Abstract
Arsenic-contaminated areas of Sanganer, Jaipur, Rajasthan, India were surveyed for the presence of metal resistant bacteria contaminated with textile effluent. Samples were collected from soil receiving regular effluent from the textile industries located at Sanganer area. The properties like pH, electrical conductivity, organic carbon, organic matter, exchangeable calcium, water holding capacity and metals like arsenic, iron, magnesium, lead and zinc were estimated in the contaminated soil. In total, nine bacterial strains were isolated which exhibited minimum inhibitory concentration (MIC) of arsenic ranging between 23.09 and 69.2mM. Four out of nine arsenic contaminated soil samples exhibited the presence of arsenite hyper-tolerant bacteria. Four high arsenite tolerant bacteria were characterized by 16S rDNA gene sequencing which revealed their similarity to Microbacterium paraoxydans strain 3109, Microbacterium paraoxydans strain CF36, Microbacterium sp. CQ0110Y, Microbacterium sp. GE1017. The above results were confirmed as per Bergey's Manual of Determinative Bacteriology. All the four Microbacterium strains were found to be resistant to 100μg/ml concentration of cobalt, nickel, zinc, chromium selenium and stannous and also exhibited variable sensitivity to mercury, cadmium, lead and antimony. These results indicate that the arsenic polluted soil harbors arsenite hyper-tolerant bacteria like Microbacterium which might play a role in bioremediation of the soil.
Keywords: Arsenic, bacteria, hyper-tolerance, metal-resistant
INTRODUCTION
Industrial uses of metals have generated large amount of aqueous effluents that contain high levels of heavy metals. At present, metal-polluted industrial effluents are mostly treated by the chemical methods, such as chemical precipitation, electrochemical treatment and ion exchange.[1] These methods provide only partially effective treatment and are expensive. Bioremediation of heavy metals using microorganisms has received a great deal of attention in recent years not only as a scientific novelty but also for its potential application in the industry.[2]
Arsenic is one of the most abundant toxic metals present in the environment. The average abundance of arsenic in the earth crust is 1.8 ppm; in soil it is 5.5 to 13ppm; in streams it is less than 2μg/l and in ground water it is generally less than 100 μg/l.[3] The major concern of arsenic enriched environment is its potential for mobilization and transport to ground water and thereby polluting drinking water supply.[4] The ubiquity of arsenic in the environment has led to the evolution of arsenic defense mechanism in microbes. Most studies of arsenic resistant bacteria have been conducted in environmental locations, which contain high concentrations of arsenic like Deinococcus indicus and Bacillus indicus from aquifers at West Bengal, India, Staphylococcus and Citricoccus from gold mine reactor.[5] However, research shows that common microorganisms such as E.coli, Pseudomonas aerugenosa and Staphylococcus aureus also exhibit arsenic resistance.
The objective of the present study was to isolate and identify bacteria which possess tolerance to high concentrations of arsenite from heavily polluted soil (receiving effluent from textile industries).
MATERIALS AND METHODS
Soil collection
Nine soil samples were collected from the selected sites receiving effluent from the textile (printing and dying) industries situated at Sanganer area, Jaipur (Rajasthan, India) at different distances (100-200 m) from the source of effluent discharge and at various depths (10,20,30,40-60 cm), in a previously ethanol cleaned polypropylene zip lock bags. The soil samples were stored at 4°C for physico-chemical characterization. The microbial analysis was started within 2 h of sample collection.
Soil properties and chemical analysis
The soil pH was measured by pH meter (Electronic India, digital pH meter model-III E). Electrical conductivity was measured in the supernatant with the help of conductivity meter (Elico CM-180). Organic carbon and organic matter were determined by rapid dichromate oxidation method. Exchangeable calcium was determined by EDTA titrimetry method. Water holding capacity was estimated with 50 g of oven dried (105°C) soil.[3,6]
Metal estimation
Soil samples were digested using microwave digestion system (Anton Paar Mutiwave 3000) for metal estimations by atomic absorption spectrophotometer (model A Analyst 100; Perkin Elmer).
Isolation of arsenite resistant bacteria
Soil dilution technique was used to isolate arsenite resistant bacteria. Nine resistant strains were isolated from soil samples by culturing them in nutrient broth followed by nutrient agar supplemented with 1g/l of sodium arsenite and incubated at 37°C for 24-48 h at 120 rpm (for Nutrient Broth).The pure colonies were obtained with repeated spreading and streaking . The strains were preserved in 15 % v/v of glycerol and 24 h Nutrient broth culture at 4°C.
