Abstract
Snake envenomation is a global public health problem, with highest incidence in Southeast Asia. Inadequate health services, difficult transportation and consequent delay in antisnake venom administration are the main reasons for high mortality. Adverse drug reactions and inadequate storage conditions limit the use of antisnake venom. The medicinal plants, available locally and used widely by traditional healers, therefore need attention. A wide array of plants and their active principles have been evaluated for pharmacological properties. However, numerous unexplored plants claimed to be antidotes in folklore medicine need to be studied. The present article reviews the current status of various medicinal plants for the management of snake bite.
Keywords: Antisnake venom, medicinal plants, pharmacological activity, snake bite
INTRODUCTION
Snake envenomation is an important global health issue. It constitutes an occupational hazard mainly in the field of agriculture. Highest incidence and mortality due to snake bites is reported from South and Southeast Asian countries having extensive agricultural practices and diversity in snake species.[1] Poor health services, difficult transportation, delay in the antisnake venom administration especially in rural areas are the important factors responsible for high mortality.
It is estimated that in India alone, there are more than 2,00,000 venomous bites per year, of which 35,000–50,000 are fatal.[2] The estimates are arbitrary as majority of deaths are unreported. In rural areas, where most of the bites take place, the victims are mostly taken to traditional healers, who neither report them to the authorities nor document the cases, hence paucity of reliable epidemiological data.
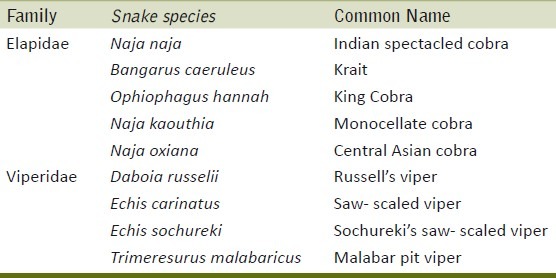
There are more than 3000 known species of snakes of which around 300 are poisonous. In India out of 216 species, approximately 53 are poisonous.[2] Bites are primarily due to the venomous species of the families Elapidae and Viperidae. The common poisonous snakes in India mainly include Indian spectacled cobra (Naja naja), common krait (Bungarus caeruleus), Russell's viper (Daboia russelii) and saw-scaled viper (Echis carinatus) [Table 1]. Hump-nosed pit vipers (Hypnale hypnale and H. nepa) have been reported from South India.[3]
Table 1.
Common poisonous snakes in India
The snake venom is a complex mixture of enzymes including the procoagulants, non-enzyme proteins, peptides, carbohydrates, amines, lipids and metal ions. The venom exerts neurotoxic, cytotoxic and hemotoxic effects. The administration of antisnake venom (ASV), the only specific treatment for snake bite, however, is associated with many drawbacks.
Antisnake venom and its limitations
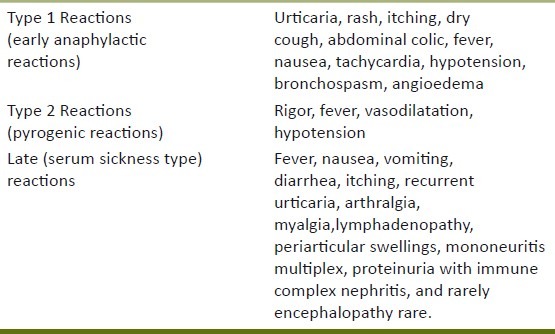
Antivenom for ophidian bites is a suspension of antibodies, prepared mainly from horses. Animals are hyper-immunized against the venom of a given species (monovalent) or venoms from several different species at the same time (polyvalent). Infusion of ASV may lead to adverse reactions ranging from early reactions (pruritus, urticaria) to potentially fatal anaphylaxis[4] [Table 2]. The reported incidence of these reactions varies from 5 to 80%.[5–7] There are also pyrogen reactions due to endotoxin contamination. Serum sickness may also develop in certain cases.
Table 2.
Adverse drug reactions profile of antisnake venom
Availability and issues in stockpiling
The production and supply of antivenom is associated with logistical, marketing, storage and economic difficulties. The development is a costly, time-consuming process requiring ideal storage conditions. The liquid form of ASV has a half life of 2 years. Storage at 0–4°C is necessary, otherwise rapid deterioration sets in rendering it unfit for use. However, the lyophilized form retains potency up to 5 years when stored in any cool, dark place. In India, polyvalent antisnake venom is prepared from horses, which are hyper-immunized against the venom of the four common poisonous snakes (Cobra, Krait, Russell's Viper and Saw-scaled Viper).
Issue of specificity
Absolute specificity is an issue in management with ASV. The geographic and taxonomic diversity in species leads to a significant variation in composition and antigenic reactivity of venom.[8,9] So the use of a particular ASV may get restricted to a geographical area of relevant specificity. Thus, monovalent ASV would be the solution. But in view of the cost and diverse specificity, polyvalent ASV would provide answers to an extent, considering the medically important species. However, because of paucity of reliable literature on distribution and diversity of venomous species, ASV is not available for all species. This is one of the main obstacles of immunotherapy.
Herbs as an alternative to antisnake venom
The plant kingdom provides an alternative to ASV. Medicinal plants have been used as folk medicine for treatment of snake bites. Reliance on medicinal plants is primarily due to their safety, effectiveness, cultural preferences, inexpensive nature and dependence on neighboring forests.
Globally, traditional healers are practicing herbal medicine to cure snake envenomations; however, the practice is not really recognized by modern medicine. The number of studies evaluating the pharmacologically active principles against snake bites are few.[10,11] Though novel phytotherapeutic agents have been isolated from plants due to vital leads from ethnic groups, yet validation is still an issue. Emphasis should be on proper design of both in vivo and in vitro studies, so that they relate exactly to the clinical situations.[12]
This review is an attempt to present a comprehensive account of numerous herbals used world over for the treatment of snake bite. A thorough literature survey highlights the fact that plant kingdom has tremendous resources which can be exploited for unidentified novel compounds with antivenin activity or those supplementing the action of antisnake venom.
Herbal plants with antitoxin activity
The indigenous systems of medicine use medicinal plants for the treatment of snake bites. There is a huge repository of plants reported to possess antisnake venom activity.[13,14] Investigation of therapeutic potential of plants used for snake bites shows the presence of different phytochemicals [Table 3].
Table 3.
Phytochemicals isolated from plants used for snakebites

Screening of plants used in traditional medicine and determination of their active principles and different activities is being undertaken. The active principles isolated have been associated with various pharmacological properties and may provide a substantial contribution to the modern therapeutics of snake bite.
