Abstract
BACKGROUND & AIMS
Staging inadequately predicts metastatic risk in patients with colon cancer. We used a gene expression profile derived from invasive, murine colon cancer cells that were highly metastatic in an immunocompetent mouse model to identify patients with colon cancer at risk of recurrence.
METHODS
This phase 1, exploratory biomarker study used 55 patients with colorectal cancer from Vanderbilt Medical Center (VMC) as the training dataset and 177 patients from the Moffitt Cancer Center as the independent dataset. The metastasis-associated gene expression profile developed from the mouse model was refined with comparative functional genomics in the VMC gene expression profiles to identify a 34-gene classifier associated with high risk of metastasis and death from colon cancer. A metastasis score derived from the biologically based classifier was tested in the Moffitt dataset.
RESULTS
A high score was significantly associated with increased risk of metastasis and death from colon cancer across all pathologic stages and specifically in stage II and stage III patients. The metastasis score was shown to independently predict risk of cancer recurrence and death in univariate and multivariate models. For example, among stage III patients, a high score translated to increased relative risk of cancer recurrence (hazard ratio, 4.7; 95% confidence interval, 1.566–14.05). Furthermore, the metastasis score identified patients with stage III disease whose 5-year recurrence-free survival was >88% and for whom adjuvant chemotherapy did not increase survival time.
CONCLUSION
A gene expression profile identified from an experimental model of colon cancer metastasis predicted cancer recurrence and death, independently of conventional measures, in patients with colon cancer.
Keywords: Gene Expression Profiling, Colon Cancer Prognosis, Predictive Gene Signature, Mouse Model
Colorectal carcinoma is the third most commonly occurring noncutaneous carcinoma and the second leading cause of cancer-related death in the United States.1 Although it is well established that adjuvant chemotherapy (CTX) for patients with stage III colon cancer results in a survival benefit for the group, careful review of clinical trials data shows that 40%–44% of patients with stage III colon cancer enrolled in “surgery-only” groups did not recur in 5 years even without adjuvant treatment.2 Therefore, this subgroup of patients with stage III colon cancer, if prospectively identifiable, is unlikely to achieve benefit from and could be spared adjuvant CTX. In addition, clinical trials have failed to show the benefit of adjuvant CTX when applied to unselected patients with stage II colon cancer.3–5 However, some studies suggest that a subset of patients with high-risk stage II colon cancer may benefit from adjuvant therapy.6– 8 Much of these data for patients with stage II colon cancer comes from meta-analyses, and the question of whether adjuvant treatment really improves outcomes in stage II patients with “high-risk” features (eg, T4 lesions, poorly differentiated histology, or lymphovascular invasion) has not been answered in prospective clinical trials to date. Thus, an accurate and reliable method that identifies patients at greatest and least risk (eg, “high-risk” stage II and “low-risk” stage III colon cancer) could improve the selection of individualized therapy within these groups.
In this study, an experimental mouse model of metastasis was used to develop a gene expression profile that discriminated high versus low risk of cancer recurrence and death in patients with colon cancer. This biologically based model identified distinct subsets of patients with stage II and III colon cancer at greater risk of cancer recurrence and death. Using a metastasis score derived from the gene expression profile, we found that stage III patients with low metastasis score did not gain significant benefit from adjuvant CTX, suggesting that these patients could have been spared the potentially toxic and costly effects of these treatments. Conversely, patients with stage III disease with a high metastasis score treated with adjuvant CTX had markedly improved outcomes compared with patients with a high metastasis score who did not receive adjuvant CTX. Thus, our biologically based gene expression profile provides a potential platform to facilitate selection of patients with colon cancer who may benefit from adjuvant systemic therapy.
Materials and Methods
Cell Culture and Mouse Model
MC-38 mouse adenocarcinoma cells were obtained from the American Type Culture Collection (Manassas, VA).9 MC-38 cells were transfected with firefly luciferase gene (pGL3 basic; Promega, Madison, WI) and selected (G418; Invitrogen, Carlsbad, CA). To enrich for invasive cells, 7.5 × 105 cells were seeded onto 6-well, 8.0-micron pore transwell polycarbonate membrane inserts (Costar, Cambridge, MA) coated with 2.5 mg/mL matrigel, and incubated with serum-free Dulbecco’s modified Eagle’s medium in the upper chamber and complete Dulbecco’s modified Eagle’s medium in the bottom well. After 12 hours, cells that invaded were aseptically harvested by brief, gentle trypsinization and transferred to new dishes,10 and 6 serial passages through matrigel-coated Boyden chambers ensued. The selected invasive cells and the parental luciferase-expressing MC-38 cells were injected into the tail vein, and development of lung metastases was assessed.11 Development of metastases was determined by bioluminescence imaging.12,13 The MC-38met cells were derived by culture of tumor cells from a metastatic lung tumor.14 The Vanderbilt Institutional Animal Care and Use Committee approved all animal work.
