Abstract
Cyclophosphamide (CYC) as an anticancer alkylating agent has been known as a male reproductive toxicant. This study was aimed to evaluate the protective effect of rutin (RUT) on CYC-induced reproductive toxicity. Sexually mature Wistar rats (weighing 199 ± 10 g with five animals in each group) were given CYC (15 mg/kg) and/or RUT (30 mg/kg) twice a week via gavage for 4 weeks. The sperm counts, sperm motility, sperm morphology, daily sperm production (DSP), testicular, and epididymal antioxidant systems: superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), glutathione reductase (GR), glutathione-S-transferase (GST), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and testicular steroidogenic enzymes (3β-hydroxysteroid dehydrogenase and 17β-HSD and spermatogenesis marker enzymes (lactate dehydrogenase (LDH), sorbitol dehydrogenase (SDH), alkaline phosphatase (ALP), acid phosphatase (ACP) in the testes, epididymis and seminal vesicles were investigated at the end of the fourth week. By the end of the fourth week, RUT prevented lower sperm counts, sperm motility, DSP, and higher abnormal sperm numbers induced by CYC. In testes, RUT decreased SOD, LDH, and SDH and increased CAT, 3β-HSD, 17β-HSD, ALP, and ACP induced by CYC. In epididymis, RUT increased SOD, CAT, GSH, GSH-Px, GR, GST SDH, ALP and ACP and decreased MDA and LDH induced by CYC. In seminal vesicles, marker enzymes were unchanged in rats given CYC alone or in combination with RUT. It appears that RUT ameliorates CYC reproductive toxicity at the investigated dose.
Keywords: Antioxidant system, cyclophosphamide, rats, reproductive toxicity, rutin, sperms
INTRODUCTION
More than 50% of male patients receiving chemotherapy for several solid tumors sustain severe and sometimes irreversible damage to the seminiferous epithelium.[1] Since many of these patients are treated with chemotherapeutic agents before and during their reproductive years, and cure rates for several types of cancers are high, sterility caused by treatment is a very significant concerned.[2] The cytotoxic effects of cyclophosphamide and other chemotherapeutic drugs result in part from their interaction with DNA leading to defective DNA, abnormal cell function and cell death.[3] However, cyclophosphamide is not specific for cancer cells and will affect all dividing cells, including those in the immune system, reproductive systems and the gastrointestinal tract. Cyclophosphamide interfares with the formation of eggs in the ovaries and the formation of sperm in the testicles and may cause infertility in both sexes.[4] Hence, if you are man and you are prescribed cyclophosphamide, you may wish to consider sperm banking if you are interested in having children in the future. And if you are a woman with a normal menstrual cycle, and you have the option, you may consider banking your ovarian eggs to be used in the future.[4]
Numerous studies have shown that CYC exposure can disrupt the redox balance of tissues to suggest that biochemical and physiological disturbances may result from oxidative stress.[5,6] It is therefore important to continue the search for an effective model compound that will protect against cyclophosphamide-induced reproductive toxicity and reduce toxicity issues associated with chemotherapy. In the light of this, several chemopreventive strategies have been presented which have beneficial impacts on the cyclophospamide-experimental model of testicular injury.[6,7] These studies suggest that natural compounds with antioxidative properties may have the potential to ameliorate cyclophosphamide-mediated testicular injury. In the past decade, the bioactivities of flavonoids on human health have given rise to much attention, especially the antioxidant activity.[8] The protective role of flavonoids involves several mechanisms of action: direct antioxidant effect, inhibition of enzymes of oxygen-reduction pathways and sequestration of transient metal cations.[9,10] Epidemiologic and experimental data support the hypothesis on cancer prevention via interference with the biochemical and molecular basis of carcinogenesis with functional food components.[11]
RUT is a flavonol glycoside composed of the flavonol quercetin and disaccharide rutinose. Possible activities of rutin can be mostly accounted for by the above-described mechanisms, common to the flavonoid group of chemicals.[9,10] Antioxidant power of RUT was corroborated by several studies.[12,13] This study was intended to examine whether co-administration of RUT can prevent CYC-induced reproductive toxicity in rats and how oxidative stress/free radicals play a role. To achieve this aim, rats were given CYC and/or RUT by oral gavage for 4 weeks, after which their sperm counts, sperm motility, sperm morphology, antioxidant system, and spermatogenesis marker enzymes were assessed. These results would help to provide protection against chemotherapy-induced oxidative damage in the investigated tissues.