Anti-inflammatory activity
Ethanolic extracts of Bixa orellana, Brownea rosa-de-monte, Dracontium croatii, Struthanthus orbicularis, Gonzalagunia panamensis, and Trichomanes elegans are reported to inhibit edema due to Bothrops asper venom.[15] Decrease of edema formation with aqueous extracts of Casearia sylvestris Sw. has been noted in rats injected with lethal doses of Bothropic venoms. Ellagic acid has inhibited edematogenic activity due to total venom and phospholipase A2 (PLA2) from Bothrops jararacussu.[16]
Methanolic extract of seeds of Vitis vinifera L. has shown promise for the treatment of local effects of viperine bites. The extract neutralized edema-inducing property of venom.[17] Cordia verbenacea extract significantly reduced paw edema, induced by Bothrops jararacussu snake venom.[18]
Different doses of Tamarindus indica seed extract upon preincubation with venom before assays significantly neutralized edema.[19] Anacardium occidentale bark extract has also been shown to neutralize edema induced by viper venom.[20]
Lupeol acetate from roots of Hemidesmus indicus R.Br. is documented to significantly neutralize edema induced by Russell's Viper, in experimental animals, besides the cardiotoxicity, neurotoxicity and respiratory changes induced by Naja kaouthia venom.[21]
Antiophidian properties are reported to be associated with triterpenoid saponins. Glycyrrhizin, isolated from the roots of Glycyrrhiza glabra, has been found to be anti-inflammatory.[22] Inhibition of edema due to Naja naja venom is reported with turmerin isolated from Curcuma longa.[23]
Bidens pilosa is documented to have anti-inflammatory potential.[24] Strychnos nux-vomica used by tribals for snake bites is reported to be anti-inflammatory.[25] Extracts of Andrographis paniculata and Aristolochia indica have shown significant decrease in edema.[26] Partial inhibition of edema has been reported with the aqueous extracts of Pentaclethra macroloba.[27]
Anti-hemorrhagic and anticoagulant activity
Prolongation of clotting time of blood plasma was observed with Brownea rosa-de-monte, Pleopeltis percussa, Bixa orellana and Heliconia curtispatha, Trichomanes elegans, after pre-incubation with venom.[15] Methanolic extracts of Mouriri pusa Garden, Byrsonima crassa Niedenzu, Davilla elliptica St. Hills upon evaluation have shown complete neutralization of local hemorrhage. Flavonoids namely myricetin, quercetin, amenthoflavone have been attributed the antihemorrhagic potential. Quercetin is a potent lipoxygenase inhibitor.[28] Tamarindus indica seed extract has neutralized the hemorrhage, indirect hemolysis and degradation of Bbeta chain of human fibrinogen, caused by viper venom in experimental animals.[19]
The aqueous extract of leaves of Schizolobium parahyba significantly inhibited the coagulant, hemorrhagic and fibrinogenolytic activities induced by Bothrops pauloensis and Crotalus durissus terrificcus venoms and their isolated toxins after preincubation with venoms and toxins before assays.[29] In vivo tests with polyphenols of Areca catechu L and Quercus infectoria Oliv showed inhibition of the hemorrhagic activity of Calloselasma rhodostoma Kuhl venom and dermonecrotic activity of Naja kauothia venom.[30]
Prolongation of clotting time of Echis carinatus venom-treated blood has been observed with the aqueous extracts of Mucuna pruriens, Strophanthus hispidus, and Strophanthus gratus.[31] Activation of coagulative activity by Mucuna pruriens seed extract is well documented in literature.[32] Inhibition of fibrinocoagulation activity induced by Bothrops jararaca venom is reported with the extracts of Masypianthes chamaedrys.[33] Neutralization of hemorrhage and partial inhibition of procoagulant activity of venom and abolition of degradation of Aalpha and Bbeta chains of human fibrinogen has been observed with Vitis vinifera seed extract; against viper venom induced local effects.[17]
The organic acid from root extract of Hemidesmus indicus significantly antagonized hemorrhagic, coagulant and anticoagulant activities in experimental rodents, induced with viper venom.[34] Lupeol acetate from the plant has neutralized hemorrhage and defibrinogen induced by Russell's Viper.[21] Inhibition of hemorrhage and dermonecrotic activities of venoms in vivo is reported with methanolic extracts of leaves of Camellia sinensis. The action has been attributed to complexation and chelation of plant phenolic compounds and venom proteins.[35]
Eclipta prostrata is used for snake bites in China and Brazil. The aqueous extract of Eclipta prostrata and wedelolactone, a potent and selective 5-lipoxygenase inhibitor isolated from the plant, has shown anti-hemorrhagic activity against Bothrops jararaca, Bothrops jararacussu venoms and myotoxins, bothropstoxin and crotoxin.[36,37] Partial inhibition of hemorrhagic activity has been observed with the butanolic extracts of Eclipta prostrata containing demethylwedelolactone as the main constituent.[38]
Glycyrrhizin a thrombin inhibitor, from the roots of Glycyrrhiza glabra has shown anti-thrombotic properties in vivo. Prevention of venom-induced changes in hemostasis, both in vivo and in vitro, have also been noted.[22] Neutralization of hemorrhagic, fibrinolytic and proteolytic activities of metalloproteases from Bothrops snake venoms is reported with a triterpenoid saponin isolated from Pentaclethra macroloba.[39]
Ar-turmerone from Curcuma longa roots has shown neutralization of the lethal effect of Crotalus durissus terrificus and hemorrhagic effect of Bothrops jararaca venoms.[40] Tannic acid is documented to neutralize hemorrhage due to Crotalus adamanteus venom.[41] Baccharis trimera has yielded clerodane diterpenoid, possessing anti-hemorrhagic properties against snake venoms.[42] Neutralization of hemorrhage due to viper venom is documented with seed extract of Strychnos nux-vomica.[43]
Enzyme inhibitory activity
Enzyme inhibiting and protein binding properties have been associated with chemically active compounds of flavonoids, polyphenols, terpenoids, xanthene etc. The phytochemicals also inhibit PLA2 activities of viper and cobra venom.[44] Phenolics, especially polyphenols, like some tannins bind proteins, acting upon components of venom directly and disabling them to act on receptors. They could also act by competitive blocking of the receptors.[45] Tannic acid has been found to be a potent inhibitor of hyaluronidase.[41]
