Abstract
Although current postexposure prophylaxis rabies virus (RV) vaccines are effective, ~40,000–70,000 rabies-related deaths are reported annually worldwide. The development of effective formulations requiring only 1–2 applications would significantly reduce mortality. We assessed in mice and nonhuman primates the efficacy of replication-deficient RV vaccine vectors that lack either the matrix (M) or phosphoprotein (P) gene. A single dose of M gene–deficient RV induced a more rapid and efficient anti-RV response than did P gene–deficient RV immunization. Furthermore, the M gene–deleted RV vaccine induced 4-fold higher virus-neutralizing antibody (VNA) levels in rhesus macaques than did a commercial vaccine within 10 days after inoculation, and at 180 days after immunization rhesus macaques remained healthy and had higher-avidity antibodies, higher VNA titers, and a more potent antibody response typical of a type 1 T helper response than did animals immunized with a commercial vaccine. The data presented in this article suggest that the M gene–deleted RV vaccine is safe and effective and holds the potential of replacing current pre- and postexposure RV vaccines.
Rabies virus (RV) infections in humans are controlled by limiting infections in domestic and wild animals and through the use of rabies postexposure prophylaxis (PEP), which is administered after a person comes into contact with a potentially infected animal. Current vaccines consist of inactivated RV strains and are very effective in PEP settings when administered properly in combination with rabies immunoglobulin. The efficacy of these vaccines is diminished in developing countries where a combination of high cost and complex delivery regimens makes it difficult to utilize available vaccines. As a result, the annual number of rabies-related deaths worldwide has been conservatively estimated to be between 40,000 and 70,000 [1]. A recent study in Tanzania indicated that the number of rabies-related deaths may be 100 times higher [2], and estimates suggest that only 3% of rabies cases in humans are reported [3]. Untreated rabies results in 100% mortality, and 40% of rabies cases involve children, making rabies the seventh most important global infectious disease [4]. In Africa, a person (usually a child) dies of rabies every 20 min [5]. In China, cases of rabies in humans increased by 27% from 2005 to 2006, becoming the leading cause of mortality in 2006 [6].
In addition to the human loss caused by rabies, treating exposed individuals also presents a financial burden to both developed and developing countries. Each year an estimated 10 million people worldwide receive PEP [1], and in some regions of the world the number of people requiring PEP is increasing. In Thailand alone the number of patients receiving PEP increased from 90,000 in 1991 to >400,000 in 2003 [7]. The cost of RV vaccines in Africa and Asia is almost $600 million per year [3] and is expected to rise.
Cases of rabies in humans in the United States are rare because PEP is readily available and measures to control rabies in domestic and wild animals have been largely successful. Up to almost 40,000 people have been reported to receive PEP in the United States per year [8], and estimates in South Carolina indicated that the frequency of PEP was higher than reported, suggesting that rabies PEP is underreported at a national level [9]. In the United States, the major reservoir of and potential source for RV exposure among humans is wildlife. Between 2006 and 2007 there was almost a 5% increase in the number of animals with rabies reported to the Centers for Disease Control and Prevention [10]. While it might be argued that the increase was due to improved surveillance, the data highlight the concern for and difficulty of containing zoonotic viral infections and emphasize the need for effective and inexpensive RV PEP in humans even in developed countries.