Estimation of minimum inhibitory concentration (MIC) of arsenite resistant bacteria
The pure bacterial colonies were inoculated in nutrient broth supplemented with increasing doses of sodium arsenite (1-9 g/l) at 120 rpm, 37°C for 24, 48 and 72 h of incubation. The MIC of each bacterial strain was determined on the basis of negligible growth in terms of optical density at 600nm.[7]
Identification of bacterial isolates based on 16 S rDNA sequencing
Four arsenite hyper-tolerant bacteria were used for further study. DNA was isolated from the culture using QIAamp DNA Purification Kit (Qiagen).Its quality was evaluated on 1.2% Agarose Gel, a single band of high-molecular weight DNA was observed. The 16S rDNA gene fragment was amplified by PCR from genomic DNA using 16S rDNA gene universal primers: 8F and 1492R[8,9] 8F: (5’ AGA GTT TGA TCC TGG CTC AG 3’), 1492R: (5’ ACG GCT ACC TTG TTA CGA CTT 3’) and a single discrete PCR amplicon band of 1500 bp was observed when resolved on Agarose Gel [Figure 1]. The PCR amplicon was purified using Qiagen Mini elute Gel extraction kit according to the manufactures protocol to remove contaminants.
Figure 1.
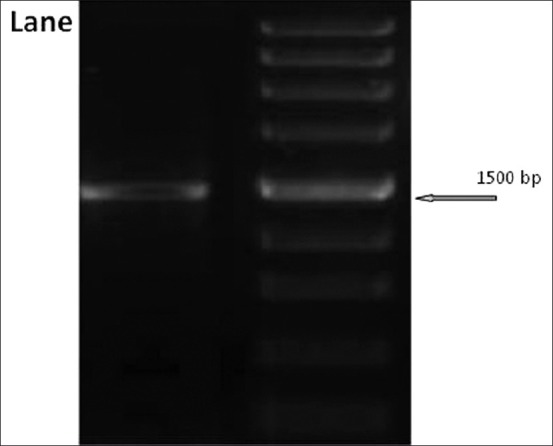
Gel Image of 16S rDNA amplicon Lane 1: 16S rDNA amplicon band Lane 2: DNA Marker
The concentration of the purified DNA was determined and was subjected to automated DNA sequencing on ABI 3730xl Genetic Analyzer (Applied Biosystems, USA). Sequencing was carried out using BigDye® Terminator v3.1 Cycle sequencing kit, following manufacturer's protocol. Electrophoresis and data analysis was carried out on the ABI 3730xl Genetic Analyzer using appropriate Module, Basecaller, Dyeset/Primer and Matrix files. Consensus sequence of 1403 bp for IB1and IK1, 1412 bp for IM2 and reverse sequence of 787 bp for IR-1 rDNA gene was generated from forward and reverse sequence data using aligner software. The 16S rDNA gene sequence was used to carry out BLAST with the nrdatabase of NCBI genebank database. Based on maximum identity score, first ten sequences were selected and aligned using multiple alignment software program Clustal W and Distance matrix was generated using RDP database. The evolutionary history was inferred using the Neighbor-Joining method.[10] The bootstrap consensus tree inferred from 500 replicates was taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches[11] [Figure 2]. The evolutionary distances were computed using the Kimura 2-parameter method[12] and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (complete deletion option). Phylogenetic analyses were conducted in MEGA4[13] [Figure 2]. To support the 16S rDNA sequencing results, biochemical tests were performed for Microbacterium using Bergeys Manual of Determinative Bacteriology, (1994).
Figure 2.