Human Tissue and Microarray Platforms
The protocols and procedures for this study were approved by the institutional review boards at the University of Alabama-Birmingham Medical Center, Vanderbilt Medical Center (VMC), the Veterans Administration Hospital (Nashville, TN), and the H. Lee Moffitt Cancer Center (MCC; Tampa, FL). Representative sections of fresh tissue specimens were flash frozen in liquid nitrogen and stored at −80°C until RNA isolation. Quality assessment slides were obtained to verify the diagnosis. Stage was assessed by American Joint Commission on Cancer guidelines. RNA was purified with the use of the RNeasy kit (QIAGEN, Valencia, CA). Mouse and human samples were hybridized to Affymetrix arrays (Mouse Genome 430 2.0 GeneChip Expression and Human Genome U133 Plus 2.0 GeneChip Expression Arrays, respectively; Santa Clara, CA).
Statistical Methods and Identification of VMC High-Risk Patients
The 300-gene metastasis-associated signature was determined with the limma package in Bioconductor,15 based on 3 criteria: (1) fold change >2; (2) false discovery rate based on the moderated t test followed by Benjamini and Hochberg’s multiple-test adjustment <0.01; and (3) log odds ratio of differential expression (B statistic) >1. Directional concordance between MC-38met cells and 19 patients from VMC with poor outcome (17 patients with stage IV and 2 patients with stage III who developed metastatic recurrence; n = 19) was determined (P ≤ .10, exact binomial test) to refine the metastasis-associated signature to the 34-gene recurrence classifier. Integration of mouse and human microarray datasets was conducted.16 Clustering (Pearson’s correlation coefficient) was applied to the integrated dataset.
Clinical Outcomes and Testing of the 34-Gene–Based Metastasis Score
The association between individual gene expression level and clinical endpoint (overall survival [OS], disease-specific survival [DSS], and disease-free survival [DFS]) were first analyzed with the use of a Cox proportional hazard (PH) model,17–20 and definitions for each are provided in Supplementary Methods. A compound score was calculated for each patient by summation of a Cox PH weighted sum (Wald score) of log-2 gene expression for each gene in the classifier.20 The compound score was used to measure the effect of the 34-gene classifier on survival (OS and DSS) and recurrence (DFS). A Vanderbilt-derived Cox model and re-sampling Wald tests were used to rule out over-fitting of the model. The estimated coefficients of the Cox PH model in the VMC data (n = 55 patients) were applied to the MCC dataset (n = 177 patients). In addition, a censored C index21 was computed to validate the predictive value of the identified expression classifier.
The compound scores were used in a univariate analysis (eg, log-rank test) to segregate patients into higher-than-median and lower-than-median compound score groups. The compound score, age, sex, and tumor grade were adjusted in the multivariate Cox model for both DSS and DFS. Adjusted P values as well as the adjusted 95% confidence intervals of the hazard ratios (HRs) from the Cox model were reported. Fisher’s exact test was used for the T4, histologic, microsatellite instability status, and the stage III metastasis score/CTX analyses.
Results
Development of an Immunocompetent Mouse Model of Colon Cancer Metastasis
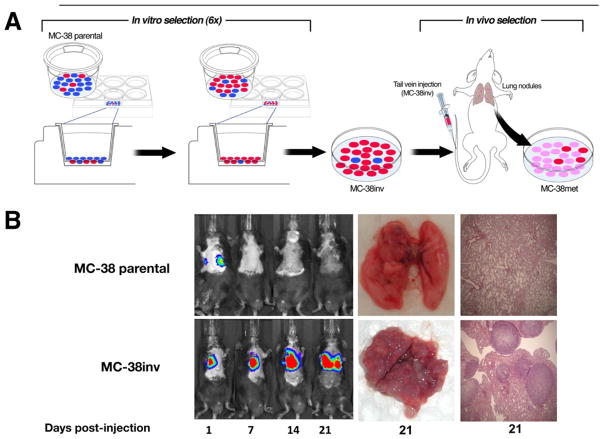
Tumors are a heterogeneous mixture of cells with varied invasive and metastatic potentials. Therefore, we used a conventional invasion assay to enrich for a subpopulation of highly invasive MC-38 mouse colon cancer cells (Figure 1A, MC-38inv). After 6 serial passages through matrigel, MC-38inv cells were more invasive than MC-38 parental cells both in vitro and in vivo in a tail vein injection assay (Figure 1B and Supplementary Table 1; P < .001). Lung tumors derived from MC-38inv cells were cultured to derive a highly metastatic cell line, MC-38met. MC-38met cells were injected into the tail vein and spleen and produced extensive metastatic tumors in the lung and liver, respectively (see Supplementary Figure 1 and Supplementary Table 2).
Figure 1.
Cell culture and mouse model: murine model of metastasis, in vivo monitoring, and ex vivo proof of metastases. (A) MC-38 parental cells (heterogeneous, blue and red) were subjected to 6 sequential passages through matrigel-coated transwells (enrichment of invasive subpopulations of MC-38 cells [red]) called “MC-38inv.” After in vivo passage, a stabilized cell line (pink cells) called “MC-38met” was established. (B) MC-38inv cells were tested alongside MC-38 parental cells for the ability to form lung metastasis in a tail vein assay. The figure shows representative tumor progression in live mice by bioluminescent imaging (days 1–21) and at the time of autopsy (day 21).