Current PEP in the United States for nonimmunized individuals consists of wound cleaning and 1 dose of passive rabies immunoglobulin followed by 5 doses of inactivated RV vaccine (reviewed in Manning et al [8]). In developing countries, regimens approved by the World Health Organization have been implemented to reduce the cost of vaccination. Experimental approaches are also being investigated, including DNA vaccines [11–16], viral vectors [17–22], and prime-boost combinations with DNA vaccines and viral vectors [23]. Among these strategies are replication-deficient RV-based vaccines that may replace current inactivated vaccine formulations. This approach is advantageous because the major antigenic determinant for RV, the glycoprotein (G), is expressed by highly immunogenic replication-deficient RV vaccine strains that elicit acquired and innate immune responses. Here we investigate the utility of phosphoprotein (P) gene– and matrix (M) gene–deleted RVs as potential RV vaccines for humans. Each is propagated in a cell line stably expressing the RV P or M gene and therefore contains the structural proteins necessary to infect cells and express antigen. However, P gene–deleted RVs are replication deficient because of the critical role played by the RV P in virus transcription and replication, and gene expression relies solely on the input RV P gene. M gene–deleted viruses are replication impaired because of a defect in budding but contain intact polymerase machinery and therefore produce higher levels of antigen expression. Both vaccines are safe because they do not spread into the central nervous system (CNS) of immunodeficient (recombination activating gene 2 [RAG2] knockout) mice. Thus, replication-deficient RV-based vaccines have the potential to be highly efficacious.
MATERIALS AND METHODS
Replication-deficient RV vaccine vectors
A replication-deficient P gene–deficient RV vaccine vector (SPBN-ΔP) was designed as described elsewhere [17]. A replication-deficient M gene–deficient RV vaccine vector (SPBN-ΔM) was constructed using a polymerase chain reaction (PCR) strategy and standard molecular techniques. Using SPBN [24] as our template, a ~700-bp fragment spanning from the RV P C-terminus to the stop codon in the RV P gene was amplified (PCR 1) using forward primer RP306 (5′-TTTCCATGGTGAGATAGCCAAGG-3′; the NcoI site is underlined) and reverse primer RP307 (5′-AAAACGCGTTTAGCAAGATGTATAGCGATTC-3′; the MluI site is underlined, and the RV P gene stop codon is in boldface). A ~1.2-kb fragment corresponding to the transcriptional stop/start sequence between the M and G genes (including sequences from the RV G gene) was amplified (PCR 2) using forward primer RP308 (5′-TTTACGCGTTCAGATTATATCCCGCAAATTT ATC-3′; the MluI site is underlined) and reverse primer RP309 (5′-AAAATCGATTGACTCTTCTA CAACATG-3′; the ClaI site is underlined). PCR 1 and 2 products were digested with MluI and ligated, and the resulting product was used as the template for an additional PCR (PCR 3) using the primer pair RP306/RP309. The PCR 3 product (1.9 kb) was digested with NcoI and PpuMI and inserted into SPBN, which was also digested with NcoI and PpuMI, resulting in SPBN-ΔM. Because of the replication deficiency of the M gene–deleted virus, the protocol for the recovery of infectious recombinant virus [25] was modified. Briefly, BSR cells (baby hamster kidney–derived cell line) stably expressing bacteriophage T7 RNA polymerase were transfected with 4 different plasmids encoding the RV proteins N, P, M, and L and 1 plasmid encoding antigenomic RNA of SPBN-ΔM. Three days after transfection, supernatants were transferred to BSR cells expressing RV M (gift of K. Conzelmann, University of Munich) [26]. Virus recovery was confirmed by immunostaining with a fluorescein isothiocyanate–conjugated anti–RV N antibody [25]. Viral stock RNA was isolated and converted to DNA by reverse-transcription PCR. The deletion within SPBN-ΔM was confirmed by sequencing.
Characterization of recombinant RV in vitro
Expression of RV G on the surface of cells infected with SPBN, SPBN-ΔP, or SPBN-ΔM was analyzed by means of flow cytometry, as described elsewhere [17]. Growth kinetics of recombinant RVs were determined as described elsewhere [27] with modifications to account for growth on complementing cell lines. Briefly, BSR cells, BSR cells expressing RV P [17], or BSR cells expressing RV M were plated in 60-mm dishes and 16 h later infected with SPBN, SPBN-ΔP, or SPBN-ΔM, respectively, at a multiplicity of infection of 2. After incubation at 37°C for 1 h, inocula were removed and cells were washed 4 times with phosphate-buffered saline, to remove unadsorbed virus. Three milliliters of complete medium was added back, and 100 µL of tissue culture supernatants was removed at the indicated time points after infection. Viral aliquots were titered in duplicate using their respective virus-specific cell lines.
