Abstract
Aim and Objective:
The objective was to determine the activity of methanol extract of fruit of Trichosanthes cucumerina in doxorubicin-induced cardiotoxicity in rats.
Materials and Methods:
The methanol extract of fruit of T. cucumerina was prepared. Male Wistar rats were divided in four groups. Group I was vehicle control. Group II animals received doxorubicin 4 mg/kg i.p. on days 21, 28, 35, and 42. Group III and IV animals were treated with methanol extract of T. cucumerina (500 and 1000 mg/kg, respectively) for 49 days. Doxorubicin was administered on days 21, 28, 35, and 42 days. The parameters of study were body weight, serum biomarkers, ECG, blood pressure, and left ventricular function. At the end of the study, the histology of heart, liver, and kidney was carried out.
Results:
Cardiac toxicity by doxorubicin was manifested as body weight loss, elevated serum LDH and CK-MB, increased ST, QT and QRS complex, reduced blood pressure, and left ventricular function. The methanol extract of T. cucumerina significantly decreased LDH and CK-MB, reduced ST, QT interval and QRS complex, increased heart rate, restored blood pressure, and left ventricular function. Doxorubicin caused liver and kidney necrosis, cellular infiltration, and vascular changes that indicated injury.
Conclusion:
T. cucumerina (1000 mg/kg) reduced the severity of doxorubicin-induced cardiac damage especially in heart. It is concluded that doxorubicin-induced cardiotoxicity is reduced by pretreatment with methanol extract of fruit of T. cucumerina.
Keywords: Cardiotoxicity, doxorubicin, Trichosanthes cucumerina
INTRODUCTION
Doxorubicin (DOX), an anthracycline antibiotic and is widely used as anticancer agent. In spite of its high antitumor efficacy, its use in clinical chemotherapy is limited due to its diverse toxicities, including renal, hematological, testicular and most importantly cardiac toxicity that eventually ends in cardiomyopathy and heart failure.[1,2,3] The cardiac toxic effects of DOX may occur immediately after a single dose, or several weeks to months of repetitive DOX administration. Several explanations account for the doxorubicin cardiotoxicity e.g., free radical production, calcium overloading, mitochondrial dysfunction and peroxy nitrite formation.[4]
Different plant extracts like Lagenaria sisceraria.[5] Cranbery,[6] aged garlic extract[7] and chemical agents Metallothionein,[8] alpha lipolic-acid[9] were studied for the preventive role of doxorubicin induced cardiotoxicity.
T. cucumerina linn. (Family: Cucurbitaceae) commonly named as snake gourd, padval, serpent is widely distributed in Asian countries. T. cucumerina is used in the treatment of headache, alopecia, fever, abdominal tumors, bilious, boils, acute colic, diarrhea, haematuria, and skin allergy. T. cucumerina is used as an abortifacient, vermifuge, refrigerant, purgative, malaria, laxative, hem agglutinant, emetic, cathartic, bronchitis, and anthelmintic. A novel isoflavone glucoside, 5, 6, 6’-trimethoxy-3’, 4’- methylene - dioxyisoflavone 7- O -beta- D- (2”-O-pcoumaroylglucopyranoside) has been characterized from the seeds of T. cucumerina.[10] The whole plant including root, fruit, leaf, and seed have medical properties. T. cucumerina has anti-inflammatory activity and antidiabetic activity.[11,12] It has been reported that fruit pulp of T. cucumerina has the antioxidant activity.[13] The aerial parts of T. cucumerina have both hepatoprotective[14] and gastroprotective[15] activities. T. cucumerina is rich source of proteins, fat, fibres, carbohydrates, and vitamin A and E. The chemical constituents present in T. cucumerina are cucurbitacin B, cucurbitacin E, isocucurbitacin B, 23, 24 -dihydroisocucurbitacin B, 23,24-dihydrocucurbitacin E, sterols 2 β-sitosterol, stigmasterol, α-carotene and β-carotene.[16] The presence of useful phytochemicals constituents present in the plant that possesses the potent antioxidant effect necessitates study to investigate its effect in animal models of cardiotoxicity as there is paucity of research work. The objective of the study was to determine the activity of methanol extract of TC in doxorubicin induced cardiotoxicity in rats.