MATERIALS AND METHODS
Sexually mature male Wistar rats (weighing approximately 199 ± 10 g) were obtained from the Department of Veterinary Anatomy, University of Ibadan, Ibadan, Oyo State, Nigeria. The animals were housed in plastic cages, fed a standard laboratory diet and water ad libitum, exposed to a 12 h light/dark cycle. The experimental designs and protocols for study received the approval of Institutional Animal Ethics Committee in accordance with the standard guide for the care and use of laboratory animals. CYC (500 mg; Endoxan) was purchased from Korean United Pharma Inc. Chungnam, Korea. Rutin was obtained from Sigma Chemicals (St. Louis, MO). CYC was administered twice week at a dose of 15 mg/kg b.wt. per day in corn oil. RUT was administered twice a week at a dose of 30 mg/kg b.wt per day in corn oil. The same dose of CYC and RUT as described above in a suspension of corn oil was administered twice a week to the RUT plus CYC-treated rats. However, the control rats were administered corn oil 0.2 mL per animal, twice a week. The first day the animals were treated was accepted as experimental day 0. All treatments were applied by gavage. At the end of the fourth week (28 days) of treatment, the five rats in each group were sacrificed and dissected and one pair of testes and epididymis and seminal vesicle (wet) were excised immediately, washed with ice-cold physiological saline and homogenized in 10 mM Tris-HCl buffer (pH- 7.4). Aliquot of a 10% tissue homogenate were processed for the assessment of various biochemical parameters: lipid peroxidation was determined by the method of Ohkawa et al.[14] MDA formed as an end product of the peroxidation of lipids served as an index of the intensity of oxidative stress. The activity of SOD using epinephrine as substrate was assayed according to the method of Misra and Fridovich.[15] CAT was assayed by the reduction of dichromate in acetic acid to chromic acetate when heated in the presence of hydrogen peroxide; the chromic acetate thus produced is measured calorimetrically at 610 nm.[16] The enzyme GST was assayed by the method of Harbig et al.[17] and the specific activity expressed as μmol GSH-CDNB (1-chloro-2,4-dinitrobenzene) conjugate formed/min/mg protein using an extinction coefficient of 9.6 mM–1cm–1.GSH was determined as per the method described by Jollow.[18] GSH-Px was assayed as described by Rotruck et al.[19] GR, which utilizes NADPH to convert oxidized glutathione (GSSG) to the reduced form (GSH), was assayed as described by Staal et al.[20] For assaying the ACP activity, the method of Moss[21] was used. The ALP activity was determined in tissue samples according to the method of Principato et al.[22] The LDH activity was determined by the method of Cabaud and Wroblewski[23] and was based on the interconversion of pyruvate and lactate. The SDH assay was based on the conversion of D-sorbitol to D-fructose.[24] The activity levels of testicular 3β-HSD (conversion of NAD to NADH) and 17β-HSD (conversion of NADPH to NADP) were measured by the method of Bergmeyer.[25] Protein content in the tissue samples was determined by the method of Lowry et al.[26] The other pair of the testis and epididymis was taken to determine testicular sperm counts, epididymal sperm numbers, epididymal sperm motility, and epididymal sperm morphology as per the methods described previously by us.[27] The data were expressed as the mean ± SD and were analyzed by means of one-way analysis of variance (ANOVA). Statistical evaluation of data were done following Student's t-test. A difference was considered significant at P < 0.05.