Inhibition of enzymatic activity is reported with extracts of Casearia sylvestris in experimental animals, injected with lethal doses of Bothropic venoms.[16] Significant inhibition of PLA2 activity induced by Bothrops pauloensis and Crotalus durissus terrificcus venoms is documented with the leaf extract of Schizolobium parahyba.[29] Neutralization of Vipera russelii venom enzymes namely phospholipase, protease and hyaluronidase is reported with the bark extract of Anacardium occidentale in a dose-dependent manner.[20]
Abolition of hyaluronidase and proteolytic activities of viper venom with methanolic extract of seeds of Vitis vinifera has been reported.[17] Edunol, a pterocarpan isolated from Harpalyce brasiliana was found to be antiproteolytic and an inhibitor of PLA2.[46] Inhibition of azocaseinolytic activity of Bothrops jararaca venom has been seen with the extract of Masypianthes chamaedrys.[33]
Lupeol acetate from roots of Hemidesmus indicus significantly neutralized PLA2 activity induced by Russell's Viper.[21] Antihyaluronidase activity is reported with Mimosa pudica against Naja naja, Vipera russelii and Echis carinatus venoms.[47]
Methanolic leaf extract of Azadirachta indica has shown significant inhibition of PLA2 enzymes of Cobra and Russell's Viper venoms.[48] Withania somnifera has yielded a glycoprotein inhibitor, found to be effective in cobra and viper bite. The compound inhibited the PLA2 activity of Naja naja.[49] 4-nerolidylcatechol has been isolated from Piper species. Various species of the plant are reported to inhibit activity of PLA2 from venoms of Bothrops species.[50]
Plant extracts of Andrographis paniculata and Aristolochia indica effectively inhibited the main toxic enzymatic effects of Echis carinatus, responsible for a large number of deaths in India. Inhibition of PLA2 and neutralization of procoagulant activity was observed with both the extracts.[26] Aristolochic acid from Aristolochia radix is reported to inhibit the enzymatic and pharmacological activities of PLA2 induced by Vipera russelii venom.[51,52]
In vitro tests with polyphenols from Areca catechu L and Quercus infectoria Oliv showed inhibition of PLA2, proteases, hyaluronidase and L-amino acid oxidase of Naja naja kaouthia and Calloselasma rhodostoma venoms.[30] Edunol, from Harpalyce brasiliana is reported to be an inhibitor of PLA2.[46]
Tamarindus indica has shown potent venom neutralizing properties. Main hydrolytic enzymes responsible for the early effects of envenomation by Russell's Viper (inflammation, local tissue damage, and hypotension) have been inhibited by the seed extract, in a dose-dependent manner.[19]
Methanolic extract of fresh leaves of Camellia sinensis showed inhibition of PLA2, hyaluronidase, L-amino acid oxidase in venoms of Naja naja kaouthia and Calloselasma rhodostoma, by in vitro neutralization.[35] Pentacyclic triterpenes, betulin and betulinic acid extracted from Betula alba have demonstrated activity against PLA2.[53]
A triterpenoid saponin from Gymnema sylvestre, potassium salt of gymnemic acid has inhibited ATPase induced by Naja naja venom.[54] Neutralization of PLA2 activity has been documented with seed extract of Strychnos nux-vomica.[43] Eclipta alba is documented to inhibit PLA2 activity.[55]
Antibacterial and antiparasitic activity
Mikania laevigata and Mikania glomerata, having antiophidian, antibacterial and antiparasitic activity, have been used in Brazil for the treatment of snake bites.[56] A broad spectrum of antibacterial activity has been associated with root extract of Aristolochia bracteata in snake bites.[57] Extracts of Delonix elata and Mollugo cerviana and Merremia tridentata have shown significant antibacterial activity. Medicinal properties may be attributed to bioactive compounds like alkaloids, glycosides, tannins found in these plants. They have been used for various ailments including snake bites.[58]
Antimyotoxic activity
Ellagic acid from Casearia sylvestris aqueous extract has shown inhibition of myotoxic activity in rats when tested against effects, from both total venom and PLA2 from genus Bothrops.[16] Neutralization of myotoxic effects of Vipera russelii venom is reported with the bark extract of Anacardium occidentale.[20] Significant inhibition of myotoxicity induced by Bothrops pauloensis and Crotalus durissus terrificcus venoms and their isolated toxins by aqueous extract of leaves of Schizolobium parahyba has been documented.[29]
Methanolic extract of seeds of Vitis vinifera has shown neutralization of myonecrotic properties of viper venom.[17] Edunol from Harpalyce brasiliana was found to be antimyotoxic.[46] Myotoxicity induced by Bothrops jararacussu snake venom and its main PLA2 homologues is reported to be inhibited with Cordia verbenacea extract.[18]
Significant neutralization of myotoxic effects due to Russell's Viper have been observed with extracts of Tamarindus indica.[19] The aqueous extract and wedelolactone, from Eclipta prostrata, has shown antimyotoxic activity against Bothrops jararaca, and Bothrops jararacussu venoms and two isolated myotoxins bothropstoxin and crotoxin.[36,37] The extracts of genetically modified E.alba inhibited myotoxicity induced by PLA2 from the venoms of Crotalus durissus terrificus and Bothrops jararacussu.[55]
Dried root extracts of Mimosa pudica have inhibited the myotoxicity due to Naja kaouthia venom.[59] Curcuma longa has shown inhibition of myotoxicity due to Naja naja venom.[23] Partial inhibition of myotoxic activity has been reported with the Pentaclethra macroloba.[27]
Antivenin activity
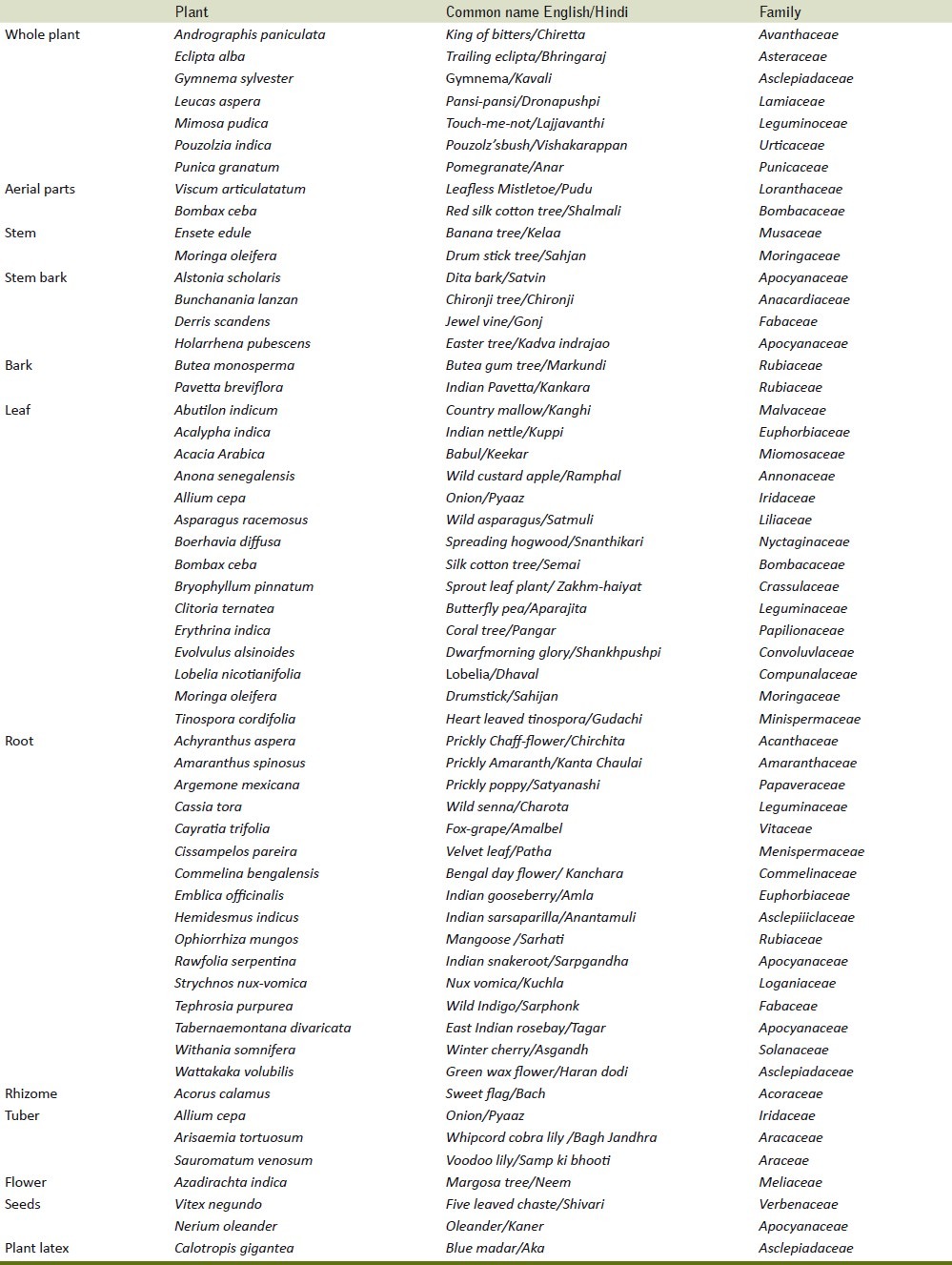
There is huge repository of medicinal plants used for treating snake bites [Table 4]. Many plants have been conserved and used as antidotes for snake envenomations.[60] Increase in survival rates of rats has been observed with Casearia sylvestris extract.[16] . Different species of Echinacea are used in North America for treating snake bites.[61] The plant contains echinacoside, cichoric acid, ketoalkenes, alkyl amides and polysaccharides.[62] Anisodamine, an alkaloid isolated from Anisodus tanguticus with the chemical structure and pharmacological action similar to atropine and scopolamine, has been proposed to be an effective drug for snake bites.[63]
Table 4.
