under ether anesthesia before final culling of mice for estimating hematological parameters namely total leukocyte count [TLC] and differential leukocyte count [DLC e.g., monocytes, granulocytes, and lymphocyte], and biochemical parameters, namely, total protein, serum globulin and serum albumin using standard kits (Merck System).
Immunological evaluation
Animals were immunized by injecting 0.2 mL of 5×109 SRBC suspensions intraperitoneally, 7 days prior to sacrifice the experimental animals.
Assessment of humoral immune response (antibody titer)
Serum was separated from blood to determine the antibody titer by the hemagglutination test. Antibody titer was carried out as described by Puri et al.,[3] in briefly, twofold dilution of test serum was made in 0.15 M phosphate buffer saline (PBS) and aliquoted in “U” bottomed microtiter plates. One percent SRBC suspended in PBS was dispended in each well and mixed thoroughly. The plates were incubated for 4 h at 37°C and then observed visually for hemagglutination. The highest dilution of the test serum giving hemagglutination was taken as antibody titer.
Assessment of cell-mediated immune response
Cell-mediated immune response was assessed as described by Lagrange et al.,[4] in briefly, all the animals under various groups were immunized by injecting 20 μL of 5×109 SRBC per mL subcutaneously into the right footpad on 19th day of treatment. Thickness of left footpad was measured using Vernier calipers on the 26th day of treatment. The mice were then challenged by injecting 20 μL of 5×109 SRBC per mL intradermally on the left hind foot pad (time 0). Foot pad thickness was measured after 24 and 48 h of challenge. The difference between the thickness of left foot just before and after challenge in millimeter was taken as a measure of delayed type hypersensitivity (DTH).
Mice were sacrificed after taking body wt. and the weight of Spleen and Thymus were taken at necropsy for calculation of organ:body wt. ratio. O:BW ratio was calculated by dividing organ weight with body weight [g]
O:BW=Organ weight [g]/Body weight [g]×100
Statistical analysis
One-way-analysis of variance (ANOVA) was used to compare the effects of treatment of various doses of Ace on different biochemical, hematological, and immunological variables in control and treated mice. Differences between means were considered significant for P<0.05 and highly significant for P<0.01.[5]
RESULTS AND DISCUSSION
This study was conducted to see the effect of various doses of Ace on the hematobiochemical and immunological profile of BALB/c mice. An approximate LD50 of Ace, that is, 351 mg/kg b. wt. in mice, was taken into consideration for the calculation of doses of Ace to be administered to mice.[2] The effect of administration of Ace at the rate of 1/40th of LD50 [8.78 mg/kg b. wt.], 1/30th of LD50 [11.70 mg/kg b. wt.] and 1/20th of LD50 [17.55 mg/kg b. wt.] once daily for 28 days on body weight, hematological parameters, protein metabolism, and immunological functions have been investigated in the present study. TP, total albumin and total globulin were measured to see the effect of Ace on protein metabolism, and organ: body weight ratio of immune organs, serum antibody titer, and skin thickness were measured for monitoring the immunological function.
There were no apparent clinical signs of toxicity at all dose level tested up to 14 days of experiment. After second week, clinical symptoms were observed in the mice of high-dose Ace-treated group, that is, T3 [17.55 mg/kg]. The most common signs were sudden onset of depression, reduced feed intake, dullness, and rough hair coat in high-dose Ace-treated mice. While in medium-dose Ace-treated group [T2, 11.70 mg/kg body weight] animals were dull and depressed. Mice of control group [C1] and low-dose Ace-treated group [T1] did not show any visible clinical signs of toxicity.
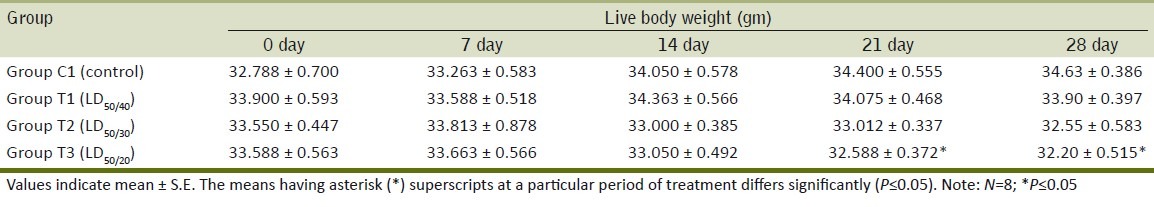
After 14 days, Ace-treated mice at different doses showed a nonsignificant dose-related decrease in the live body weight gain as compared to control group [C1]. At 21st and 28th day of experiment, progressive increase in live body weight was significantly reduced in the mice of group T3 [Table 1] that had received Ace at highest concentration. The decrease in live body weight of mice gives an indication of stress due to given doses of Ace.