RESULTS AND DISCUSSION
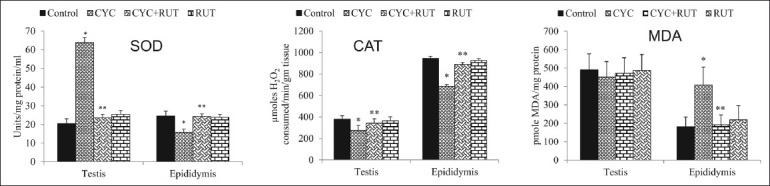
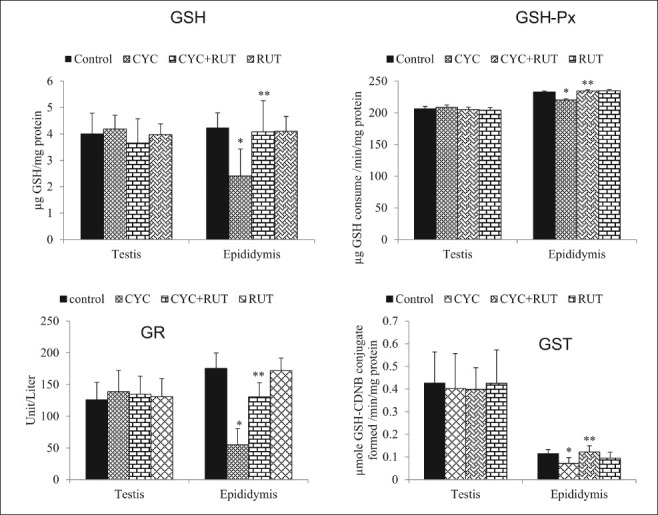
The RUT-treated group did not differ significantly from the control group at the end of the fourth week in terms of testicular and cauda epididymal sperm counts, epididymal sperm motility, abnormal sperm morphology, and numbers of sperm production daily but the CYC-treated rats had significantly lower sperm counts, total epididymal sperm motility and higher abnormal sperm morphology than the control group. However, the CYC plus RUT-treated rats had significantly higher sperm counts, better sperm motility and lower abnormal sperm morphology rates than the CYC-treated rats [P < 0.05; Table 1]. DSP in the CYC-treated rats was significantly decreased (42%) compared with the control group. However, the CYC plus RUT-treated rats had significantly higher DSP (57%) than the CYC-treated rats [P < 0.05; Table 1]. The MDA concentrations were unchanged while the activities of SOD increased and CAT decreased in the testis of CYC-treated rats. In epididymis, MDA levels significantly increased (125.41%) while the activities of SOD and CAT were significantly [Figure 1] decreased (P < 0.05). At the end of the fourth week, the RUT-treated and control rats did not differ significantly in terms of their MDA concentration, SOD and CAT activities in both organs. As shown in Figure 2, CYC given to rats at a dose of 15 mg/kg, oral gavage, did not changed the GSH levels and the activities of GSH-Px, GR, and GST activities in the testis compared with the control rats (P>0.05). Also, the RUT-treated and CYC plus RUT-treated rats did not differ significantly from the control group or the CYC-treated group at the end of the fourth week in terms of GSH levels, GSH-Px, GR, and GST activities in the testis. In epididymis, the RUT-treated group did not differ significantly from the control group at the end of the fourth week in terms of GSH homeostasis as both GSH levels, GSH-Px, GR, and GST activities remain unchanged, but the CYC-treated rats had significantly decreased GSH levels, GSH-Px, GR, and GST activities compared with control [P < 0.05; Figure 2]. However, the RUT plus CYC-treated rats had significantly higher GSH levels, GSH-Px, GR and GST activities than the CYC-treated group [P < 0.05; Figure 2]. In the testis and epididymis, the RUT-treated and control rats did not differ significantly in terms of LDH, SDH, ACP, and ALP activities but the CYC-treated rats had significantly increased LDH and SDH activities while ACP and ALP activities were significantly decreased in the testis compared to control [Figure 3]. However, in the epididymis, CYC administration increased the LDH activity while SDH, ACP, and ALP activities were significantly decreased compared to control. In addition, the presence of RUT in combination with CYC maintained the enzyme activities of marker spermatogenesis enzymes (SDH, LDH, ALP, and ACP) to near normal values compared to control group and also significantly different with the values seen in the CYC-treated rats in both organs. None of these marker enzymes were affected in the seminal vesicles either in the CYC-treated rats or in the CYC plus RUT-treated rats [P > 0.05; Figure 3]. At the end of the fourth week, the RUT-treated and control rats did not differ significantly in terms of their 3β-HSD and 17β-HSD activities but the CYC-treated rats had significantly decrease 3β-HSD and 17β-HSD activities than the control group [Figure 4]. However, the CYC plus RUT-treated rats had significantly increased 3β-HSD and 17β-HSD activities than the CYC-treated rats [P < 0.05; Figure 4]. This study reveals that CYC administration decreased spermatid count per testis, sperm count per epididymis, DSP/gram testis, sperm motility, and significantly increased abnormal sperm rates. This has been reported previously in rat's testis after administration of CYC orally, weekly for 4 and 8 weeks.