The objective of the study was to determine activity of methanol extract of TC in doxorubicin induced cardiotoxicity in rats.
MATERIALS AND METHODS
Materials
Doxorubicin hydrochloride was obtained as gift sample from Intas Biopharmaceutics, Ahmedabad, India.
T. cucumerina fruit was identified and authenticated at Agharkar Research Institute, Pune, India. Whole fruit was cut into small flakes and dried on tray dryer at a temperature of 30–40°C. The dried flakes were pulverized to make fine powder. The powder was mixed with methanol (ratio 1:7) the flask was shaken at regular intervals for 7 days at room temperature. The extract was filtered through muslin cloth. The filtrate was dried on tray dryer at a temperature of 30–40°C for 48 h. Percentage yield of the extract was 22%.
Preparation of solution
Weighed quantity of methanolic extract of TC was suspended in distilled water. The route of administration was oral.
Weighed quantity of doxorubicin was dissolved in normal saline. The route of administration was intraperitoneal.
Experimental animals
The research protocol was approved by the Institutional Animal Ethics Committee (IAEC). Male Wistar rats (160–240 gm body weight) were used for the study. The animals were housed at an ambient temperature of 25±2°C and relative humidity of 50±2% and light and dark cycle (12 h light/dark). The animals had access to pellet diet (Chakan Oil Mills, Pune) and water ad libitum.
Experimental design and protocol
Thirty two male Wistar rats were divided into four groups consisting of eight animals in each group.
Group I: Control group, animals received distilled water as a vehicle for 49 days and normal saline (i.p.) on 21st, 28th, 35th, and 42nd day.
Group II: DOX group, animals received doxorubicin (4 mg/ kg, i.p) on 21st, 28th, 35th, and 42nd day.
Group III: TC 500+DOX group, animals received TC extract (500 mg/kg, p.o.) for 49 days and doxorubicin (4 mg/kg, i.p.) on 21st, 28th, 35th, and 42nd day.
Group IV: TC1000+DOX group, animals received TC extract (1000 mg/kg, p.o.) for 49 days and doxorubicin (4 mg/kg, i.p.) on 21st, 28th, 35th, and 42nd day. Total cumulative dose of DOX in Groups II, III, and IV was 16 mg/kg i.p.
Body weight parameters
Body weight of each rat was recorded weekly till the completion of 49-day study.
Serum parameters
On the last day, blood was withdrawn from the retro-orbital plexus of each rat anaesthetized with ether.
Serum was separated; lactate dehydrogenase (LDH) and creatine phosphokinase-MB isoenzyme (CK-MB) were measured by using kits (Merk Specialities Pvt. Ltd., India).
Electrocardiographic and hemodynamic parameters
All rats were fasted overnight and had free access to water after the last dose administration of the drug on 49th day of study. At the end of experiment (i.e., on day 50th) animals were anaesthetized by urethane (1.25g/kg, i.p.). ECG was recorded by the power lab instrument (AD Instruments) having LABCHART-6 pro software. Needle electrodes were inserted under the skin for the limb lead at position II in rats of each group for each ECG tracing, QRS complexes, ST interval, PR interval, and heart rate (HR) were measured.
The right carotid artery of each rat was cannulated. The cannula was filled with heparinized saline and connected to pressure transducer. After 30 min of stabilization, the hemodynamic parameters were recorded by power lab instrument. The hemodynamic parameters were systolic blood pressure (SBP), diastolic blood pressure (SBP) and mean arterial blood pressure (MABP).
After recording the hemodynamic parameters, Millar catheter was inserted in right carotid artery. After 15 min of stabilization, the parameters max dp/dt, min dp/dt and left ventricular systolic pressure (LVSP) were recorded by Power lab.