Table 1.
Effect of daily oral administration of acephate on progressive increase in live body weight of BALB/c mice
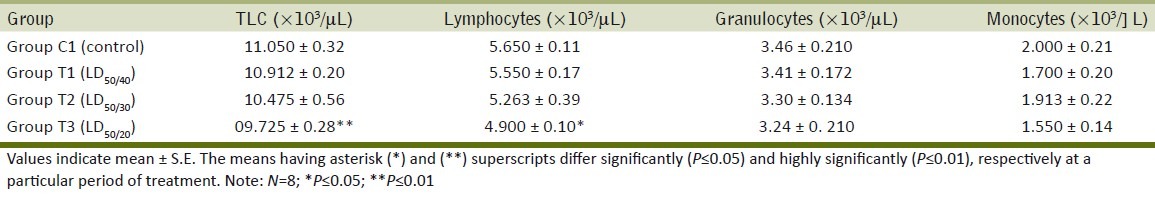
Significant reductions were obtained in TLC values in mice of treatment group T3 that had received Ace at highest concentration [Table 2]. Besides, there were nonsignificant decrease in granulocytes count and monocytes count after 28 days oral administration in comparison to control group [Table 2]. The significant reduction in TLC suggests that Ace at high concentration has immunotoxic potential and might suppress leucopoiesis.
Table 2.
Effect of daily oral administration of acephate on hematological parameter of BALB/c mice
Estimates of total serum proteins and the albumin/globulin ratio may give useful information about the liver and lymphocyte function. There was significant decrease in serum total protein and serum globulin in the mice of high-dose Ace-treated group [T3] whereas nonsignificant decrease was observed on the medium- [T2] and low-dose group [T1] as compared to the control group [C1]. Acephate treated mice at different doses showed a nonsignificant decrease in the serum albumin level after 28 days oral administration in comparison to the control group [Table 3]. Similarly, the serum globulin level was decreased in all treated groups following feeding of broiler chicks with 20-ppm fenvalerate, 2 ppm monocrotophos and 2 ppm endosulfan.[6] The fall in total serum protein and serum globulin could be due to the stressogenic effect of Ace, or general toxic action that also leads to decrease in weight gain in the insecticides treated mice.
Table 3.
Effect of daily oral administration of acephate on serum biochemical parameter and organ:body weight ratio of BALB/c mice
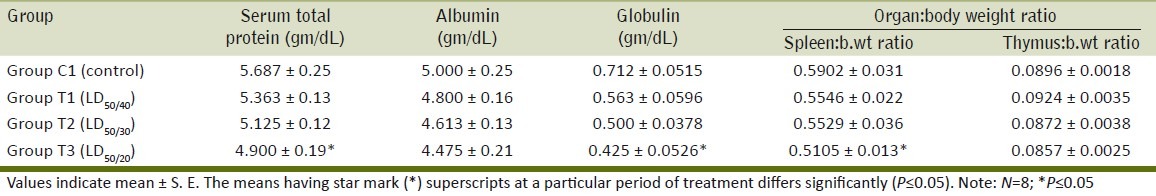
The weight and gross morphology of lymphoid organs are the first parameters studied in assessing toxicity, as the response to injury is often expressed as changes in tissue or organ weight, size, color, and gross appearance. There was significant decrease in the spleen:body weight ratio in the mice of the high-dose Ace-treated group [T3] in comparison to control group; however, a nonsignificant effect was observed in medium- and low-dose groups. Nonsignificant reduction in the thymus:body weight ratio was observed in the mice of treatment groups [T1, T2, and T3] as compared to the control group [Table 3]. A decrease in spleen:body wt. ratio suggests that Ace might be immunotoxic at higher doses used in this study [Table 3]. This reduction in organ:body weight ratio of immune organs suggests that there might be some toxic effects on immune system of mice after their exposure to Ace at higher concentrations.