Immunization and challenge
Groups of 5 female BALB/c mice aged 6–8 weeks were inoculated intramuscularly with a single dose of serial 10-fold dilutions of SPBN-ΔM or SPBN-ΔP. Five weeks after immunization, mice were challenged intramuscularly with 100 times the median lethal dose (LD50) of pathogenic challenge virus strain (CVS) N2c (a mouse-adapted subclone of CVS-24 RV [28]) and observed for 4 weeks for clinical signs of rabies. Mice were euthanized at the onset of neurological symptoms. The experiments were approved and conducted in accordance with Institutional Animal Care and Use Committee (IACUC) requirements.
Immunization of rhesus macaques
Four adult male rhesus macaques (designated DK07, DK86, DE72, and DG69) were immunized intramuscularly in the cranial aspect of the thigh with 6 × 107 foci-forming units (FFUs) of SPBN-ΔM. Two additional adult male animals (designated DG78 and N432) were immunized intramuscularly with a full human dose (1 mL) of commercially available inactivated human diploid cell vaccine (HDCV), and animals from both groups were given booster immunizations 5 days later in a similar fashion. These animals were housed at the Tulane National Primate Research Center (TNPRC), and experiments were approved and conducted in accordance with IACUC requirements. The TNPRC is accredited by the American Association of Laboratory Animal Care.
Antibody enzyme-linked immunosorbent assay
RV G–specific immunoglobulin G, G1, and G2a (IgG, IgG1, and IgG2a, respectively) [17] antibody responses were determined in serum samples from immunized mice by enzyme-linked immunosorbent assay (ELISA), as described elsewhere [17, 29]. RV G–specific IgG and subclass-specific antibody responses were determined in immunized macaques by means of a similar method, except that goat anti–rhesus IgG–horseradish peroxidase (1:1000) (SouthernBiotech) and mouse anti–human IgG1–HRP (1:1000) (SouthernBiotech) were used as secondary antibodies.
Neutralizing antibody assay
Virus-neutralizing antibody titers were determined using the rapid fluorescent focus inhibition test, as described elsewhere [17].
Antibody avidity determination using a sodium thiocyanate ELISA
RV G–specific antibody avidity was determined by sodium thiocyanate ELISA as described elsewhere [30], except that 96-well ELISA plates were coated with 100 ng/well of RV G in a coating buffer (5 mmol/L Na2CO3 [pH, 9.6]) overnight at 4°C.
Safety and dissemination of SPBN-ΔM RV in RAG2 knockout mice
We previously reported on the spread and safety of SPBN-ΔP in B and T cell–deficient RAG2 knockout mice [17]. The safety of SPBN-ΔM was assessed in a similar fashion. Briefly, groups of 5 female RAG2 knockout mice (Taconic Farms) aged 6–8 weeks were injected intramuscularly with 105 FFUs of SPBN-333 or SPBN-ΔM in the gastrocnemius muscle. SPBN-333 [31] is a highly attenuated vaccine strain that is apathogenic after direct intracranial inoculation of immunocompetent mice, yet it retains residual pathogenicity in RAG2 knockout mice after a peripheral inoculation and served as a positive control. Clinical signs of rabies in mice inoculated with SPBN-333 were used as an indicator for when to collect samples from SPBN-ΔM–inoculated mice, since the possibility existed that this vector would not cause overt disease even in severely immunodeficient mice. Mice were monitored daily for weight change and signs of rabies. At 24 days after infection mice were euthanized, and muscles, brains, and spinal cords were harvested. Total RNA was isolated from tissues, and quantitative reverse-transcription PCR was performed as described elsewhere [32].