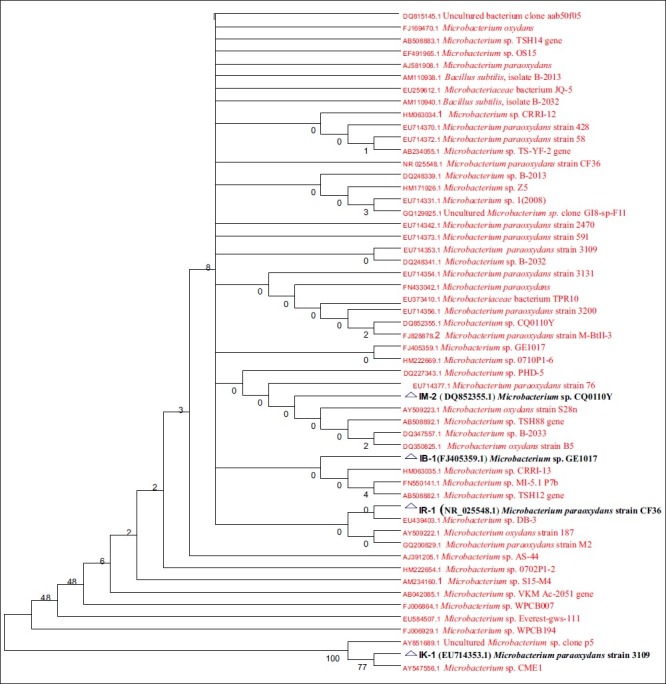
Phylogenetic tree based on 16S rDNA gene sequencing of the isolated arsenite resistant strains (IM-2, IB-1, IR-1, IK-1)
Metal Sensitivity Test
For the metal sensitivity test, bacterial lawns on nutrient agar media were treated with absorbent discs (Whatman No.1) soaked overnight in deionised solution containing 100μg/ml of different metal salts. The plates were incubated for 48 h at 37°C. The zone of inhibition produced by salts was measured and scored as very resistant (>2.0 mm), moderately resistant (2.0-10.0 mm) or sensitive (>10.0mm).
RESULTS
Soil properties and chemical analysis
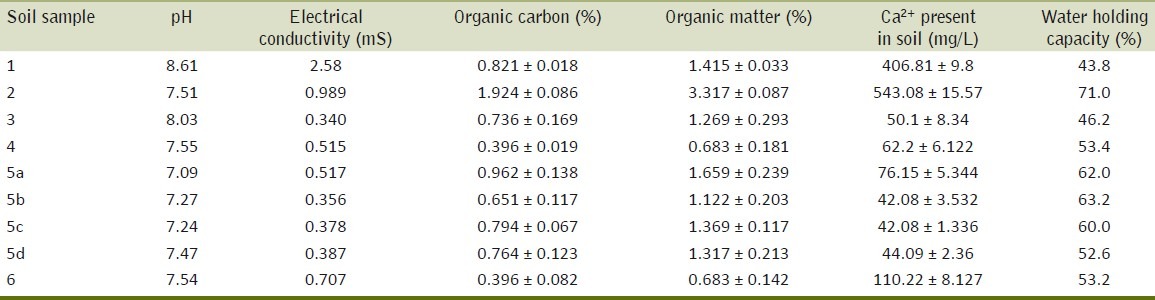
The physicochemical properties of the contaminated soil are summarized in Table 1. The table shows the pH ranged between 7.09 and 8.61, electrical conductivity between 2.58 and 3.40 mS., organic carbon ranged between 0.396 and 1.924% and while organic matter from 0.683 to 3.317%. Exchangeable Ca2+ ranged from 42.08 to 543.08 mg/l. Water holding capacity was observed between 43.8-71.0% in the soil samples. The sample#1 (within 100 m of source of effluent discharge, depth 0-10 cm) showed highest pH and electrical conductivity while the sample#2 (within 100 m of textile discharge, depth 0-20 cm) showed highest organic carbon, organic matter along with highest exchangeable Ca2+ and water holding capacity.
Table 1.
Properties and chemical analysis of soil contaminated with textile effluent
Metal estimation
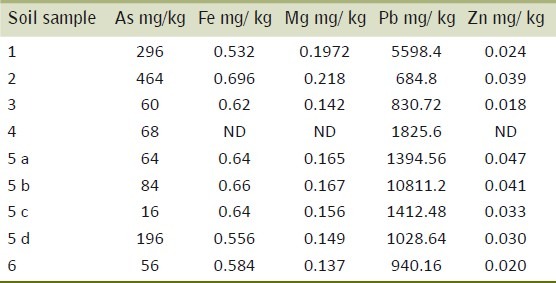
The soil samples exhibited high arsenic and lead concentrations (16.0-464.0 mg/kg and 684.8-10811.2 mg/kg respectively) while iron and magnesium ranged between 0.532-0.696 and 0.137-0.218 mg/kg. Zinc was at the lowest (0.018-0.047 mg/kg) [Table 2].
Table 2.