Discovery of a Gene Expression Profile Associated With Metastasis: Mouse to Man
A flow diagram of the derivation of the metastatic gene expression signature and its refinement and testing is provided in Figure 2A. MC-38 parental and MC-38met cell mRNA expression profiles were examined by microarray and directly compared to evaluate the gene expression changes associated with invasion and metastasis. Gene elements from this microarray profile were mapped to 11,465 corresponding human orthologs. Initial cluster analysis for the 300 genes is shown in Supplementary Figure 2. To refine the signature with relevance to cancer recurrence, each of these 300 genes was scored for directional concordance (see Materials and Methods) with gene expression data from 19 high-risk VMC patients with colon cancer who either had metastatic disease or had died of cancer progression (see Materials and Methods). Thirty-four genes (Table 1) from the metastasic gene signature exhibited directional concordance in 13 of the 19 high-risk patients. Because the 300 genes had been selected with stringent statistical criteria, we did not further attempt to determine the minimum number of genes that could discriminate outcomes.
Figure 2.
(A) Recurrence classifier development. VMC 2-step schematic for enrichment and establishment of the 34-gene recurrence classifier for colon cancer. Mouse genes were mapped to human orthologs, and 300 differentially expressed genes (MC-38 parental versus MC-38met) were identified. These 300 genes were next refined with 19 high-risk patients from the VMC training dataset for concordance. This analysis showed 34 genes with concordant expression among the 19 high-risk patients and the MC-38met cells. The 34-gene recurrence classifier was then applied to the independent MCC database to determine whether it could be used to discriminate patients on the basis of outcomes. (B) Functional genomic cluster analysis of the 34-gene recurrence classifier. Mean-centered gene expression data (rows) clustered with individual VMC patients (columns) results in 2 distinct patient groups (cluster 1, pink; cluster 2, green). The 19 VMC high-risk patients used in the concordance analysis are marked with a red asterisk.
Table 1.
Characterization of the 34 Gene Recurrence Classifier
| Gene symbol | Fold change | Processes/networks |
|---|---|---|
| Up-regulated genes | ||
| CXCR7 | 3.9 | Cancer, survival/growth, and chemotaxis |
| AK1 | 2.8 | Nucleotide binding |
| ACTB | 2.8 | Cancer, cell morphology and motility, growth, polarization, and adhesions |
| MGP | 2.8 | Cell–cell signaling, branching, migration |
| HES1 | 2.8 | Cancer, endocrine function, cell death |
| TMEM14A | 2.6 | Cell proliferation (target of CREB) |
| EGR1 | 2.5 | Cancer, endocrine function, cell death |
| VDR | 2.4 | Cancer, endocrine function, cell death |
| C6orf64 | 2.4 | Membrane dynamics |
| NQO1 | 2.4 | Cancer, cell death |
| STOX2 | 2.3 | Putative stem cell marker |
| ACYP2 | 2.2 | Mutated in aromatic rice |
| SPRY4 | 2.1 | Cancer; cell migration, proliferation, differentiation |
| DCTD | 2.1 | Nucleotide biosynthesis |
| TACC2 | 2.1 | Cancer; biogenesis, morphology, proliferation |
| PDLIM5 | 2.0 | Cancer, actin binding |
| Down-regulated genes | ||
| CRABP1 | −21.0 | Cancer, CpG island methylation |
| MMP13 | −17.3 | Cell–cell signaling, immune response |
| MYOT | −7.1 | Cancer, actin filaments and stress fibers |
| DFNB31 | −5.1 | Cell–cell signaling |
| HPSE | −4.7 | Cell–cell signaling, immune response |
| TEX11 | −3.7 | Cell cycle/division |
| SYT17 | −3.1 | Membrane protein |
| MUM1L1 | −2.8 | Unknown |
| SLC25A30 | −2.6 | Oxidative stress |
| CSN3 | −2.5 | Cell cycle, membrane dynamics |
| NMNAT3 | −2.4 | Nucleotide biosynthesis |
| DENND2A | −2.4 | Unknown |
| CIRBP | −2.3 | Cancer, nucleotide binding |
| SPDYA | −2.3 | Putative cell cycle |
| S100A3 | −2.2 | Cancer |
| PRTN3 | −2.1 | Cell–cell signaling, immune response |
| C20orf74 | −2.1 | GTPase regulation |
| HS3ST5 | −2.0 | Putative epigenetic regulation |
Genes that were up-regulated or down-regulated in both MC-38met–derived cells and in 19 patients from VMC with poor prognosis are shown. Gene symbols and associated fold-change in gene expression for MC-38 parental versus MC-38met as determined by microarray are given. Known relationships (connectedness) between up-regulated and down-regulated genes were determined independently, and, when networks existed, functional enrichment within the networks was determined in Ingenuity Pathways Analysis (www.ingenuity.com), PubMed, and on the Affymetrix website (see Supplementary Methods).