Plants used for treating snakebites
Mucuna pruriens seeds are reported to neutralize toxicity due to Echis carinatus. A glycoprotein with functional oligosaccharide chains isolated from the plant is said to be responsible for the neutralization of venom-induced actions.[64] The seeds of the plant have been used as oral prophylactics for snake bite in Nigeria. Experimental studies on rats pretreated with extract and challenged with different snake venoms were done to investigate the effectiveness of anti-Mucuna pruriens antibody, to neutralize the toxicity of snake venoms in vitro. It was observed that pretreatment provided effective protection against lethality of venoms of Naja sputatrix and moderate protection against Calloselasma rhodostma, showing involvement of immunological neutralization.[65]
Significant neutralizing capacity against Macrovipera lebetina venom has been observed with the dichloromethane extract of Artemisia campestris.[66] Antiophidian activity of Masypianthes chamaedrys is reported in literature.[33] 12-methoxy-4-methylvoachalotine isolated from Tabernaemontana catharinensis has inhibited the lethal activity of crotoxin, the main toxin of Crotalus durissus terrificus.[67]
2-hydroxy-4-methoxy benzoic acid isolated form Hemidesmus indicus has shown antisnake venom activity in experimental models. It increased the lethal action neutralization of venom by polyvalent antiserum, suggesting the use of herbal antagonists for snake bites. It also reduced venom-induced free radical generation and showed antiserum action potentiation.[68,69] Leaf extract of Guiera senegalensis detoxified venom of Echis carinatus and Naja nigricollis in vitro. Albino mice given reconstituted venom incubated with the extract, intraperitoneally, showed reduction in mortality when compared with those given venom alone.[70] Root bark of Ehretia buxifolia is shown to possess antisnake activity. Ehretianone, a quinonoid xanthene is the active compound isolated from the plant.[44] Lipid peroxidation induced by viper venom in experimental animals is reported to be inhibited with Strychnos nux-vomica seed extract. The plant effectively neutralized viper venom lethality.[43] It contains caffeic acid and monomeric caffeic acid, an antidote for snake bites.
Allium cepa containing sulfurous volatile oils is used in South America for snake bites.[71] Protective effects of resverotrol (3, 4’, 5-trihydroxy trans stilbene) from Cissus assamica is well documented.[72] Stem bark extract of Parkia biglobosa has shown neutralization of venoms of Naja nigricollis and Echis ocellatus in experimental models.[73] Snake venom neutralization has been associated with leaf extract of Acalypha indica.[74]
Inhibitory activity is reported with salireposide and benzoylsalireposide, phenolic glycosides from Symplocos racemosa.[75] Bredemeyeroside D and B, triterpenoid saponins isolated from Bredemeyera floribunda have exhibited antisnake venom activity.[76,77] Cordia ecalyculata and Echinodorus grandiflorus are reportedly used in Brazil for various conditions including snake bites.[78] Pentacyclic triterpenes or glycosides shown to possess antisnake venom activity have been found in Alstonia scholaris, Aegle marmelos, Centipeda minima, Aloe vera, Elephentopus scaber etc.[79] Antivenin activity has been reported with extracts of Crinum jagus and Hibiscus aethiopicus Linn.[80,81]
Indian herbs with potential antivenin activity
Many Indian herbs have been used for the treatment of snake bites.[82–84] An ethno botanical survey of folk plants used in snake bites in southern parts of Tamil Nadu reports the use of 72 medicinal plants in snake bites. Plant extracts of Aristolochia indica (terpenoids), Hemidesmus indica (phenols), Gloriosa superba (esters), Strychnos nux-vomica, Rauwolfia serpentina (alkaloids), Eclipta prostrata (wedelolactone), Achyranthes aspera (glycosides) and Andrographis paniculata (terpenoids) have shown potent venom neutralizing effect. The plant extracts and partially purified fractions were administered orally to rats, envenomed with rattle snake venom. Significant protection against venom-induced changes in serum SOD and LPx levels were seen, after administration of purified fractions.[85]
Trichosanthes tricuspidata is used in Bastar district of Chhattisgarh for snake bites. The plant mainly contains pharmacologically important phytochemicals trichotetral, cucurbitane glycosides and cucurbitacins.[86] Root bark of Ehretia buxifolia is shown to possess antisnake activity.[44] Enicostemma axillare containing tannins is applied locally in snake bites.[58] Inhibition of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom has been reported with aqueous and alcoholic root extracts of Mimosa pudica.[59]
Plants used for snake bites in Assam include Amaranthus spinosus (roots/stem), Amaranthus viridis (stem, leaves), Argemone mexicana, Bryophyllum pinnatum (leaves), Commelina bengalensis (roots), Pouzolzia indica, Cassia tora (roots).[87] In the Malwa region of Madhya Pradesh, Eclipta alba (whole plant), Moringa oleifera (root, bark), Rauwolfia serpentina (spiral roots) and Tephrosia purpurea (root) have been used in snake bites, with latter two having excellent potential.[88]
Documentation of an ethno botanical survey and traditional medicines used by snake charmers in Haryana highlighted the use of 19 different medicinal plants for snake bites.[89] An ethno medicinal survey in Karnataka reports that mainly root extracts of some medicinal plants were used either alone or as a formulation for snake envenomations.[90]
The Zingiberaceae family comprising of rhizomatous medicinal plants is characterized by the presence of volatile oils and oleoresins. Various species of Curcuma, used extensively in Indian households, find use in a wide range of disorders. Curcuma aromatica and Curcuma longa have been used as an antidote in snake bites.[91] Amongst the various other Indian medicinal plants used and recommended for treatment of snake bites, Vitex negundo, Emblica officinalis significantly neutralized the Vipera russelii and Naja kaouthia venom-induced effects both in vitro and in vivo studies. Triterpenoids from the root extract of Emblica officinalis and Vitex negundo are believed to significantly neutralize antisnake activity of Vipera russelii and Naja kauothia.[92]
β–sitosterol and stigmasterol from the root extract of Pluchea indica along with antiserum have been proposed to help in neutralization of venom-induced effects.[93] Steroids form complexes with venom, held together by “Vander Waals” and hydrophobic forces.