After recording of all the parameters, animals were euthanized by cervical dislocation. Heart from each rat removed and placed in 10% formaline solution. The organ specimens were subjected to dehydration with xylene (1 h each) and alcohol of strength 70%, 90%, and 100%, respectively each for 2 h. The infiltration and impregnation was carried out twice by treatment with paraffin, each time for 1 h. Paraffin wax was used to prepare paraffin L molds. Specimens were cut into section of 3-5mm thickness and stained with hematoxylin and eosin (H and E stain). The sections were mounted by diestrene phthalate xylene (DPX).
Heart, liver, and kidney section were assessed for histopathology parameters including inflammation, necrosis, coagulation, and cellular infiltration. The grading system used for assessment of parameters was (00: No changes, +: Less than 25% changes, ++: Less than 50% changes, +++: Less than 75% changes and ++++: More than 75% changes).
Statistical analysis
Data was expressed as mean±SEM. Statistical analysis was carried out by one-way ANOVA followed by post-Tukey's test and two-way ANOVA followed by post-Bonferroni test for parameters using GraphPad InStat version 5.00 for Windows Vista™ BASIC, GraphPad Software, SanDiego, CA, USA.
RESULTS
Body weight parameter
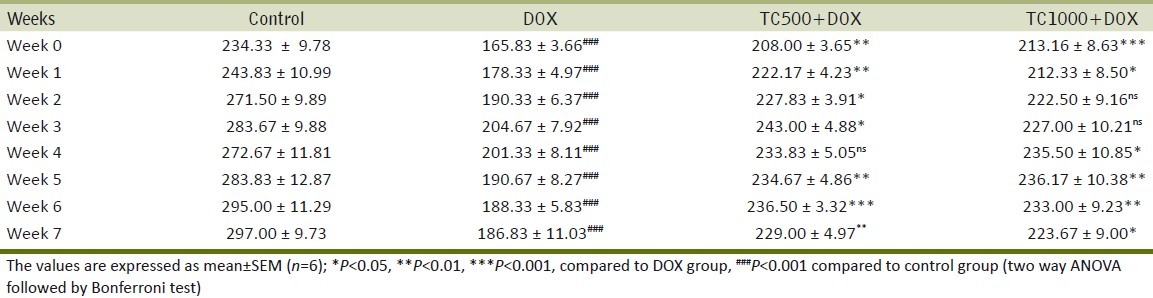
Animals of the DOX group showed significant decrease compared to the control group. In the T. cucumerina Groups III and IV body weight of animals significantly increased compared to DOX Group II [Table 1].
Table 1.
Body weight of control, DOX and TC treated rats
Serum parameters
Dox group showed significant increase in LDH and CK-MB compared to control. Rats treated with TC500 and TC1000 showed significant decrease in LDH and CK-MB compared to DOX group. The results thus indicated effectiveness of T. cucumerina in preventing rise of CK-MB and LDH by doxorubicin [Table 2].
Table 2.
LDH and CK-MB in control, DOX and TC treated rats

ECG parameters
Significantly increase of ST interval, QT interval and QRS complex compared to control was observed in DOX group. In the T. cucumerina Groups III and IV ST interval, QT interval, and QRS complex were significantly decreased compared to DOX group [Table 3].
Table 3.
ECG parameters recorded in control, DOX and TC treated rats

Doxorubicin decreased the heart rate compared to control group. Pretreatments with TC500 and TC1000 increased heart rate significantly (P<0.05 and P<0.01) compared to the DOX group [Table 3].
Hemodynamic parameters
DOX reduced SBP, DBP, and MABP nonsignificantly compared to the control group. T. cucumerina treatment restored the SBP, DBP, and MABP. However, dose-dependent effect was not observed [Table 4].
Table 4.
Effect of DOX and TC on blood pressure

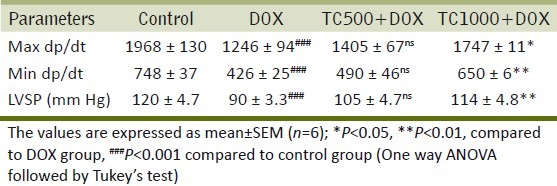
The max dp/dt, min dp/dt and LVSP decreased significantly in the DOX group compared to control, whereas in rats treated with T. cucumerina (1000 mg/kg, group IV) significant increase in max dp/dt (P<0.05), min dp/dt (P<0.01), and LVSP (P<0.01) was observed compared to the DOX group [Table 5].