Metal estimation of the soil contaminated with textile effluent
Arsenite resistant bacteria
Nine arsenite resistant bacterial strains were isolated from soil samples 4, 5a, 5b and 5c collected at the depth 10-40 cm and distance of 200 m from textile effluent. These soil samples exhibited presence of iron, zinc, lead and magnesium as well as showed near neutral pH and medium range of electrical conductivity, exchangeable calcium and water holding capacity [Tables 1 and 2]. These soil samples were also found contaminated with arsenic ranging between 16 and 68 mg/kg of soil.
Identification
Bacterial colonies were yellow, cream or white colored having circular shape with smooth or lobulated margins. Eight bacterial strains were Gram positive whereas one was Gram negative bacilli [Table 3].
Table 3.
MIC, morphological studies and staining properties of the isolated strains
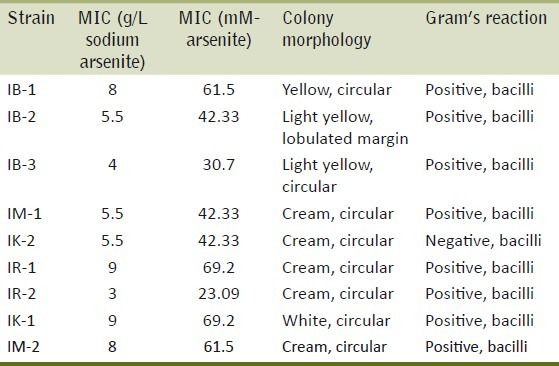
Minimum inhibitory concentration (MIC)
The isolated resistant strains exhibited MIC in range of 3-9 gm/l (23.09-69.2mM) [Table 3]. Four arsenite hyper-tolerant bacteria (IB-1, IR-1, IK-1, IM-2) were selected for the further study on the basis of highest MIC as 8, 9, 9, 8 gm/l respectively of sodium arsenite. Based on nucleotide homology and phylogenetic analysis of the 16S rDNA gene sequence in Figure 1, the bacterial strain IB-1 with MIC of 8 gm/l was found to be Microbacterium sp. GE1017 (GenBank Accession Number: FJ405359.1), IR-1 with MIC of 9 gm/l was identified as Microbacterium paraoxydans strain CF36 (GenBank Accession Number: NR_025548.1), IK-1 with MIC of 9gm/l was identified as Microbacterium paraoxydans strain 3109 (GenBank Accession Number: EU714353.1) and IM-2 with MIC of 8 gm/l was identified as Microbacterium sp. CQ0110Y (GenBank Accession Number: DQ852355.1) [Figure 2].
Biochemical characterization
The selected four bacterial strains were tested for their morphological and biochemical properties. The four strains appeared as slender rods in young cultures arranged singly or in pairs, some arranged at an angle to give V formations. These were Gram positive, non acid fast, nonsporing, aerobic and metabolism primarily respiratory, acid not produced from carbohydrates (sucrose, dextrose, xylose), catalase positive, oxidase and nitrate reductase negative.
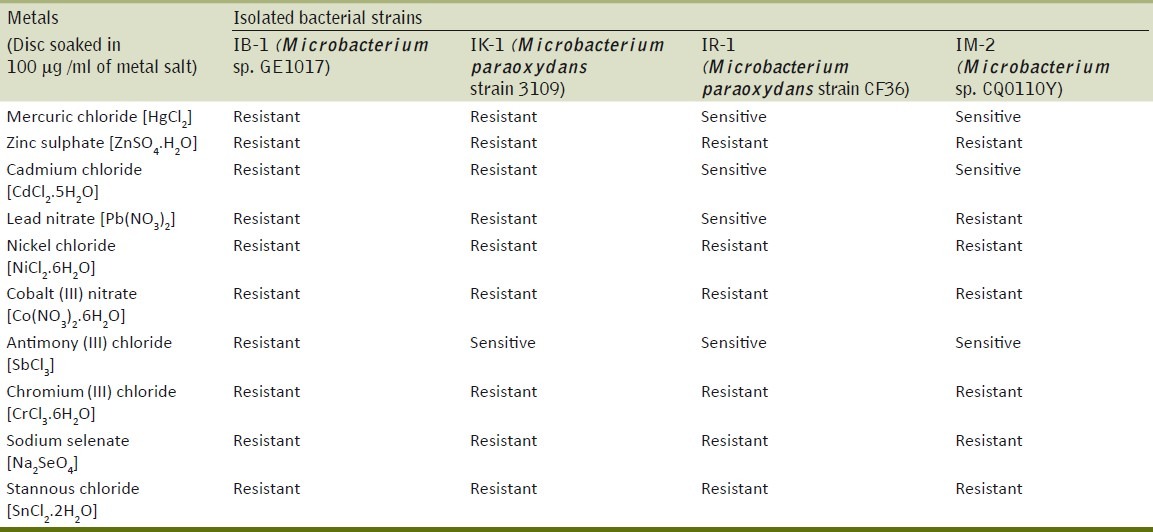
Metal sensitivity test
The strains (IM-2, IB-1, IR-1, IK-1) have been isolated from soil contaminated with arsenic, lead, zinc and iron exhibit resistance not only to arsenite but also to heavy metals like cobalt, nickel, zinc, chromium, selenium and stannous (100μg/ml). The strain IR-1 was found to be sensitive to mercury, cadmium and antimony and IM-2 exhibited sensitivity to mercury, cadmium and antimony [Table 4]. This polymetal resistance can be explained as adaptation to the polluted soils.