The resultant 34-gene expression pattern was designated the recurrence gene classifier and was next integrated with the entire VMC human microarray dataset and subjected to unsupervised cluster analysis. The recurrence gene classifier separated patients into 2 clusters (Figure 2B). The cluster associated with MC-38met cells (cluster 2) contained 17 of the 19 high-risk patients used in the signature refinement process.
Recurrence Classifier Identifies Patients With Colon Cancer With Poor Outcome in an Independent Colon Cancer Dataset
An independent human colon cancer gene expression and clinical database from MCC was used to test the ability of the recurrence classifier to discriminate patients at increased risk of cancer recurrence and death. The demographics for the training (VMC) and test (MCC) sets are shown in Table 2. Two hundred five patients were available for analysis in the MCC group and 195 of these patients had complete demographic, histologic grade, stage, and differentiation information. Of this 195-patient group, we focused on patients with colon cancer (n = 177) to avoid potential confounding effects of neoadjuvant and radiation therapies in tumor samples from patients with rectal cancer and outcomes. A method for weighted scoring of the gene expression pattern for the recurrence classifier was applied (see Materials and Methods), and metastasis scores were created. Patients were segregated into higher- and lower-than-median metastasis score groups, and survival analysis was performed between the 2 groups. To rule out over-fitting of the model, 3 separate statistical approaches were applied. First, we developed the metastasis score based on the Cox PH model in the VMC data (n = 55 patients) and then applied the estimated coefficients of the Cox PH model from VMC data to the MCC dataset (n = 177 patients). Thus, the sign and magnitude of the coefficient was completely based on the training dataset (VMC) and then tested in the MCC dataset. Patients with colon cancer with a high metastasis score across all stages in the MCC dataset had significantly worse OS and DSS than did the patients with a low metastasis score (Figure 3A and B; P = .003 and P = .04, respectively). Second, multiple permutation testing was performed with the 34-gene recurrence classifier, and the metastasis score was also robust in this model (see Supplementary Figure 3). Third, a C-index was calculated to validate the predictive value of the 34-gene metastasis score. The C-index is a probability of concordance between predicted and observed survival, with C = 0.5 for random predictions and C = 1 for a perfectly discriminating model.
Table 2.
Study Demographics
| VMC training dataset (n = 55)a | MCC testing dataset (n = 177) | |
|---|---|---|
| Age, y, mean ± SD | 62.3 ± 14.1 | 65.5 ± 13.1 |
| Sex, male, n (%) | 30 (54.5) | 96 (54.2) |
| Stage I, n (%) | 4 (7.3) | 24 (13.6) |
| Stage II, n (%) | 15 (27.3) | 57 (32.2) |
| Stage III, n (%) | 19 (34.5) | 57 (32.2) |
| Stage IV, n (%) | 17 (30.9) | 39 (22) |
| Median follow-up, mo (minimum–maximum) | 50.2 (0.4–111.3) | 48.1 (0.92–142.6) |
| No. of deaths (%) | 20 (36.3) | 73 (41.2) |
| White, n (%) | 50 (90.9) | 151 (85.3) |
| Black, n (%) | 4 (7.3) | 9 (5.1) |
| Other, n (%)b | 1 (1.8) | 17 (9.6) |
NOTE: All patients had colorectal adenocarcinoma (stages I–IV) according to current guidelines from the American Joint Commission on Cancer. One hundred seventy-seven of 205 MCC patients met the criteria of having stage I–IV colon cancer, as well as complete demographic, histologic grade, stage, and differentiation information. MCC, Moffitt Cancer Center; VMC, Vanderbilt Medical Center.
Includes 14 patients from the University of Alabama-Birmingham Medical Center (tumors provided by M.J.H.).
Other in the VMC medical record indicates not otherwise specified and implies Hispanic ethnicity.
Figure 3.
The 34-gene recurrence classifier as tested in the MCC dataset across all stages. Kaplan–Meier estimates of overall and disease-specific survival in the MCC test set. Expression data for probes corresponding to the 34-gene recurrence classifier were used to build the Cox proportional hazard model from patient data in the Vanderbilt dataset. Plots represent survival analyses in the MCC patient dataset, based on β and Wald statistics (see Supplementary Methods) from the Vanderbilt dataset. (A) Overall and (B) disease-specific survival analyses were performed.