Table 5.
Effect of DOX and TC on left ventricular function
Histopathology parameters
The histopathology of heart in control group did not show any remarkable change. Doxorubicin administration showed myocardial necrosis, hyperemia, and cellular infiltration. The heart of TC500 and TC1000 showed decreased myocardial necrosis, hyperemia and cellular infiltration [Figure 1] [Table 6].
Figure 1.

Photomicrographs of histological changes of rat heart. Red arrow showing hypermia, black arrow showing necrosis, blue arrow showing cellular infiltration. H and E stain ×400
Table 6.
Assessment of toxicity in heart of various groups of rats

DISCUSSION
Doxorubicin (DOX; Adriamycin) is an excellent antitumor drug for treating several types of solid cancer, leukemia and lymphomas. Acute and chronic toxicity are the major limiting complication. Acute cardiotoxicity represented symptoms, such as arrhythmias while chronic toxicity can develop into irreversible cardiomyopathy, which affects approximately 30–40% of the patients who receive 500 mg/mm2 total dose.[17] DOX is toxic agent for all cellular components including genetic material, kidney, heart and liver and genetic material of myocardial tissues. DOX causes acute renal failure that is a limiting factor of its usefulness. Therefore, novel therapeutic agents within proved efficacy seem to be considerable for clinical approach.[18]
From cardiac hypertrophy to heart failure, activation of compensatory mechanisms including the sympathetic nerve system and the rennin angiotensin system takes place. These responses collectively lead to extensive biochemical, physiological and molecular changes.[1] For that reason we have selected doxorubicin induced cardiac toxicity.
The animals were treated with Doxorubicin permits a marked exposure of bone marrow cells. Although chemotherapy affects virtually every organ system in the body, the cell population typically exhibit rapid cell turnover, such as those of the bone marrow and gastrointestinal mucosa.[19] However, the majority of authors dealing with this problem consider that cardiomyopathy and nephropathy makes the most important contribution to the mortality of experimental animals after long-term treatment of DOX. In our experiment, excessive fluid accumulation was found in pleural, pericardial and peritoneal cavities rats treated with DOX. Intra peritoneal administration of DOX at the dose of 20 mg/ kg induced cardiac toxicity, characterized by oxidant injury[18] as well as dose of 25 mg/kg and 18 mg/kg.[17] also induced cardiac toxicity. In the current study, doxorubicin was injected four times in equal doses, resulting in total cumulated dose of 16 mg/kg. Our results showed that 16 mg/kg cumulative dose of i.p. DOX produced signs of cardiac toxicity in rats. The elevation of the level of different enzymes by doxorubicin treatment is probably a reflection that the drug induces cardiac toxicity, whereas LDH and CK-MB are rather specific for myocardial damage.[17]
In this study, DOX caused increase in the activities of all these enzymes in plasma. They are not specific for myocardial injury individually however; evaluation of these enzymes together may be an indicator of myocardial injury.[18] These results agreed with previous doxorubicin induced cardiac toxicity studies demonstrated by various scientists.[6,7,20] In our experiment the significant increase in LDH and CK-MB caused by DOX was decreased in treatment group TC500 and TC1000.
Another form of doxorubicin cardiotoxicity is arrhythmia that may occur at any time and after any dosage.[21] We have noted the significant increase in ST, QT and QRS interval in only DOX group. ECG changes are related to the prolongation of action potential duration, but it is considered that the recovery phase of the transmembrane action potential is most prominently affected with DOX, influencing preferentially Ca2+ movements across the cellular membrane.[22] In the TC500 and TC1000 group DOX administration did not increase ST, QT and QRS interval.