Viper venom induced lipid peroxidation is reported to be inhibited in experimental animals by Strychnos nux-vomica seed extract.[43] Costus speciosus roots which contain diosgenin and starch in the rhizome have been used for snake bites.[91] Ipomoea digitata contains triterpenoids, phenolic compound and flavonoids and is used in snake bites. The root extract of the plant has shown antioxidant activity.[94] Ethnic people have conserved plants like Acorus calamus, Buchanania lanzan (stem bark), Moringa oleifera (stem, leaves), Achyranthes aspera, and Gynandropsis gynandra, Bombax ceiba, whose rhizome paste is an antidote for snake bite and scorpion sting.
CONCLUSION
Keeping in view the various limitations of ASV, herbal therapeutics for snake envenomations seem to be a viable alternative. However, there are only a few species of plants, believed to be effective for snake bites in traditional medicine whose pharmacological evaluation has been undertaken so far. In view of a plethora of active compounds in the plant kingdom, an in-depth scientific investigation is warranted to evaluate their antisnake venom potential, to derive therapeutically effective natural products for snake bites.
Integration of traditional healers who rely mainly on medicinal plants is necessary. Exploration of their materia medica for better alternatives for venom antidotes is essential. The benefit of phytotherapy is controversial because pharmacological and toxicological activities are not well studied and documented.
Complete phytochemical investigation of extracts and analysis of active principles to be used as potent therapeutic agents along with well-designed studies evaluating the pharmacologically active principles are necessary. Further, standardization of the basic active compound along with toxicity and safety studies is mandatory. In view of the conflict of healthcare practitioners with traditional medicine, validation of various reports may be carried out by corroborating their results, and thus selecting plants with potential. Scientific validation of the conventional therapies should be the long-term goal in order to test the veracity of the claims.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snakebite in South Asia: A review. PloS Negl Trop Dis. 2010;4:e603. doi: 10.1371/journal.pntd.0000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bawaskar HS. Snake venoms and antivenom issues: Critical supply issues. J Assoc Physicians India. 2004;52:11–3. [PubMed] [Google Scholar]
- 3.Joseph JK, Simpson ID, Menon NC, Jose MP, Kulkarni KJ, Raghavendra GB, et al. First authenticated cases of life threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans R Soc Trop Med Hyg. 2007;101:85–90. doi: 10.1016/j.trstmh.2006.03.008. Epub 2006 Jul 12. [DOI] [PubMed] [Google Scholar]
- 4.Mahadevan S. Antisnake venom (ASV)-A perspective. 2005. http://www.jipmer.edu/charu/Issue-2.pdf .
- 5.Williams DJ, Jensen SD, Nimorakiotakis B, Müller R, Winkel KD. Antivenom use, premedication and early adverse reactions in the management of snake bites in rural Papua New Guinea. Toxicon. 2007;49:780–92. doi: 10.1016/j.toxicon.2006.11.026. Epub 2006 Dec 2. [DOI] [PubMed] [Google Scholar]
- 6.Cannon R, Ruha AM, Kashani J. Acute hypersensitivity reactions associated with administration of crotalidae polyvalent immune Fab antivenom. Ann Emerg Med. 2008;51:407–11. doi: 10.1016/j.annemergmed.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Dhanya SP, Bindu LR, Hema CG, Dhanya TH. Antisnake venom use: A retrospective analysis in a tertiary care centre. Cal Med J. 2009;7:e2. [Google Scholar]
- 8.Shashidharamurthy R, Kemparaju K. Region specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern regional venom: A comparative study of the venoms from three different geographical distributions. Int Immunopharmacol. 2007;7:61–9. doi: 10.1016/j.intimp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Tsai IH, Tsai HY, Wang YM, Tun-Pe, Warrell DA. Venom phospholipases of Russell's vipers from Myanmar and eastern India- cloning,characterization and phylogeographic analysis. Biochim Biophys Acta. 2007;1774:1020–8. doi: 10.1016/j.bbapap.2007.04.012. Epub 2007 May 5. [DOI] [PubMed] [Google Scholar]
- 10.Coe FG, Anderson GJ. Snakebite ethnopharmacopoeia of Nicaragua. J Ethnopharmacol. 2005;96:303–23. doi: 10.1016/j.jep.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Veronese EL, Esmeraldino LE, Trombone AP, Santana AE, Bechara GH, Kettelhut I, et al. Inhibition of myotoxic activity of Bothrops jararacussu venom and its 2 major myotoxins, Bth TX-I and BthTX-II by the aqueous extract of Tabernaemontana catharinensis A. DC. (Apocyanaceae) Phytomedicine. 2005;12:123–30. doi: 10.1016/j.phymed.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Harvey A. Testing of natural remedies for natural toxins. Toxicon. 2003;41:939. doi: 10.1016/s0041-0101(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 13.Martz W. Plants with a reputation against snakebite. Toxicon. 1992;30:1131–42. doi: 10.1016/0041-0101(92)90429-9. [DOI] [PubMed] [Google Scholar]
- 14.Houghton PJ, Osibogun IM. Flowering plants used against snakebite. J Ethnopharmacol. 1993;39:1–29. doi: 10.1016/0378-8741(93)90047-9. [DOI] [PubMed] [Google Scholar]
- 15.Nunez V, Otero R, Barona J, Saldarriaga M, Osorio RG, Fonnegra R, et al. Neutralization of edema-forming, defibrinating and coagulant effects of Bothrops asper venom by extracts of plants used by healers of Colombia. Braz J Med Biol Res. 2004;37:969–77. doi: 10.1590/s0100-879x2004000700005. Epub 2004 Jun 22. [DOI] [PubMed] [Google Scholar]
- 16.da Silva SL, Calgarotto AK, Chaar JS, Marangoni S. Isolation and characterization of ellagic acid derivatives isolated from Casearia sylvestris SW aqueous extract with antiPLA2 activity. Toxicon. 2008;52:655–56. doi: 10.1016/j.toxicon.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Mahadeswaraswamy YH, Devaraja S, Kumar MS, Goutham YN, Kemparaju K. Inhibition of local effects of Indian daboia / Vipera russelli venom by the methanolic extract of grape (Vitis vinifera L.) seeds. Indian J Biochem Biophys. 2009;46:154–60. [PubMed] [Google Scholar]