[28,29] These observations are in agreement with this study and also agree with the results obtained with other chemotherapeutic drugs.[30] DSP is a quantitative index of the ability to produce sperm.[31] Sperm morphologies are qualitative indexes of spermatogenesis.[31] In this study, CYC affected these two indexes of spermatogenesis in the same manner as observed with other testicular toxicants.[27] These results suggest that CYC inhibits spermatogenesis. The sperm abnormalities observed in CYC-treated rats are a serious problem of reproductive function, since abnormal sperm cannot reach the oviduct after intravaginal ejaculation.[31] Lipid peroxidation is one of the main manifestations of oxidative damage, which plays an important role in the toxicity of many xenobiotics. The present findings showed that CYC exposure did not affect the MDA concentrations in the testis. This was also accompanied with unchanged GSH homeostasis as both GSH levels and activities of GSH-Px, GR, and GST were not significantly impaired. However, antioxidative enzymes such as SOD and CAT were altered. The joint action of these enzymes decreases the toxicity produced by the superoxide anion (O2•–) and radicals generated by action of different toxics during their metabolism. We noted that SOD increased whereas CAT decreased in the testis. It has been demonstrated that CYC reduces the CAT activity in the testis.[32] The SOD activity may be induced in cells exposed to oxidative stress as protective responses to eliminate xenobiotics.[33] Thus, the existence of an inducible antioxidant system may reflect an adaptation to oxidative stress and have been established in several systems.[29,34] On the contrast, MDA levels were elevated in the epididymis. This was accompanied by concomitant decrease in the activities of SOD and CAT. These suggest that while the testis antioxidant system was capable to protect against CYC-induced oxidative damage, the epididymal antioxidant was not efficient to protect against CYC-induced oxidative stress. It is therefore possible that the epididymis is more vulnerable to CYC-induced oxidative damage. To support our observations, it was reported that the epididymal epithelial is not as rich in some antioxidant molecules as the testis.[35] CYC might accelerate enzymatic degradation of CAT and SOD in the epididymis. This hypothesis may partially explain the lower SOD and CAT values in the epididymis from the animals exposed to testicular toxicant when compared to the level measured in controls and found in testis.[36] Hence, the epididymis expresses smaller antioxidant potential, which undergoes depletion upon chronic contact with CYC or its metabolites. Thus, the epididymis, where matured spermatozoa are stored seems to be an important factor in the aetiology of male infertility induced by CYC. Further studies are required to determine if low levels of individual antioxidant molecules and high oxidative stress in the epididymis itself are associated with poor fertility in CYC-treated rats. The reduction in the activity of CAT in the epididymis may reflect the inability of epididymal mitochondria, microsomes, and cytosol to eliminate the hydrogen peroxide produced by SOD. This may also be attributed to enzyme inactivation caused by excess ROS production.[37] The reduction in the activities of these enzymes and increase in MDA could reflect the adverse effect of CYC on the antioxidant system in epididymis.
Table 1.
Effect of rutin on sperm counts, numbers of sperm production daily, sperm motility, sperm morphology in cyclophosphamide - treated rats
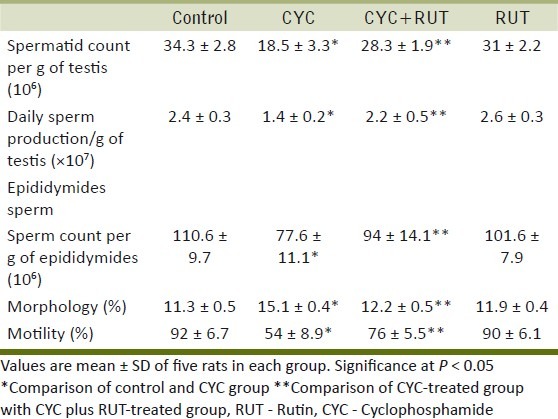
Figure 1.
Superoxide dismutase (SOD) and catalase (CAT) activity and Malondialdehyde (MDA) levels in the testis and epididymis of cyclophosphamide (CYC) and rutin (RUT) treated rats. Values are mean ± SD for five rats in each group. Values are statistically significant at P < 0.05. *comparison of control and CYC-treated group; **comparison of CYC-treated group with RUT plus CYC-treated group
Figure 2.