RESULTS
Vaccine construction and characterization
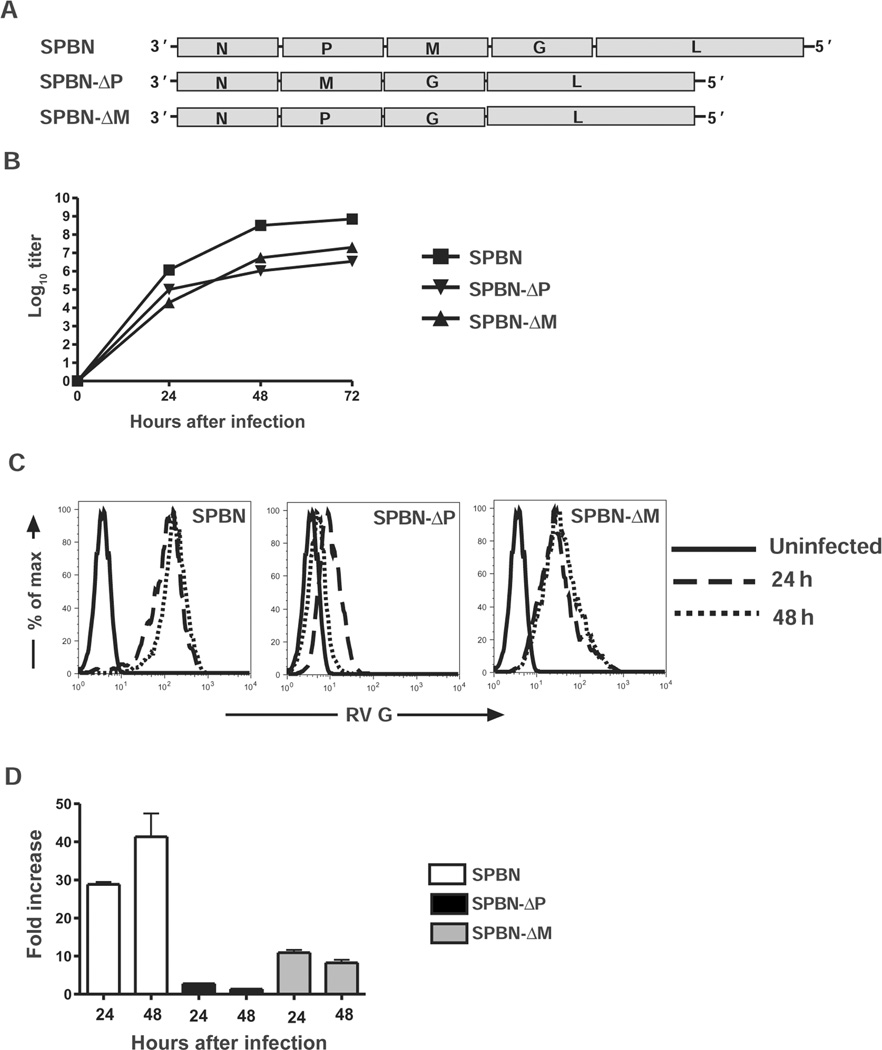
Replication-deficient RV-based vaccine vectors were constructed using PCR-directed deletion of either the M gene (SPBN-ΔM) or the P gene (SPBN-ΔP) [17] (see Figure 1A and Materials and Methods). Infectious virus was recovered and propagated in BSR cells expressing RV M or RV P. Despite similar growth kinetics and titers between SPBN-ΔM and SPBN-ΔP grown in trans-complementing cell lines (Figure 1B), SPBN-ΔM expressed almost 4–6-fold more RV G on the surface of neuroblastoma cells than did SPBN-ΔP at 24 and 48 h after infection (Figures 1C and 1D). For comparison, the parental replication-competent vector SPBN grew to titers 10–50-fold greater than those for replication-deficient RVs and expressed up to 30- and 5-fold more RV G on the surface of infected cells than on cells infected with either SPBN-ΔP or SPBN-ΔM, respectively.