- 18.Ticli FK, Hage LI, Cambraia RS, Pereira PS, Magro AJ, Fontes MR, et al. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): Antiserum action potentiation & molecular interaction. Toxicon. 2005;46:318–27. doi: 10.1016/j.toxicon.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Ushanandini S, Nagaraju S, Harish Kumar K, Vedavathi M, Machiah DK, Kemparaju K, et al. The anti snake venom properties of Tamarindus indica (leguminosae) seed extract. Phytother Res. 2006;20:851–8. doi: 10.1002/ptr.1951. [DOI] [PubMed] [Google Scholar]
- 20.Ushanandini S, Nagaraju S, Nayaka SC, Kumar KH, Kemparaju K, Girish KS. The anti-ophidian properties of Anacardium occidentale bark extract. Immunopharmacol Immunotoxicol. 2009;31:607–15. doi: 10.3109/08923970902911909. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee I, Chakravarty AK, Gomes A. Daboia russellii and Naja kaouthia venom neutralization by lupeol acetate isolated from the root extract of Indian sarsaparilla Hemidesmus indicus R.Br. J Ethnopharmacol. 2006;106:38–43. doi: 10.1016/j.jep.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Assafim M, Ferreira MS, Frattani FS, Guimarães JA, Monteiro RQ, Zingali RB. Counteracting effect of glycyrrhizin on the haemostatic abnormalities induced by Bothrops jararaca snake venom. Br J Pharmacol. 2006;148:807–43. doi: 10.1038/sj.bjp.0706786. Epub 2006 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chethankumar M, Srinivas L. New biological activity against phospholipase A2 by turmerin, a protein from Curcuma longa L. Biol Chem. 2008;389:299–303. doi: 10.1515/BC.2008.024. [DOI] [PubMed] [Google Scholar]
- 24.Jager AK, Hutchings A, Stadenvan J. Screening of Zulu medicinal plants used for medicinal purposes. J Ethnopharmacol. 1996;52:95–100. doi: 10.1016/0378-8741(96)01395-5. [DOI] [PubMed] [Google Scholar]
- 25.Chaurasia S. Anti-inflammatory and antioxidant activity of Strychnos nux vomica Linn. Am Eurasian J Sustain Agric. 2009;3:244–52. [Google Scholar]
- 26.Meenatchisundaram S, Parameswari G, Subbraj T, Michael A. Studies on antivenom activity of Andrographis paniculata and Aristolochia indica plant extracts against Echis carinatus venom. The Internet J Toxicol. 2009;6 [Google Scholar]
- 27.da Silva JO, Coppede JS, Fernandes VC, Sant’ana CD, Ticli FK, Mazzi MV, et al. Antihemorrhagic, antinucleolytic and other antiophidian properties of the aqueoua extract from Pentaclethra macroloba. J Ethnopharmacol. 2005;100:145–52. doi: 10.1016/j.jep.2005.01.063. Epub 2005 Apr 20. [DOI] [PubMed] [Google Scholar]
- 28.Nishijima CM, Rodrigues CM, Silva MA, Lopes-Ferreira M, Vilegas W, Hiruma-Lima CA. Anti- hemorrhagic activity of four Brazilian vegetable species against Bothrops jararaca venom. Molecules. 2009;14:1072–80. doi: 10.3390/molecules14031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendes MM, Oliveira CF, Lopes DS, Vale LH, Alcântara TM, Izidoro LF, et al. Antisnake venom properties of Schizolobium parahyba (Caesalpinoideae) aqueous leaves extract. Phytother Res. 2008;22:859–66. doi: 10.1002/ptr.2371. [DOI] [PubMed] [Google Scholar]
- 30.Leanpolchareanchai J, Pithayanukul P, Bavovada R. Antinecrosis potential of polyphenols against snake venoms. Immunopharmacol Immunotoxicol. 2009;31:556–62. doi: 10.3109/08923970902821702. [DOI] [PubMed] [Google Scholar]
- 31.Houghton PJ, Skari KP. The effect on blood clotting of some west African plants used against snake bite. J Ethnoparmacol. 1994;44:99–108. doi: 10.1016/0378-8741(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 32.Guerranti R, Aguiyi JC, Errico E, Pagani R, Marinello E. Effects of Mucuna pruriens extract on activation of prothrombin by Echis carinatus venom. J Ethnopharmacol. 2001;75:175–80. doi: 10.1016/s0378-8741(00)00401-3. [DOI] [PubMed] [Google Scholar]
- 33.Castro KN, Carvalho AL, Ameida AP, Oliveira DB, Borba HR, Costa SS, et al. Preliminary in vitro studies on the Marsypianthes chamaedrys (boia-caa) extracts at fibrinoclotting induced by snake venoms. Toxicon. 2003;41:929–32. doi: 10.1016/s0041-0101(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 34.Alam MI, Auddy B, Gomes A. Isolation, purification and partial characterization of viper venom inhibiting factor from the root extract of the Indian medicinal plant sarsaparilla (Hemidesmus indicus R.Br.) Toxicon. 1994;32:1551–57. doi: 10.1016/0041-0101(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 35.Pithayanukul P, Leanpolchareanchai J, Bavovada R. Inhibitory effect of tea polyphenols on local tissue damage induced by snake venoms. Phytother Res. 2010;S1:S56–62. doi: 10.1002/ptr.2903. [DOI] [PubMed] [Google Scholar]
- 36.Melo PA, do Nascimento MC, Mors WB, Suarez-Kurt G. Inhibition of myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrate (Asteraceae) extracts and constituents. Toxicon. 1994;32:595–603. doi: 10.1016/0041-0101(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 37.Mors WB. Plants against snakebites. Mem Inst Oswaldo Cruz. 1991;86(Suppl 2):193. doi: 10.1590/s0074-02761991000600044. [DOI] [PubMed] [Google Scholar]
- 38.Pithayanukul P, Laovachirasuwan S, Bavovada R, Pakmanee N, Suttisri R. Anti venom potential of butanolic extract of Eclipta prostrata against Malayan pit vipervenom. J Ethnopharmacol. 2004;90:347–52. doi: 10.1016/j.jep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 39.da Silva JO, Fernandes RS, Ticli FK, Oliveira CZ, Mazzi MV, Franco JJ, et al. Triterpenoid saponins, new metalloprotease snake venom inhibitors isolated from Pentaclethra macroloba. Toxicon. 2007;50:283–91. doi: 10.1016/j.toxicon.2007.03.024. Epub 2007 Apr 13. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira LA, Henriqyes OB, Andreoni AA, Vital GR, Campos MM, Habermehl GG, et al. Antivenom & biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae) Toxicon. 1992;30:1211–8. doi: 10.1016/0041-0101(92)90437-a. [DOI] [PubMed] [Google Scholar]
- 41.Kuppuswamy UR, Das N. Protective effects of tannic acid and related natural compounds on Crotalus adamenteus subcutaneous poisoning in mice. Pharmacol Toxicol. 1993;72:290–5. doi: 10.1111/j.1600-0773.1993.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 42.Januario AH, Santos SL, Marcussi S, Mazzi MV, Pietro RC, Sato DN, et al. Neo-clerodane diterpenoid, a new metalloprotease snake venom inhibitor from Baccharis trimera (Asreraceae):anti-proteolytic and anti-hemorrhagic properties. Chem Biol Interact. 2004;150:243–51. doi: 10.1016/j.cbi.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee I, Chakravarty AK, Gomes A. Antisnake venom activity of ethanolic seed extract of Strychnos nux vomica Linn. Indian J Exp Biol. 2004;42:468–75. [PubMed] [Google Scholar]