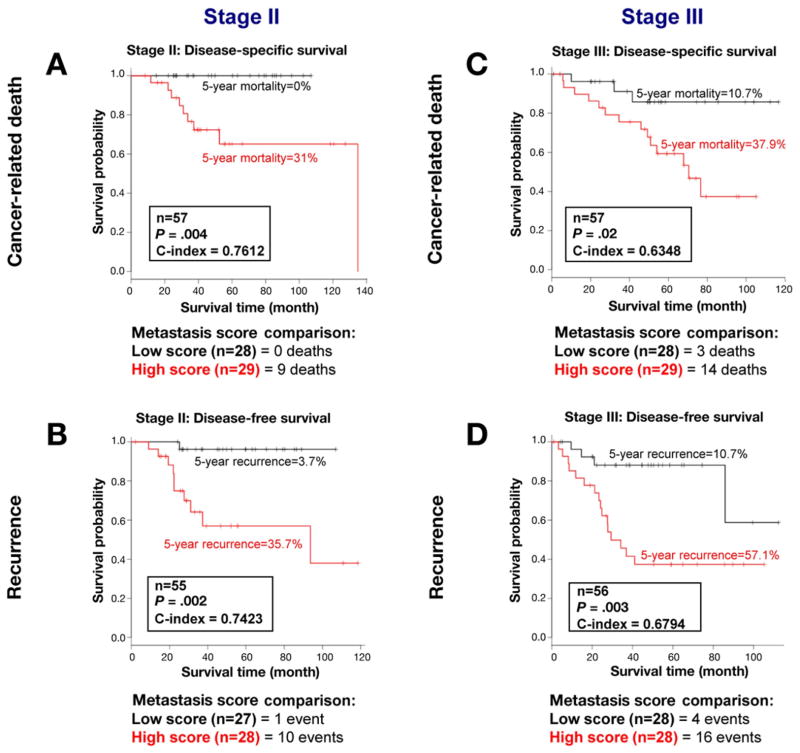
To determine whether the metastasis score could be used to identify patients with stage II and III colon cancer at high risk of recurrence, we tested it on patients with stage II and III colon cancer in the MCC dataset. Patients with a low metastasis score in each group (stage II alone, Figure 4A and B; stage III alone, Figure 4C and D) showed significantly better outcomes than did patients with a high metastasis score. As can be seen in the figure, this finding held true in both analyses that used the endpoints of DSS (cancer-related death; Figure 4A and C) as well as DFS (recurrence; Figure 4B and D).
Figure 4.
Kaplan–Meier estimates from 114 patients with colon cancer (stages II and III) under study at MCC were analyzed with the 34-gene–based metastasis score. Lower-than-median metastasis score is denoted in black and higher-than-median metastasis score is noted in red. A low score was associated with better disease-specific and disease-free survival in patients with stage II (A cancer-related death, n = 57 patients; high scores [9 of 9 total deaths] and B disease-free survival; n = 55; high scores [10 of 11 total events]). Similarly, a low score was associated with better disease-specific and disease-free survival in patients with stage III colon cancer (C cancer-related death, n = 57 patients, high scores [14 of 17 total deaths] and D disease-free survival, n = 56, high scores [16 of 20 total events]). Five-year mortality and recurrence rates are shown for patients with stage II and III colon cancer (A to D).
Notably, there were no cancer-related deaths and only one recurrence event in patients with stage II colon cancer with a low metastasis score (see Figure 4A and B). At 5 years, 31% of the patients with stage II colon cancer with high metastasis scores had died of cancer versus none of the patients with low scores. For the patients with stage III colon cancer, the 5-year mortality rate was 10.7% for patients with low metastasis scores compared with 37.9% for the patients with high metastasis scores (see Figure 4C). The median survival time for stage III colon cancer patients a high metastasis score was 29.4 months. In sharp contrast, the patients with stage III colon cancer with a low metastasis score as a group fared so well that this group had not reached the threshold to calculate their median survival time. We noted no association with either the high or the low metastasis score on available data about T4 lesions, lymph nodes retrieved, histologic grade, or microsatellite instability status in the MCC dataset (see Supplementary Results). These data show that the 34-gene metastasis score can discriminate patients with stage II and III colon cancer who have a low- or high-risk of cancer recurrence and death.
High Metastasis Score in Univariate and Multivariate Analyses Predicts Recurrence and Survival
The metastasis score was tested in the MCC patient dataset to determine the relative risk of recurrence and cancer-related death. Patients with a high metastasis score had increased relative risk of recurrence, as measured by HR across all stages (Table 3; HR, 4.9; P < .001). Patients with stage II colon cancer with a high score were also at increased relative risk of recurrence (HR, 13.1; P = .01). Finally, the relative risk of recurrence in patients with stage III colon cancer with a high score was in-creased in this analysis (HR, 4.7; P = .006). These data show that the metastasis score based on the 34-gene classifier is a strong predictor of recurrence and cancer-related death in patients with colon cancer.
Table 3.
The 34-Gene Metastasis score Associates With Increased Risk of Recurrence
| HR (95% CI) | P | |
|---|---|---|
| Disease-free survival | ||
| All stages | 4.9 (2.157–11.27) | <.001 |
| Stage II | 13.1 (1.660–103.1) | .01 |
| Stage III | 4.7 (1.566–14.05) | .006 |
Univariate analysis was done with metastasis scores to segregate patients from the MCC dataset into higher-than-median and lower-than-median score groups. Hazard ratios (HRs) were calculated for each patient group related to disease-free survival.