Figure 1.
Replication-deficient viral vectors. A, Diagram of the recombinant replication-deficient rabies virus (RV) vaccines with deleted RV phosphoprotein (SPBN-ΔP) or matrix (SPBN-ΔM) genes. Infectious virus was recovered as described in Materials and Methods and propagated on BSR cells stably expressing RV P or RV M. B, Growth kinetics of recombinant vectors, determined by preparing a 1-step growth curve using BSR cells expressing RV P or RV M infected at a multiplicity of infection of 2 with either SPBN-ΔP or SPBN-ΔM, respectively. For comparison, a 1-step growth curve was prepared in parallel using BSR cells infected with the parental replication-competent vector, SPBN. C, Uninfected (solid lines) neuroblastoma cells or SPBN-, SPBN-ΔP–, or SPBN-ΔM–infected neuroblastoma cells (multiplicity of infection, 3) examined for RV glycoprotein (G) expression on the NA cell surface after 24 h (dashed lines) and 48 h (dotted lines). Cells were incubated with polyclonal rabbit anti–RV G antiserum followed by a Cy2-conjugated anti–rabbit antibody. Surface expression was determined by flow cytometry, and fluorescence intensity was plotted. The histograms are representative of 2 independent experiments. D, Fold increase in mean fluorescent intensity over uninfected cells, plotted using data from panel C and including the average and standard error of the mean of values from the 2 independent experiments conducted in duplicate. L, polymerase; N, nucleoprotein.
Immunogenicity of SPBN-ΔM and SPBN-ΔP in mice
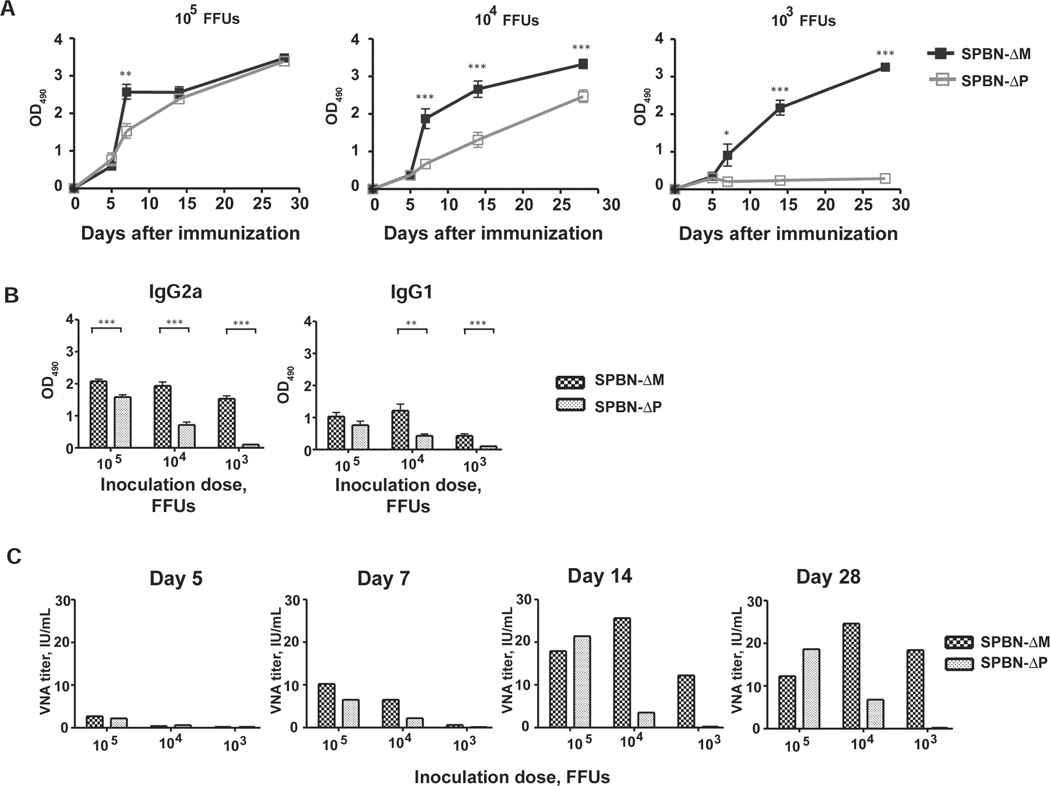
Mice immunized intramuscularly with a single dose of 105, 104, or 103 FFUs of SPBN-ΔM had significantly higher anti–RV G antibody levels 7 days after inoculation than did mice immunized with SPBN-ΔP (for 105, P = .001; for 104, P < .001; for 103, P = .028) (Figure 2A). Mice immunized with 104 or 103 SPBN-ΔM FFUs had significantly higher antibody responses than did mice immunized with SPBN-ΔP at all time points thereafter (P < .001). The antigenic potency of SPBN-ΔM was highlighted by the similarity in antibody response after immunization with different doses, including 103 FFUs. Conversely, the antibody response detected in SPBN-ΔP–immunized mice was dose dependent, and seroconversion to RV G did not occur in mice immunized with 103 FFUs. As shown in Figure 2B, mice immunized with SPBN-ΔM mounted statistically higher