Glutathione (GSH) level, and glutathione peroxidase (GSH-Px), glutathione reductase (GR), and glutathione-S-transferase (GST) activity in the testis and epididymis of cyclophosphamide (CYC) and rutin (RUT) treated rats. Values are mean ± SD for five rats in each group. Values are statistically significant at P < 0.05. *comparison of control and CYC-treated group; **comparison of CYC-treated group with RUT plus CYC-treated group
Figure 3.
Lactate dehydrogenase (LDH), sorbitol dehydrogenase (SDH), alkaline phosphatase (ALP) and acid phosphatase (ACP) activity in the testis, epididymis and seminal vesicle of cyclophosphamide (CYC) and rutin (RUT) treated rats. Values are mean ± SD for five rats in each group. Values are statistically significant at P < 0.05. *comparison of control and CYC-treated group**comparison of CYC-treated group with RUT plus CYC-treated group
Figure 4.
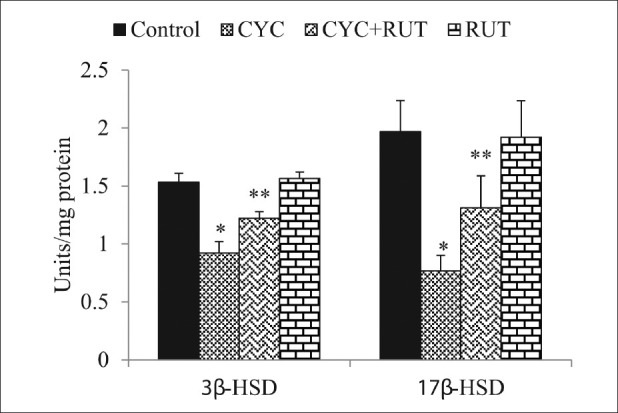
3β-hydroxysteroid dehydrogenase (3β-HSD) and 17β-hydroxysteroid dehydrogenase (17β-HSD) activity in the testis of cyclophosphamide (CYC) and rutin (RUT) treated rats. Values are mean ± SD for five rats in each group. Values are statistically significant at P < 0.05. *comparison of control and CYC-treated group; **comparison of CYC-treated group with RUT plus CYC-treated group
While SOD and CAT protect a greater extent against severe oxidative damage, the glutathione cycle means a notable source of protection against low levels of oxidative stress.[38] The alteration in the activities of these enzymes, together with the alteration in the GSH-redox system (i.e., decreased GSH, GSH-Px, GR, and GST activities), supports the observation that CYC can induce alterations in the oxidative system of the epididymis. Toxic lipid hydroperoxides and hydrogen peroxide produced during oxidative stress are detoxified by GSH-Px using GSH. GR recycles GSH from GSSG (oxidized glutathione) whereas GST catalyzes the detoxification of electrophilic compounds to protect cells against peroxidative damages.[39] Thus, this system plays an important role in the detoxification of xenobiotics and in the antioxidation of free radicals and ROS.
Activities of testicular marker enzymes such as SDH, LDH, ACP, and ALP are considered functional indicators of spermatogenesis. In this study, LDH and SDH showed significant increase whereas ACP and ALP showed a significant decrease in the testis. The same pattern of expression where observed in the epididymis except for the decreased SDH activity. The seminal vesicle was not responsive to CYC treatment. LDH and SDH are associated with the maturation of germinal epithelial layer of seminiferous tubules. SDH is primarily associated with pachytene spermatocyte maturation of germinal epithelium. Its activity is related to the function of germ cells and decreases during the depletion of germ cells.[40] SDH is also responsible for providing energy to sperm cells by converting sorbitol to fructose. The increased activity of SDH in the testis and its decreased activity in the epididymis of CYC-treated rats suggest an altered cellular physiology of the germinal elements in seminiferous tubules resulting from interference of CYC with the process of spermatogenesis. Since the activity of LDH is closely associated with spermatogenesis and male testicular development, the increased activity of this enzyme in CYC-administered animals represents a strategy to improve spermatogenesis and testicular maturation in the already compromised spermatogenic event.[41] The decrease in the activities of ALP and ACP may be due to the decrease in the secretory activity of the male accessory sex glands.[40] The decrease in the activities of both ACP and ALP in the testis and epididymis coincided with the decrease of spermatozoa quality of rats treated with CYC. Moreover, the alteration in ACP and ALP enzymes activity may lead to the destruction of seminiferous epithelium and loss of germinal elements, resulting in the reduction of the number of spermatids associated with the decrease in the daily sperm production in the testes.[40] This particular pattern in the activity of these marker enzymes as observed in this study is characteristics of testicular atrophy associated with damage to germ cells and sertoli cells by many xenobiotics in the literature.[6,42]