Table 4.
Plate diffusion method used to assay the tolerance of arsenic (III) resistant bacteria to metals
DISCUSSION
The results revealed that soil samples exhibited high arsenic and lead concentrations while iron and magnesium were present at lower concentration with zinc at the lowest concentration. As the pH of the contaminated soil was near neutral, it is possible that arsenite is in unionized form. Thus it can passively move across the membrane bilayer or can be transported by a carrier protein similar to those that transport unionized organic compounds.[14]
Nine arsenite resistant bacterial strains were isolated from soil samples (4, 5a, 5b and 5c). The possible reason for the occurrence of arsenite hyper-tolerant bacteria from soil might be favorable conditions of growth in medium range of pH, calcium and electrical conductivity and development of adaptation to arsenic. Microbial resistance to arsenite has been frequently observed from various arsenic contaminated sites.[5,15]
Arsenite-resistant Microbacterium species was detected to tolerate arsenite up to 80 mM from arsenic contaminated site.[16] These arsenite resistance Microbacterium might play a role in the biogeocycling of arsenic in the environments. The strains isolated from soil exhibit resistance not only to arsenite but also to heavy metals like cobalt, nickel, zinc, chromium, selenium and stannous. The strain IR-1 was found to be sensitive to mercury, cadmium and antimony and IM-2 exhibited sensitivity to mercury, cadmium and antimony. This polymetal resistance can be explained as adaptation to the polluted soils. The dissimilarity between the level of resistance exhibited by the isolated Microbacterium strains and the reported strains may have resulted from the variations in the levels of metal contamination, source of contamination, period of metal exposure, the characteristics of soil and variations of metal bioavailability.
The isolates may have developed metal resistance systems in an attempt to protect sensitive cellular components. The mechanisms by which microorganism exhibited resistance include exclusion of metal ions, extra cellular precipitation, binding of metal ions to the outer surface of bacteria, enzymatic transformation, precipitation by oxidation/reduction reaction and biosynthesis of metal binding proteins or extracellular polymers, whereas environmental factors may include pH and redox potential, metal speciation, soil particulates, and soluble organic matters.[17]
Although arsenic is generally toxic to living organisms, it has been reported that microorganisms utilize arsenic compounds as electron donors or electron acceptors, and can possess arsenic detoxification mechanisms.[18] Bacteria evolved a variety of mechanisms for coping with arsenic toxicity, including minimizing the amount of arsenic that enters the cell (e.g. through increased specificity of phosphate uptake), oxidizing the arsenite (through the activity of arsenite oxidase), or arsenite peroxidation with membrane lipids. Resistance to arsenic species in both Gram-positive and Gram-negative organisms results from energy-dependent efflux of either arsenate or arsenite from the cell, mediated by the ars operon.[19] The existence of a bacterium with an arsC gene that is responsible for the conversion of As(V) to As(III), which may be either extruded from the cells or sequestered in the intracellular compartment in its free form and/or in conjugation with glutathione (GSH) or other thiols has been confirmed earlier.
The results revealed that soil receiving textile effluents of Sanganer area, Jaipur are highly contaminated with different metals like arsenic iron, magnesium, lead and zinc. Metal resistant bacteria can have potential to remove these metals from effluents so that they are less available and in turn less toxic.[20] It is suggested that metal accumulation ability of these resistant species should be evaluated. We hypothesized that these arsenite tolerant bacteria could possess transformation ability and could represent a good candidate for bioremediation of native polluted soil. Future studies will be needed to elucidate the role of these metal resistant bacteria in view of their potentially practical exploitation.