Doxorubicin is an inhibitor of both systolic and diastolic cardiac function. Furthermore, metabolite doxorubicinol is a more potent inhibitor than doxorubicin of three ion pumps. Moreover, doxorubicinol abolished calcium loading activity of cardiac sarcoplasmic reticulum vesicles. Doxorubicin is known to cause decrease in heart functionality in both human and laboratory animals.[23] In present study decrease in systolic and diastolic pressure, max dp/dt, min dp/dt and LVSP was observed in DOX group that was reversed in treatment group of TC500 and TC1000.
TC contains cucurbitacin B, cucurbitacin E, carotenoids and ascorbic acid that are reported to have antioxidant activity.[24–27] Antioxidants by delay the oxidation of other molecules by inhibition of oxidative chain reaction by free radicals and also reduce oxidative damage.[28] The oxidative damage may result in development of cancer and cardiovascular disease.[29] TC contains many different antioxidant components that provide protection against harmful free radicals that are strongly associated with reduced risk of cardiovascular diseases.
CONCLUSION
It is concluded that TC at the doses of 500 and 1000 mg/ kg has a potential cardioprotective activity by reducing doxorubicin induced cardiotoxicity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Adebooye OC. Phyto-constituents and anti-oxidant activity of the pulp of snake tomato (Trichosanthes cucumerina L.) Afr J Tradit Complement Altern Med. 2008;5:173–9. doi: 10.4314/ajtcam.v5i2.31270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Majed AA, Gdo AM, Al-Shabanah OA, Mansour MA. Alpha-lipolic acid ameliorate myocardial toxicity induced by doxorubicin. Pharmacol Res. 2002;46:499–503. doi: 10.1016/s1043661802002311. [DOI] [PubMed] [Google Scholar]
- 3.Arawwawala M, Thabrew I, Arambewela L, Handunnetti S. Anti-inflammatory activity of Trichosanthese cucumerina Linn. in rats. J Ethanopharmacol. 2010;131:538–43. doi: 10.1016/j.jep.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Arawwala M, Thabrew I, Arambewela L. Gastroprotective activity of Trichosanthese cucumerina in rats. J Ethanopharmacol. 2010;127:750–54. doi: 10.1016/j.jep.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Ateşşahin A, Karahan I, Türk G, Gür S, Yilmaz S, Ceribaşi AO. Protective role of lycopene on cisplatin-induced changes in spermcharacteristics, testicular damage and oxidative stress in rats. Reprod Toxicol. 2006;21:42–7. doi: 10.1016/j.reprotox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology. 2005;212:116–23. doi: 10.1016/j.tox.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 7.De Beer EL, Bottone AE, Voest EE. Doxorubicin and mechanical performance of cardiac trabeculae after acute and chronic treatment: A review. Eur J Pharmacol. 2001;415:1–11. doi: 10.1016/s0014-2999(01)00765-8. [DOI] [PubMed] [Google Scholar]
- 8.Elberry AA, Abdel-Naim AB, Abdel-Sattar EA, Nagy AA, Mosli HA, Mohamadin AM, et al. Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol. 2010;48:1178–84. doi: 10.1016/j.fct.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Hassanpour F, Bodhankar SL, Dikshit M. Cardioprotective activity of fruit of Lagenaria siceraria (Molina) Standley on doxorubicine induced cardiotoxicity in rats. Int J Pharmacol. 2008;4:466–471. [Google Scholar]