3β-HSD and 17β-HSD play a key regulatory role in testicular steroidogenic events as these are the prime enzymes in testicular androgenesis. The diminution of these enzymes by CYC treatment in this study is consistent with a previous observation.[5] These authors reported that the decreased 3β-HSD and 17β-HSD corresponded to decreased plasma testosterone levels and thus inhibited testicular androgenesis. Though we did not measure plasma testosterone levels in this study, we hypothesized that the decreased 3β-HSD and 17β-HSD activities may lead to lowered testosterone levels and as such the spermatotoxicity observed in the CYC-treated rats may be the result of lowered plasma testosterone level. Furthermore, the decrease steroidogenesis may be the direct result of the decreased ALP and ACP activities as these enzymes are required in the metabolic reactions to channelize the necessary inputs for steroidogenesis.[40]
The positive effects of flavonoids on human health have attracted more attention in recent times. Most of the biological actions of flavonoids seem to be associated with its potency as an antioxidant. Our present work aimed to investigate the potential protective value of RUT on the reproductive toxicity induced by CYC. Our results showed that RUT had no deleterious effects on the rat's reproductive function at the indicated dose. The simultaneous supplementation with antioxidant RUT recovered the normal level of sperm counts, sperm morphology, sperm motility, and number of sperm production daily. These results revealed that CYC-induced spermatoxicity can be prevented by RUT. Additionally, our observations about the protective effects of RUT are in agreement with a recent study in rats that RUT protected against spermatoxicity induced by gentamicin.[43] Our study also showed that RUT pretreatment significantly reversed the upsurge in lipid peroxide levels and the altered antioxidant status in both the testis and epididymis. RUT has the ability to scavenge superoxide anion, hydroxyl radical,[44] and also chelate the ferrous ion involved in the production of hydroxyl radicals,[45] reduced lipid peroxidation and increased antioxidant enzymatic activities and expression.[43] The above reports corroborate well with our findings in experimental reproductive toxicity. Our data also revealed that RUT administration reverted to normalcy the deleterious effects of CYC on steroidogenesis as manifested by restoring the activities of the two key enzymes (3β-HSD and 17β-HSD). The way RUT reversed CYC-induced reduction of steroidogenesis might be through scavenging of CYC-induced ROS, which can affect steroidogenic enzyme activities at protein and transcription levels.[46] Administration of RUT along with CYC has been shown to prevent the testicular injury and spermatogenesis dysfunction as revealed by restoring SDH, LDH, ACP, and ALP activities in testicular and epididymal tissues. This may also provide an explanation for normalized sperm counts, sperm motility, DSP and abnormal sperm rate. It is, however, stated that probably no research work on experimental male infertility induced by CYC has been done in Nigeria since no literature could be available in this regard.
In conclusion, RUT was found to be capable of sustaining antioxidants in the epididymis and testis, leading to decreased oxidative stress and cellular damage initiated by CYC and its metabolites. Also, decreased spermatogenesis and steroidogenesis as reflected in the enzyme levels of 3β-HSD and 17β-HSD was prevented on RUT-coadministration. Also, the epididymal epithelial was found to be more vulnerable than the testis to CYC-induced oxidative damage whereas the seminal vesicles were found to be unresponsive in terms of the activity of marker spermatogenesis enzymes. Additional studies will be needed to decipher this tissue-differential response to CYC toxicity and also to confirm this protective role of RUT in other models of chemically induced reproductive injury.
ACKNOWLEDGMENTS
The technical expertise of Mr Omoko Ejiro of the Department of Veterinary Reproduction, University of Ibadan, Oyo State on spermatozoa analysis is gratefully appreciated.
Footnotes
Source of Support: This work received no financial support from any government, private or not-for-profit organization.