- 44.Selvanayagam ZE, Gnanavendhan SG, Balakrishna K, Rao RB, Sivaraman J, Subramanian K, et al. Ehretianone, a novel quinonoid xanthene from Ehretia buxifolia with antisnake venom activity. J Nat Prod. 1996;59:664–7. doi: 10.1021/np960355p. [DOI] [PubMed] [Google Scholar]
- 45.Lans C, Harper T, Georges K, Bridgewater E. Medicinal and ethnoveterinary remedies of hunters in Trinidad. BMC Complement Altern Med. 2001;1:10. doi: 10.1186/1472-6882-1-10. Epub 2001 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.da Silva AJ, Coelho AL, Simas AB, Moraes RA, Pinheiro DA, Fernandes FF, et al. Synthesis and pharmacological evaluation of prenylated and benzylated pterocarpans against snake venoms. Bioorg Med Chem Lett. 2004;14:431–5. doi: 10.1016/j.bmcl.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 47.Girish KS, Mohanakumari HP, Nagaraju S, Vishwanath BS, Kemparaju K. Hyaluronidase and protease activities from Indian snake venoms: Neutralization by Mimosa pudica root extract. Fitoterapia. 2004;75:378–80. doi: 10.1016/j.fitote.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Mukherjee AK, Doley R, Saikia D. Isolation of a snake venom phospholipase A2 (PLA2) inhibitor (AIPLAI) from leaves of Azadirachta indica (Neem): Mechanism of PLA2 inhibition by AIPLAI in vitro condition. Toxicon. 2008;51:1548–53. doi: 10.1016/j.toxicon.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Machiah DK, Girish KS, Gowda TV. A glycoprotein from a folk medicinal plant, Withania somnifera, inhibits hyaluronidase activity of snake venoms. Comp Biochem Physiol C Toxicol Pharmacol. 2006;143:158–61. doi: 10.1016/j.cbpc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Nunez V, Castro V, Murillo R, Ponce-Soto LA, Merfort I, Lomonte B. Inhibitory effects of Piper umbellatum & Piper peltatum extracts towards myotoxic phospholipases A2 from Bothrops snake venoms: Isolation of 4-nerolidycatechol as active principle. Phytochemistry. 2005;66:1017–25. doi: 10.1016/j.phytochem.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 51.Vishwanath BS, Kini RM, Gowda TV. Characterization of three edema-inducing phospholipase A2 enzymes from habu (Trimeresurus flavoviridis ) venom and their interaction with the alkaloid aristolochic acid. Toxicon. 1987;25:501–15. doi: 10.1016/0041-0101(87)90286-8. [DOI] [PubMed] [Google Scholar]
- 52.Vishwanath S, Gowda TV. Interaction of aristolochic acid with Vipera russelli phospholipase A2: Its effect on enzymatic & pathological activities. Toxicon. 1987;25:929–37. doi: 10.1016/0041-0101(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 53.Soares M, Ticli FK, Marcussi S, Lourenço MV, Januário AH, Sampaio SV, et al. Medicinal plants with inhibitory properties against snake venoms. Curr Med Chem. 2005;12:2625–41. doi: 10.2174/092986705774370655. [DOI] [PubMed] [Google Scholar]
- 54.Kini RM, Gowda TV. Studies on snake venom enzymes: Part I.Purification of ATPases from Naja naja venom and its inhibition by potassium gymnemate. Indian J Biochem Biophys. 1982;19:152–54. [PubMed] [Google Scholar]
- 55.Diogo LC, Fernandes RS, Marcussi S, Menaldo DL, Roberto PG, Matrangulo PV, et al. Inhibition of snake venoms and phospholipases A 2 by extracts from native and genetically modified Eclipta alba: Isolation of active coumestans. Basic Clin Pharmacol Toxicol. 2009;104:293–9. doi: 10.1111/j.1742-7843.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- 56.Napimoga MH, Yatsuda R. Scientific evidence for Mikania laevigata and Mikania glomerata as a pharmacological tool. J Pharm Pharmacol. 2010;62:809–20. doi: 10.1211/jpp.62.07.0001. [DOI] [PubMed] [Google Scholar]
- 57.Negi P, Anandharamakrishnan C, Jayaprakasha GK. Antibacterial activity of Aristolochia bracteata root extracts. J Med Food. 2003;6:401–3. doi: 10.1089/109662003772519994. [DOI] [PubMed] [Google Scholar]
- 58.Pavithra PS, Janani VS, Charumathi KH, Indumathy R, Potala S, Verma RS. Anticbacterial activity of plants used in Indian herbal medicine. Int J Green Pharm. 2010;4:22–8. [Google Scholar]
- 59.Mahanta M, Mukherjee AK. Neutralization of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom by Mimosa pudica root extracts. J Ethnopharmacol. 2001;75:55–60. doi: 10.1016/s0378-8741(00)00373-1. [DOI] [PubMed] [Google Scholar]
- 60.Meenatchisundaram S, Parameswari G, Subbraj T, Michael A. Antivenom activity of medicinal plants-a mini review. Ethnobot Leaflets. 2008;12:1218–20. [Google Scholar]
- 61.Huntley AL, Thompson CJ, Ernst E. The safety of herbal medicinal products derived from Echinacea species: A systematic review. Drug Saf. 2005;28:387–400. doi: 10.2165/00002018-200528050-00003. [DOI] [PubMed] [Google Scholar]
- 62.Hostettmann K. History of a plant: The example of Echinacea. Forsch Komplementarmed Klass Naturheilkd. 2003;10(Suppl 1):9–12. doi: 10.1159/000071678. [DOI] [PubMed] [Google Scholar]
- 63.Li QB, Pan R, Wang GF, Tang SK. Anisodamine as an effective drug to treat snakebites. J Nat Toxins. 1999;8:327–30. [PubMed] [Google Scholar]
- 64.Guerranti R, Aguiyi JC, Ogueli IG, Onorati G, Neri S, Rosati F, et al. Protection of Mucuna pruriens seeds against Echis carinatus venom is exerted through a multiform glycoprotein whose oligosaccharide chains are functional in this role. Biochem Biophys Res Commun. 2004;323:484–90. doi: 10.1016/j.bbrc.2004.08.122. [DOI] [PubMed] [Google Scholar]
- 65.Tan NH, Fung SY, Sim SM, Marinello E, Guerranti R, Aguiyi JC. The protective effect of Mucuna pruriens seeds against snake venom poisoning. J Ethnbopharmacol. 2009;123:356–58. doi: 10.1016/j.jep.2009.03.025. Epub 2009 Mar 26. [DOI] [PubMed] [Google Scholar]
- 66.Memmi A, Sansa G, Rjeibi I, El Ayeb M, Srairi-Abid N, Bellasfer Z, et al. Use of medicinal plants against scorpionic and ophidian venoms. Arch Inst Pasteur Tunis. 2007;84:49–55. [PubMed] [Google Scholar]