Multivariate analysis of the MCC patient data was performed to determine independent predictors of recurrence and survival. After adjusting for metastasis score, sex, tumor stage, age, and tumor grade, only the metastasis score (P < .001) and tumor stage (P = .002) were significant determinants of cancer recurrence (see Supplementary Table 3). We observed similar results with DSS as the outcome measure in univariate and multivariate models (see Supplementary Tables 4 and 5). Furthermore, the magnitude of the metastasis score was significantly associated with outcome. Hazard ratios for cancer-related death showed that across all stages, patients with high metastasis scores in the 50th, 75th, and 90th percentiles are at increased relative risk of cancer-related death (HR = 2.0, 3.1, and 4.6, respectively) than are patients with scores in the 10th percentile (see Supplementary Figure 4). Therefore, the 34-gene metastasis score is an independent predictor of cancer recurrence and death in patients with colon cancer even after adjustment for baseline characteristics available at resection (eg, age, grade, stage, and sex).
The 34-Gene–Based Metastasis Score Is Associated With Patient Benefit and Adjuvant CTX in Patients With Stage III Colon Cancer
As described above, a significantly greater proportion of patients with stage III colon cancer with a high metastasis score died of cancer than patients with a low metastasis score. Almost thirty percent (15 of 57 patients) of the stage III colon cancer patients did not receive adjuvant CTX. Therefore, we sought to determine whether there was a difference in survival between patients with high and low metastasis scores related to CTX administration. Stage III colon cancer patients with a low metastasis score had equivalent survival outcomes regardless of whether they received adjuvant CTX (Table 4; 10% with CTX versus 12.5% without CTX, P > .99). Among stage III patients with a high metastasis score, a significantly greater proportion of patients who did not receive CTX died of their cancer than patients who did receive adjuvant CTX (86% versus 36%; P = .04). There was no statistically significant difference in follow-up interval or in the proportion of patients receiving CTX between the high and low metastasis score groups (P = .576 and P = .770, respectively). These data suggest that patients with stage III colon cancer with a low metastasis score did not gain significant benefit from adjuvant CTX, whereas patients with stage III colon cancer with a high metastasis score had a better outcome after adjuvant CTX.
Table 4.
Metastasis Score Associates With Patient Benefit and Adjuvant CTX in Patients With Stage III Colon Cancer From the MCC Dataset
| Alive | Cancer-related death | P | |
|---|---|---|---|
| Metastasis score and survival | .003 | ||
| Low score, n (%) | 25 (62.5) | 3 (17.6) | |
| High score, n (%) | 15 (37.5) | 14 (82.4) | |
| Low score | >.99a | ||
| Adjuvant CTX (n = 20), n (%) | 18 (90) | 2 (10) | |
| No adjuvant CTX (n = 8), n (%) | 7 (87.5) | 1 (12.5) | |
| High score | .04 | ||
| Adjuvant CTX (n = 22), n (%) | 14 (63.6) | 8 (36.4) | |
| No adjuvant CTX (n = 7), n (%) | 1 (14.3) | 6 (85.7) |
CTX, chemotherapy; MCC, Moffitt Cancer Center.
No significant association was found between a low metastasis score and cancer-related death in patients with stage III who received chemotherapy compared with patients who did not receive chemotherapy (Fisher’s exact test).
Pathway Analysis of the 34-Gene–Based Metastasis Score
To relate our findings to basic tumor biology, we subjected the recurrence classifier to Ingenuity Pathways Analysis (Ingenuity Systems; www.ingenuity.com). Up-regulated genes formed a single network enriched for cancer and cell death, whereas the down-regulated genes formed a network enriched for cell-to-cell signaling/interaction and immune response (see Table 1 and Supplementary Results).
Discussion
In the present study, the biology of colon cancer metastasis was modeled in immunocompetent mice to develop a gene expression classifier that discriminates recurrence and survival outcomes in human patients with colon cancer. Patients with stage II and stage III primary colon cancers that reflected the recurrence-associated gene expression pattern were at greater relative risk of recurrence than patients who did not (HR = 13.1 and 4.7, respectively). This gene expression profile, tested with a recurrence scoring method, performed independently of conventional pathologic staging.
Perhaps most importantly, this metastasis score identifies patients with stage II colon cancer at high risk of recurrence and death and patients with stage III colon cancer at low risk of recurrence and death. Our biological model has identified a subset of high-risk stage II patients who may benefit from adjuvant therapy and a subset of low-risk stage II patients who may have an excellent outcome after surgical resection without adjuvant therapy. We found that the 5-year survival rate was >95% in patients with stage II colon cancer who had a low metastasis score, suggesting that adjuvant CTX would provide minimal benefit in this group of patients. In contrast, 31% of patients with stage II colon cancer with a high metastasis score died of cancer. Our preliminary analyses of these data suggest that patients with stage II colon cancer with high metastasis scores should be further studied to determine whether they will benefit from adjuvant therapy.