Conflict of Interest: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Howell ST, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update. 2001;7:363–9. doi: 10.1093/humupd/7.4.363. [DOI] [PubMed] [Google Scholar]
- 2.Meistrich ML. Hormonal stimulation of the recovery of spermatogenesis following chemo-or radiotherapy. APMIS. 2008;106:37–45. doi: 10.1111/j.1699-0463.1998.tb01317.x. discussion 45-6. [DOI] [PubMed] [Google Scholar]
- 3.Lee S, Schmitt CA. Chemotherapy response and resistance. Curr Opin Genet Dev. 2003;13:90–6. doi: 10.1016/s0959-437x(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 4.Pandse S, Ginsburg E, Singh AK. Strategies for preservation of ovarian and testicular function after immunosuppression. Am J Kidney Dis. 2004;43:772–81. doi: 10.1053/j.ajkd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Das UB, Mallick M, Debnath JM, Ghosh D. Protective effect of ascorbic acid on cyclophosphamide-induced testicular gametogenic and androgenic disorders in male rats. Asian J Androl. 2002;4:201–7. [PubMed] [Google Scholar]
- 6.Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. Protective effect of dL-β-lipoic acid in cyclophosphamide induced oxidative injury in rat testis. Reprod Toxicol. 2004;19:163–7. doi: 10.1016/j.reprotox.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Selvakumar E, Prahalathan C, Sudharsan PT, Varalakshmi P. Protective effect of lipoic acid on cyclophosphamide-induced testicular toxicity. Clin Chim Acta. 2006;367:114–9. doi: 10.1016/j.cca.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YM. Protective effect of quercetin on aroclor 1254-induced oxidative damage in cultured chicken spermatogonial cells. Toxicol Sci. 2005;88:545–50. doi: 10.1093/toxsci/kfi333. [DOI] [PubMed] [Google Scholar]
- 9.Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 10.Cotelle N. Role of flavonoids in oxidative stress. Curr Top Med Chem. 2001;1:569–90. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- 11.Hursting SD, Slaga TJ, Fischer SM, DiGiovani J, Phang JM. Mechanism-based cancer prevention approaches: targets, examples and the use of transgenic mice. J Natl Cancer Inst. 1999;91:215–25. doi: 10.1093/jnci/91.3.215. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Ishimitsu S, Tonogai Y. Effects of quercetin and rutin on serum and hepatic lipid concentrations, fecal steroid excretion and serum antioxidant properties. J Health Sci. 2000;46:229–40. [Google Scholar]
- 13.Florek E, Ignatowicz E, Wrzosek J, Piekoszewski W. Effect of rutin on total antioxidant status of rats exposed to cigarette smoke. Pharmacol Rep. 2005;57:84–9. [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 16.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 17.Habig WH, Pabst MJ, Jacob WB. Glutathione-s-transferase.The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 18.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3, 4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 19.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 20.Staal GE, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys Acta. 1969;185:39–48. doi: 10.1016/0005-2744(69)90280-0. [DOI] [PubMed] [Google Scholar]
- 21.Moss DW. In: Methods of Enzymatic Analysis. 3rd ed. Bergmeyer HU, editor. Berlin: Verlag-Chemie; 1984. pp. 92–106. [Google Scholar]
- 22.Principato GB, Asia MC, Talesa V, Rosi G, Giovannini E. Characterization of the soluble alkaline phosphatase from hepatopancreas of Squilla mantis L. Comp Bioch Physiol. 1985;80:801–4. [Google Scholar]
- 23.Cabaud PC, Wroblewski F. Calorimetric measurement of lactate dehydrogenase activity of body fluids. J Clin Pathol. 1958;30:234–6. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- 24.Gerlach U. Sorbitol dehydrogenase. In: Bergmeyer HU, Bergemeyer J, Grassi M, editors. Methods of enzymatic analysis. Berlin: Verlag Chemie; 1983. pp. 112–7. [Google Scholar]
- 25.Bergmeyer HU. Beta-Hydroxysteroid dehydrogenase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. pp. 447–89. [Google Scholar]
- 26.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 27.Abarikwu SO, Adesiyan AC, Oyeloja TO, Oyeyemi MO, Farombi EO. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch Environ Contam Toxicol. 2010;58:874–82. doi: 10.1007/s00244-009-9371-2. [DOI] [PubMed] [Google Scholar]