In this study, nonsignificant effect of Ace on serum antibody against SRBC was observed in the mice of all groups except the high-dose Ace-treated group [T3] in which significant decrease in antibody titer against SRBC was noted [Table 4]. Similarly, significant suppression of the humoral immune response was reported following administration of malathion, endosulfan and chlordimeform in mice.[7] This result led to immunosuppression at higher concentrations of Ace.
Table 4.
Effect of daily oral administration of acephate on antibody titer and skin thickness (cellmediated immunity) of BALB/c mice
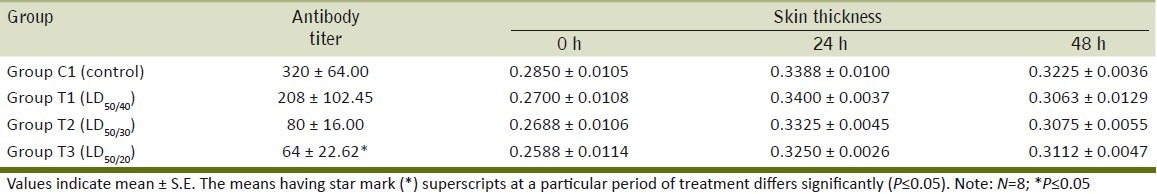
Nonsignificant increase in paw skin thickness in response to SRBC was observed in mice of treatment groups after 24 and 48 h of challenge as compared to control group. Similarly, leptophos, quinalphos, triphenyl phosphate [TPP], and aldicarb in mice[8] did not cause suppression of CMI. On the contrary, suppression of CMI was reported after exposure to Atrazine in rats.[9] This suggests that Ace may be nontoxic or marginally toxic in regards to cell-mediated immune response at dose rate administered in this study.
In conclusion, Acephate seems to be toxic for immune systems in BALB/c mice at the dose administered. Dose-dependent reduction in progressive increase in live body weight of mice suggests stressogenic effect of Ace. The reduction in organ: body weight ratio and serum protein indicative of degenerative changes in spleen and thymus. Besides, Acephate caused leukocytopenia and lymphopenia at the dose of 17.55 mg/kg/day.
ACKNOWLEDGMENTS
I would like to thank Dr. J. B. Solanki, Dean and Principal, College of Veterinary Science and Animal Husbandry, Anand Agricultural University, Anand, India for providing me the facilities to successfully complete the presented research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Spassova D, White T, Singh AK. Acute effects of acephate and methamidophos on acetylcholinesterase activity, endocrine system and amino acid concentrations in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2000;126:79–89. doi: 10.1016/s0742-8413(00)00097-9. [DOI] [PubMed] [Google Scholar]
- 2.Rattner BA, Hoffman DJ. Comparative toxicity of acephate in laboratory mice, white-footed mice, and meadow voles. Arch Environ Contam Toxicol. 1984;13:483–91. doi: 10.1007/BF01056263. [DOI] [PubMed] [Google Scholar]
- 3.Puri A, Saxena RP, Saxena KC, Srivastava V, Tandon JS. Immunostimulant activity of Nyctanthesarbortristis L. J Ethnopharmacol. 1994;42:31–7. doi: 10.1016/0378-8741(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 4.Lagrange PH, Mackaness GB, Miller TE. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974;139:1529–39. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snedecor GW, Cochran WG. In: Statistical Methods. 8th ed. Jones DH, editor. Iwoa USA: The Iowa State University Press; 1994. [Google Scholar]
- 6.Garg UK, Pal AK, Jha GJ, Jadhao SB. Haemato-biochemical and immuno-pathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides in broiler chicks. Inter Immunopharmacol. 2004;4:1709–22. doi: 10.1016/j.intimp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Shopp GM, Jr, McCay JA, Holsapple MP. Suppression of the antibody response by a formamidine pesticide: Dependence on the route of exposure. J Toxicol Environ Health. 1985;15:293–304. doi: 10.1080/15287398509530655. [DOI] [PubMed] [Google Scholar]
- 8.Jha GJ, Mahto LM, Tamang RK, Gupta MK, Chauhan HV. Evaluation of cell-mediated immunity during chronic organophosphate pesticide intoxication in mice and goats. Acta Vet Hung. 1990;38:55–60. [PubMed] [Google Scholar]
- 9.Rooney AA, Matulka RA, Luebke RW. Developmental atrazine exposure suppresses immune function in male, but not female Sprague-Dawley rats. Toxicol Sci. 2003;76:366–75. doi: 10.1093/toxsci/kfg250. [DOI] [PubMed] [Google Scholar]