A unique aspect of our study is the inclusion of sufficient numbers of patients with stage III colon cancer who did not receive adjuvant CTX in the MCC database. This enabled an evaluation of whether the molecular metastasis score could predict response to adjuvant therapy. Of the patients with stage III colon cancer with high metastasis scores who were treated with adjuvant CTX only 36.4% died of cancer, whereas 85.7% of the patients with high scores who did not receive adjuvant CTX died of cancer. Despite the small numbers in these subgroups, the differences were statistically significant. More importantly, equally low proportions of patients with stage III colon cancer with a low metastasis score died of cancer regardless of administration of CTX. Our data suggest that there is a low-risk group of patients with stage III colon cancer who could be surgically cured and spared the morbidity, expense, and potential mortality associated with adjuvant CTX. This is consistent with prior observations from randomized clinical trials that established the benefits of adjuvant CTX in stage III colon cancer whereby 40%–44% of patients enrolled in the surgery-only groups did not recur in 5 years even without adjuvant treatment.2 Determination of an objective scoring method whereby the 34-gene classifier can be tested in a prospective fashion is ongoing and will be required to determine whether the 34-gene– based metastasis score can be used clinically to guide decisions about adjuvant therapy for patients with stage III colon cancer.
Several investigative groups have reported gene expression classifiers with predictive power in breast, lung, liver, and colorectal cancers.22–27 Like the previously described colon cancer classifiers, a weakness of our model is the retrospective analysis of prospectively collected clinical data. A 43-gene poor-prognosis signature for colorectal cancer provides a classifier for patients with stage II and stage III as a molecular staging device.28 In a more recent study, a computational model was used to derive a 50-gene signature and a metastasis score for early-stage colon cancer.29 We found minimal overlap between our 34-gene classifier and the previously published, computationally derived colon cancer gene signatures. We were not surprised at this finding because the prior models were computationally determined, and ours is founded on the biology of metastasis.
We find it interesting that 13 of the 34 genes in our proposed classifier have previously described roles in cancer, and several others are involved in cell–cell signaling, immune response, cell proliferation, embryonic development, and cell migration. Inflammation plays a potent role in gastrointestinal tract tumor promotion, and tumor progression is associated with immune suppression.30–32 In this study, microarray analysis identified perturbations in gene networks related to both carcinogenesis and inflammation. Ongoing work in our laboratory aims to unravel the molecular mechanisms by which these 2 gene networks may interact to promote metastasis in our experimental model.
The cross-species functional genomics approach yields insights into the molecular mechanisms of the metastatic process. Consistent with our approach, gene expression patterns identified in wound healing have been applied successfully to breast cancer outcomes.33 In addition, cell culture and mouse models have also shown relevance to clinical outcomes in hepatocellular carcinoma with the use of gene expression profiling.34,35 Similarly, our approach has uncovered a gene classifier with prognostic significance in colon cancer.
Although a high score based on the 34-gene recurrence classifier worked well in this study, the number of significant genes reported was not based on the smallest number of genes that could discriminate the survival endpoint, but was based on the combined statistical, biological, and clinical evidence in the available data. There is also the possibility that some of the computationally derived genes discovered in human datasets would be missed in a mouse model; however, the biological basis of the 34-gene classifier derived from our mouse model seems to be a robust predictor. The possibility of achieving similar or better survival discrimination with different subsets of the genes certainly exists; however, we feel that the biological basis of our study provides a solid foundation for further translational application and testing of our model.
In conclusion, the 34-gene– based metastasis score can identify patients with stage II and III colon cancer at greater risk of colon cancer recurrence and death. Our biologically based expression classifier identifies a potential method for more appropriate selection of patients for systemic therapy after curative-intent surgical resection of colon cancer. Future prospective studies are needed to confirm whether CTX may be safely avoided in patients with stage III colon cancer with a low metastasis score and whether patients with stage II colon cancer with a high metastasis score can achieve a better outcome if they receive adjuvant CTX.
Supplementary Material
Acknowledgments
The authors thank James Goldenring, MD, PhD, Eric Neilson, MD, and Vivien Siegel, PhD for critically reviewing the manuscript in advance of submission. We thank Ramona Deal for derivation of luciferase-expressing MC-38 parental cells. We thank the Vanderbilt Microarray Shared Resource. We gratefully acknowledge the generous philanthropic support of the Ingram family. J.J.S. gratefully acknowledges support from the Society of University Surgeons-Ethicon Scholarship Award, the Vanderbilt Clinical and Translational Science Award, and the Vanderbilt Medical Scientist Training Program. The authors thank Erika Frazier (Vanderbilt Medical Center) and Joey Richardson (The University of Alabama-Birmingham Medical Center) for tissue collection/storage, clinical follow-up, and data storage. The authors thank Drs P. Wise, A. Herline, K. Sharp, and A.S. Pearson who allowed their patients to be studied. We thank Dominic Doyle and the Vanderbilt Illustration Medical Art Group for the creation of Figure 1A.