- 67.de Almeida L, Cintra AC, Veronese EL, Nomizo A, Franco JJ, Arantes EC, et al. Anticrotalic and antitumoral activities of gel filtration fractions of aqueous extract from Tabernaemontana catharinensis (Apocyanaceae) Comp Biochem Physiol C Toxicol Pharmacol. 2004;137:19–27. doi: 10.1016/j.cca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Alam MI, Gomes A. Viper venom induced inflammation and inhibition of free radical formation by pure compound (2-hydroxy-4-methoxy benzoic acid) isolated and purified from anantamul (Hemidesmus indicus R.Br.) root extract. Toxicon. 1998;36:207–15. doi: 10.1016/s0041-0101(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 69.Alam MI, Gomes A. Adjuvant effects and antiserum action potentiation by a (herbal) compound 2-hydroxy-4-methoxy benzoic acid isolated from the root extract of the Indian medicinal plant “sarsaparilla” (Hemidesmus indicus R. Br.) Toxicon. 1998;36:1423–31. doi: 10.1016/s0041-0101(98)00076-2. [DOI] [PubMed] [Google Scholar]
- 70.Abubakar MS, Sule MI, Pateh UU, Abdurahman EM, Haruna AK, Jahun BM. In vitro snake venom detoxifying action of leaf extract of Guiera senegalensis. J Ethnopharmacol. 2000;69:253–7. doi: 10.1016/s0378-8741(99)00128-2. [DOI] [PubMed] [Google Scholar]
- 71.Bennett BC, Prance GT. Introduced plants in the indigenous pharmacopoeia of northern South America. Econ Bot. 2000;54:90–102. [Google Scholar]
- 72.Yang LC, Wang F, Liu M. A study of an endothelin antagonist from a Chinese anti snake venom medicinal herb. J Cardiovasc Pharmacol. 1998;31:S249–50. doi: 10.1097/00005344-199800001-00070. [DOI] [PubMed] [Google Scholar]
- 73.Asuzu IU, Harvey AL. The antisnake venom activities of Parkia biglobosa (Mimosaceae) stem bark. Toxicon. 2003;42:763–8. doi: 10.1016/j.toxicon.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Shirwaiker A, Rajendran K, Bodla R, Kumar CD. Neutralization potential of Viper russelli (Russell's viper) venom by ethanol leaf extract of Acalypha indica. J Ethnopharmacol. 2004;94:267–73. doi: 10.1016/j.jep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 75.Ahmad VC, Abbasi MA, Hussain H, Akhtar MN, Farooq U, Fatima N, et al. Phenolic glycosides from Symplocos racemosa: Natural inhibitors of phosphodiesterase. Phytochemistry. 2003;63:217–20. doi: 10.1016/s0031-9422(03)00075-x. [DOI] [PubMed] [Google Scholar]
- 76.Pereira BMR, Daros MR, Parente JP, Matos FJA. Bredemeyeroside D, a novel triterpenoid saponin from Bredemeyera floribunda: A potent snake venom antidote activity on mice. Phytother Res. 1996;10:666–9. [Google Scholar]
- 77.Daros MR, Mtos FJA, Parente JP. A new triterpenoid saponin, Bredemeueroside from Bredmeyera floribunda. Planta Med. 1996;62:523–7. doi: 10.1055/s-2006-957962. [DOI] [PubMed] [Google Scholar]
- 78.da Silva CJ, Bastos JK, Takahashi CS. Evaluation of the genotoxic and cytotoxic effects of crude extracts of Cordia ecalyculata and Echinodorus grandiflorus. J Ethnopharmacol. 2010;127:445–50. doi: 10.1016/j.jep.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Mors WB, Nascimento MC, Pereira BM, Pereira NA. Plant natural products active against snake-bite-the molecular approach. Phytochemistry. 2000;55:627–42. doi: 10.1016/s0031-9422(00)00229-6. [DOI] [PubMed] [Google Scholar]
- 80.Ode OJ, Asuzu IU. The antisnake venom activities of the methanolic extract of the bulb of Crinum jagus (Amaryllidaceae) Toxicon. 2006;48:331–42. doi: 10.1016/j.toxicon.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Hasson SS, Al-Jabri AA, Sallam TA, Al-Balushi MS, Mothana RA. Antisnake venom activity of Hibiscus aethiopicus L. against Echis ocellatus and Naja n.nigricollis. J Toxicol. 2010;2010:837864. doi: 10.1155/2010/837864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muthu C, Ayyanar M, Raja N, Ignacimuthu S. Medicinal plants used by traditional healers in Kancheepuram district of Tamilnadu, India. J Ethnobiol Ethnomed. 2006;2:43. doi: 10.1186/1746-4269-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uniyal SK, Singh KN, Jamwal P, Lal B. Traditional use of medicinal plants among the tribal communities Chhota Bhangal, Western Himalaya. J Ethnobiol Ethnomed. 2006;2:14. doi: 10.1186/1746-4269-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Upadhyay PB, Roy S, Kumar A. Traditional uses of medicinal plants among the rural communities of Churu district in the Thar desert, India. J Ethnopharmacol. 2007;113:387–99. doi: 10.1016/j.jep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 85.Samy RP, Thwin MM, Gopalakrishnakone P, Ignacimuthu S. Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J Ethnopharmacol. 2008;115:302–31. doi: 10.1016/j.jep.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Bhandari S, Dobhal U, Sajwan M, Bisht NS. Trichosanthes tricuspidata: A medicinally important plant. Trees for Life J. 2008;3:5. [Google Scholar]
- 87.Sikdar M, Dutta U. Traditional phytotherapy among the Nath people of Assam. Ethno Med. 2008;2:39–45. [Google Scholar]
- 88.Dwivedi S, Shrivastava S, Dubey D, Kapoor S. Herbal remedies used in the treatment of scorpion sting and snake bite from the Malwa region of Madhya Pradesh. Ethnobotan Leaflets. 2009;13:326–8. [Google Scholar]
- 89.Panghal M, Arya V, Yadav S, Kumar S, Yadav JP. Indigenous knowledge of medicinal plants used by saperas community of Khetawas, Jhajjar district , Haryana, India. J Ethnobiol Ethnomed. 2010;6:4. doi: 10.1186/1746-4269-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hiremath VT, Taranath TC. Traditional phytotherapy for snake bites by tribes of Chitradurga District, Karnataka, India. Ethnobotan Leaflets. 2010;14:120–5. [Google Scholar]
- 91.Skaria BP, Joy PP, Mathew G, Mathew S. Zingiberaceous plants in traditional medicine. Proceedings of National seminar on role of medicinal and aromatic plants in ayurveda, unani and siddha systems of medicine. Hissar: CCS Haryana Agricultural University; 2005. Mar 4-5, pp. 15–20. [Google Scholar]
- 92.Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol. 2003;86:75–80. doi: 10.1016/s0378-8741(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 93.Gomes A, Saha A, Chatterjee I, Chakravarty AK. Viper and cobra venom neutralization by beta-sitosterol and stigmasterol isolated from the root of Pluchea indica Less. (Astreraceae) Phytomedicine. 2007;14:637–43. doi: 10.1016/j.phymed.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 94.Vasagam GA, Muthu KA, Kumar DS, Manavalan R. In-vitro antoioxidant activities of methanolic extract of tuberous root of Ipomoea digitata(Linn) J Pharm Res. 2010;3:639–41. [Google Scholar]