ACKNOWLEDGMENT
We thank Director, DRDE, Gwalior, for the financial assistance and Xcelris Lab, Ahmedabad, India for providing the 16S rDNA sequencing and phylogenetic analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wong MF, Chua H, Lo W, Leung CK, Yu PH. Removal and recovery of copper (II) ions by bacterial biosorption. Appl Biochem Biotechnol. 2001;91-93:447–57. doi: 10.1385/abab:91-93:1-9:447. [DOI] [PubMed] [Google Scholar]
- 2.Lei W, Chua H, Lo WH, Yu PH, Zhao YG, Wong PK. A novel magnetite--immobilized cell process for heavy metal removal from industrial effluent. Appl Biochem Biotechnol. 2000;84-86:1113–26. doi: 10.1385/abab:84-86:1-9:1113. [DOI] [PubMed] [Google Scholar]
- 3.Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58:201–35. [PubMed] [Google Scholar]
- 4.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51:257–81. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Suresh K, Prabagaran SR, Sengupta S, Shivaji S. Bacillus indicus sp. nov., an arsenic-resistant bacterium isolated from an aquifer in West Bengal, India. Int J Syst Evol Microbiol. 2004;54:1369–75. doi: 10.1099/ijs.0.03047-0. [DOI] [PubMed] [Google Scholar]
- 6.Maiti SK. Analysis of Chemical Parameters of soil.In book: Handbook of Methods in Environmental Studies: Air, Noise, Soil and Overburden analysis. Vol. 2. Jaipur, India: ABD Publishers; 2003. pp. 162–209. Chapter 11. [Google Scholar]
- 7.Courvalin P, Goldstein F, Philippon A, Sirot J. Fiches techniques d’étude pratique des antibiotiques. In L’antibiogramme. 1st ed. Paris: MPC-Videom; 1985. pp. 237–44. [Google Scholar]
- 8.Sacchi CT, Whitney AM, Mayer LW, Morey R, Steigerwalt A, Boras A, et al. Sequencing of 16S rRNA gene: a rapid tool for identification of Bacillus anthracis. Emerg Infect Dis. 2002;8:1117–23. doi: 10.3201/eid0810.020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniatis T, Sambrook J, Fritsch EF. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 10.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 12.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 14.Salam MA, Hossain MS, Ali ME, Asad MA, Ali MH. Isolation and characterization of arsenic resistant bacteria from different environment in South--West region of Bangladesh. Res J Environ Sci. 2009;3:110–5. [Google Scholar]
- 15.Dave SR, Gupta KH, Tipre DR. Diversity of arsenite--resistant cocci isolated from Hutti Gold Mine and bioreactor sample. Curr Sci. 2010;98:1229–33. [Google Scholar]
- 16.Chen S, Shao Z. Isolation and diversity analysis of arsenite-resistant bacteria in communities enriched from deep--sea sediments of the Southwest Indian Ocean Ridge. Extremophiles. 2009;13:39–48. doi: 10.1007/s00792-008-0195-1. [DOI] [PubMed] [Google Scholar]
- 17.Patel PC, Goulhen F, Boothman C, Gault AG, Charnock JM, Kalia K, et al. Arsenate detoxification in a Pseudomonad hypertolerant to arsenic. Arch Microbiol. 2007;187:171–83. doi: 10.1007/s00203-006-0182-9. [DOI] [PubMed] [Google Scholar]
- 18.Anderson CR, Cook GM. Isolation and characterization of arsenate--reducing bacteria from arsenic-contaminated sites in New Zealand. Curr Microbiol. 2004;48:341–7. doi: 10.1007/s00284-003-4205-3. [DOI] [PubMed] [Google Scholar]
- 19.Cai J, Salmon K, DuBow MS. A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology. 1998;144:2705–13. doi: 10.1099/00221287-144-10-2705. [DOI] [PubMed] [Google Scholar]
- 20.Joshi DN, Patel JS, Flora SJ, Kalia K. Arsenic accumulation by Pseudomonas stutzeri and its response to some thiol chelators. Environ Health Prev Med. 2008;13:257–63. doi: 10.1007/s12199-008-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