Funding
This work was supported by the following grants from the National Institutes of Health: CA69457, DK52334, CA068485, and CA077839 (R.D.B.); TL1 RR024978 and CA106183 (J.J.S.); CA46413, CA95103, and CA084239 (R.J.C.); CA112215 (T.J.Y.); and CA126588 and CA128323 (N.G.D.). Other sources of funding include the Society of University Surgeons-Ethicon Scholarship Award (J.J.S.).
Abbreviations used in this paper
- CTX
chemotherapy
- DFS
disease-free survival
- DSS
disease-specific survival
- HR
hazard ratio
- MCC
Moffitt Cancer Center
- OS
overall survival
- PH
proportional hazard
- VMC
Vanderbilt Medical Center
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2009.11.005.
GEO accession number is GSE17538. Complete Minimum information about a microarray experiment (MIAME)-compliant datasets for analysis is available (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17538).
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ragnhammar P, Hafstrom L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282–308. doi: 10.1080/02841860151116367. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, III, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 4.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 5.Mamounas E, Wieand S, Wolmark N, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) J Clin Oncol. 1999;17:1349–1355. doi: 10.1200/JCO.1999.17.5.1349. [DOI] [PubMed] [Google Scholar]
- 6.Gray R, Barnwell J, et al. Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 7.Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008:CD005390. doi: 10.1002/14651858.CD005390.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. J Clin Oncol. 2004;22:3395–3407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 9.Lafreniere R, Rosenberg SA. A novel approach to the generation and identification of experimental hepatic metastases in a murine model. J Natl Cancer Inst. 1986;76:309–322. [PubMed] [Google Scholar]
- 10.Poste G, Doll J, Fidler IJ. Interactions among clonal subpopulations affect stability of the metastatic phenotype in polyclonal populations of B16 melanoma cells. Proc Natl Acad Sci U S A. 1981;78:6226–6230. doi: 10.1073/pnas.78.10.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidler IJ, Nicolson GL. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976;57:1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- 12.Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins DE, Oei Y, Hornig YS, et al. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis. 2003;20:733–744. doi: 10.1023/b:clin.0000006815.49932.98. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead RH. The culture of tumour cells from human tumour biopsies. Clin Oncol. 1976;2:131–140. [PubMed] [Google Scholar]
- 15.Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Thorgeirsson SS. Genome-scale profiling of gene expression in hepatocellular carcinoma: classification, survival prediction, and identification of therapeutic targets. Gastroenterology. 2004;127(suppl):S51–S55. doi: 10.1053/j.gastro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Tukey JW. Tightening the clinical trial. Control Clin Trials. 1993;14:266–285. doi: 10.1016/0197-2456(93)90225-3. [DOI] [PubMed] [Google Scholar]
- 18.Hedenfalk I, Duggan D, Chen Y, et al. Gene-expression profiles in hereditary breast cancer. N Engl J Med. 2001;344:539–548. doi: 10.1056/NEJM200102223440801. [DOI] [PubMed] [Google Scholar]
- 19.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 20.Yanagisawa K, Shyr Y, Xu BJ, et al. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet. 2003;362:433–439. doi: 10.1016/S0140-6736(03)14068-8. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 22.Barrier A, Boelle PY, Roser F, et al. Stage II colon cancer prognosis prediction by tumor gene expression profiling. J Clin Oncol. 2006;24:4685–4691. doi: 10.1200/JCO.2005.05.0229. [DOI] [PubMed] [Google Scholar]
- 23.Shedden K, Taylor JM, Enkemann SA, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 25.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Jatkoe T, Zhang Y, et al. Gene expression profiles and molecular markers to predict recurrence of Dukes’ B colon cancer. J Clin Oncol. 2004;22:1564–1571. doi: 10.1200/JCO.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 27.Lin YH, Friederichs J, Black MA, et al. Multiple gene expression classifiers from different array platforms predict poor prognosis of colorectal cancer. Clin Cancer Res. 2007;13:498–507. doi: 10.1158/1078-0432.CCR-05-2734. [DOI] [PubMed] [Google Scholar]
- 28.Eschrich S, Yang I, Bloom G, et al. Molecular staging for survival prediction of colorectal cancer patients. J Clin Oncol. 2005;23:3526–3535. doi: 10.1200/JCO.2005.00.695. [DOI] [PubMed] [Google Scholar]
- 29.Garman KS, Acharya CR, Edelman E, et al. A genomic approach to colon cancer risk stratification yields biologic insights into therapeutic opportunities. Proc Natl Acad Sci U S A. 2008;105:19432–19437. doi: 10.1073/pnas.0806674105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Wang D, DuBois RN. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett. 2008;267:197–203. doi: 10.1016/j.canlet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–768. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 32.Kado S, Uchida K, Funabashi H, et al. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395–2398. [PubMed] [Google Scholar]
- 33.Chang HY, Sneddon JB, Alizadeh AA, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JS, Chu IS, Mikaelyan A, et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 35.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–1595. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.