- 10.Alkreathy H, Damanhouri ZA, Ahmed N, Slevin M, Ali SS, Osman AM. Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol. 2010;48:951–6. doi: 10.1016/j.fct.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 11.James YK. Molecular and cellular mechanism of carditoxicity. Environ Health Perspect. 2001;109:27–33. doi: 10.1289/ehp.01109s127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang B, Zhang L, Li M, Wu W, Yang M, Wang J, et al. Salvinolic acids prevent acute doxorubicin cardiotoxicity in mice through suppression of oxidative stress. Food Chem Toxicol. 2008;46:1510–5. doi: 10.1016/j.fct.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Kirana H, Srinivasan BP. Trichosanthese cucumerina Linn improves glucose tolerance and tissue glucose in noninsulin dependent diabetes mellitus induced rats. Indian J Pharmacol. 2008;40:103–6. doi: 10.4103/0253-7613.42301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathesh Kumar S, Ravi Kumar B, Krishna Mohan G. Hepatoprotective effect of Trichosanthes cucumerina Var cucumerina L.on carbon tetrachloride induced liver damage in rats. J Ethanopharmacol. 2009;123:347–50. doi: 10.1016/j.jep.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Lindley MG. The impact of food processing on antioxidants in vegetable oils, fruits and vegetables. Trend Food Sci Technol. 1998;9:336–40. [Google Scholar]
- 16.Antunes LM, Takahashi CS. Effects of high doses of vitamins C and E against doxorubicin-induced chromosomal damage in wistar rat bone marrow cells. Mutat Res. 1998;419:317–43. doi: 10.1016/s1383-5718(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 17.Yagmurca M, Fadillioglu E, Erdogan H, Ucar M, Sogut S, Irmak MK. Erdosteine prevents doxorubicin-induced cardiotoxicity in rats. Pharmacol Res. 2003;48:377–82. doi: 10.1016/s1043-6618(03)00185-3. [DOI] [PubMed] [Google Scholar]
- 18.Namiki M. Antioxidant/antimutagens in food. Crit Rev Food Sci Nutr. 2009;29:273–300. doi: 10.1080/10408399009527528. [DOI] [PubMed] [Google Scholar]
- 19.Olson RD, Mushlin PS, Brenner DE, Fleischer S, Cusack BJ, Chang BK, et al. Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci U S A. 1998;85:3585–9. doi: 10.1073/pnas.85.10.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osman AM, Nemnem MM, Abou-Bakr AA, Nassier OA, Khayyal MT. Effect of methimazole treatment on doxorubicin-induced cardiotoxicity in mice. Food Chem Toxicol. 2009;47:2425–30. doi: 10.1016/j.fct.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Reddy LJ, Jose B, Anjana JC, Ruveena TN. Evaluation of antibacterial activity of Trichosanthes cucumerina l. and Cassia didymobotrya fres Leaves. Int J Pharm Pharma Sci. 2010;2:153–55. [Google Scholar]
- 22.Kilickap S, Akgul E, Aksoy S, Aytemir K, Barista I. Doxorubicin induced second degree and complete atrioventricular block. Europace. 2005;7:227–30. doi: 10.1016/j.eupc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Sandhya S, Vinod KR, Chandra Sekhar J, Aradhana R, VamshiSarathNath An updated review on Tricosanthes cucumerina. Int J of Pharma Sci. Rev. Res. 2010;1:56–60. [Google Scholar]
- 24.Sarada S, Dipti P, Anuj B, Pauline T, Kain AK, Sairam M, et al. Antioxidant effect of beta- carotene on hypoxia induced oxidative stress in male albino rats. J Ethnopharmacol. 2002;79:149–53. doi: 10.1016/s0378-8741(01)00360-9. [DOI] [PubMed] [Google Scholar]
- 25.Shuai Y, Guo JB, Peng SQ, Zhang LS, Guo J, Han G, et al. Metallothionein protects against doxorubicin-induced cardiomyopathy through inhibition of superoxide generation and related nitrosative impairment. Toxicol Lett. 2007;170:66–74. doi: 10.1016/j.toxlet.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Tehila T, Margalit B, Shlomo G. Cucurbitacin glycoside: Antioxidant and free-radical scavenging activities. Biochem Biophys Res Commun. 2007;364:181–6. doi: 10.1016/j.bbrc.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 27.Teow CC, Truong V, Mcfeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007;103:829–38. [Google Scholar]
- 28.Hou XW, Jiang Y, Wang LF, Xu HY, Lin HM, He XY, et al. Protective role of granulocyte colony-stimulating factor against adriamycin induced cardiac, renal and hepatic toxicities. Toxicol Lett. 2009;187:40–4. doi: 10.1016/j.toxlet.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Hamauzu Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional andmicrowave cooking. Food Chem. 2004;88:503–9. [Google Scholar]