- 28.Rezvanfar M, Sadrkhanlou R, Ahmadi A, Shojaei-Sadee H, Rezvanfar M, Mohammadirad A, et al. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum Exp Toxicol. 2008;27:901–10. doi: 10.1177/0960327108102046. [DOI] [PubMed] [Google Scholar]
- 29.Turk G, Çeribasi AO, Sakin F, Sönmez M, Atessahin A. Antiperoxidative and anti-apoptotic effects of lycopene and ellagic acid on cyclophosphamide-induced testicular lipid peroxidation and apoptosis. Reprod Fertil Dev. 2010;22:587–96. doi: 10.1071/RD09078. [DOI] [PubMed] [Google Scholar]
- 30.Ateşşahin A, Sahna E, Türk G, Ceribaşi AO, Yilmaz S, Yüce A, et al. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res. 2006;41:21–7. doi: 10.1111/j.1600-079X.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 31.Izawa H, Kohara M, Aizawa K, Suganuma H, Inakuma T, Watanabe G, et al. Alleviative effects of quercetin and onion on male reproductive toxicity induced by diesel exhaust particles. Biosci Biotechnol Biochem. 2008;72:1235–41. doi: 10.1271/bbb.70705. [DOI] [PubMed] [Google Scholar]
- 32.Selvakumar E, Prahalathan C, Sudharsan PT, Varalakshmi P. Protective effect of lipoic acid on cyclophosphamide-induced testicular toxicity. Clin Chim Acta. 2006;367:114–9. doi: 10.1016/j.cca.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez E, Rosello-Catafau J, Jawerbaum A, Sinner D, Pustovrh C, Vela J, et al. Pancreatic nitric oxide and oxygen free radicals in the early stages of streptozotocin-induced diabetes mellitus in the rat. Braz J Med Biol Res. 2000;33:1335–42. doi: 10.1590/s0100-879x2000001100012. [DOI] [PubMed] [Google Scholar]
- 34.Pande M, Flora SJ. Lead induced oxidative damage and its response to combined administration of α-lipoic acid and succimers in rats. Toxicology. 2002;177:187–96. doi: 10.1016/s0300-483x(02)00223-8. [DOI] [PubMed] [Google Scholar]
- 35.Zini A, Schlegel PN. Identification and characterization of antioxidant enzyme mRNAs in the rat epididymis. Int J Androl. 1997;20:86–91. doi: 10.1046/j.1365-2605.1997.00039.x. [DOI] [PubMed] [Google Scholar]
- 36.Adesiyan AC, Oyejola TO, Abarikwu SO, Oyeyemi MO, Farombi EO. Selenium provides protection to the liver but not the reproductive organs in an atrazine-model of experimental toxicity. Exp Toxicol Pathol. 2011;63:201–7. doi: 10.1016/j.etp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, et al. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51:283–97. doi: 10.1016/0047-6374(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 38.Albina ML, Alonso V, Linares V, Belles M, Sirvent JJ, Domingo JL, et al. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology. 2010;271:51–6. doi: 10.1016/j.tox.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Abarikwu SO, Farombi EO, Pant AB. Biflavanone-kolaviron protects human dopaminergic SH-SY5Y cells against atrazine induced toxic insult. Toxicol In Vitro. 2011;25:848–58. doi: 10.1016/j.tiv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Sadik NA. Effects of Diallyl Sulfide and Zinc on Testicular Steroidogenesis in Cadmium-treated male rats. J Biochem Mol Toxicol. 2008;22:345–53. doi: 10.1002/jbt.20247. [DOI] [PubMed] [Google Scholar]
- 41.Erkkila K, Aito H, Aalto K, Pentikainen V, Dunkel L. Lactate inhibits germ cells apoptosis in the human testis. Mol Hum Reprod. 2002;8:109–17. doi: 10.1093/molehr/8.2.109. [DOI] [PubMed] [Google Scholar]
- 42.Pant N, Srivastava SP. Testicular and spermatotoxic effects of quinalphos in rats. J Appl Toxicol. 2003;23:271–4. doi: 10.1002/jat.919. [DOI] [PubMed] [Google Scholar]
- 43.Akondi RB, Akula A, Challa SR. Protective Effects of Rutin and Naringin on Gentamycin Induced Testicular Oxidative Stress. Eur J Gen Med. 2011;8:57–64. [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT-Food Sci Technol. 2008;41:1060–6. [Google Scholar]
- 45.Kostyuk VA, Potapovich AI. Antiradical and chelating effects in flavonoid protection against silica-induced cell injury. Arch Biochem Biophys. 1998;355:43–8. doi: 10.1006/abbi.1998.0708. [DOI] [PubMed] [Google Scholar]
- 46.Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–91. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]