IgG2a antibody titers than did mice immunized with SPBN-ΔP at all doses tested. Virus-neutralizing antibody (VNA) titers were detected in mice inoculated with 105 FFUs of SPBN-ΔM (2.7 IU/mL) and 105 FFUs of SPBN-ΔP (2.2 IU/mL) within 5 days after immunization (Figure 2C), a result consistent with the antibody titers shown in Figure 2A. By day 7, mice that received 103 FFUs of SPBN-ΔM had VNA titers of 0.6 IU/mL. The potency of SPBN-ΔM immunization was measured through day 28, when a 90-fold increase in VNA titers was observed compared with titers measured in response to vaccination with 103 FFUs of SPBN-ΔP. In parallel, we tested our vaccine against UV light–inactivated RV. At a dose equivalent to 105 FFUs, SPBN-ΔP and SPBN-ΔM induced significantly higher antibody responses against RV G compared with the responses elicited by the inactivated vaccine (for SPBN-ΔM, P = .006; for SPBN-ΔP, P = .010; data not shown). Except for mice immunized with 103 FFUs of SPBN-ΔP, which did not seroconvert, mice immunized with SPBN-ΔM and SPBN-ΔP showed higher anti–RV G antibody levels than did mice immunized with inactivated RV at all time points and doses tested (P < .001; data not shown).
Figure 2.
Inducement of rapid and potent anti–rabies virus (RV) humoral immune responses by the matrix (M) gene–deficient RV vaccine (SPBN-ΔM). Groups of 5 BALB/c mice were immunized intramuscularly with either 105, 104, or 103 FFUs of phosphoprotein (P) gene–deficient RV vaccine (SPBN-ΔP) or SPBN-ΔM, and serum samples were collected on days 5, 7, 14, 21, and 28 after immunization. A, Immunoglobulin G (IgG) titers. At each time point, serum samples from individual mice were tested for anti–RV glycoprotein (G) IgG titers by enzyme-linked immunosorbent assay (1:50 dilution). B, IgG subclass–specific titers. Serum samples from individual mice collected on day 14 after immunization were analyzed for anti–RV G IgG subclass–specific antibodies at a 1:50 dilution (left, IgG2a; right, IgG1). C, Virus-neutralizing antibody (VNA) titers. Serum samples from mice at each time point were pooled and analyzed for VNA titers. Data in panels A and B were analyzed using the unpaired Student t test, and differences with P < .05 were considered statistically significant. *P = .01–.05; **P = .001–.01; ***P = .001. OD490, optical density read at 490 nm.
Protection against pathogenic RV challenge in mice
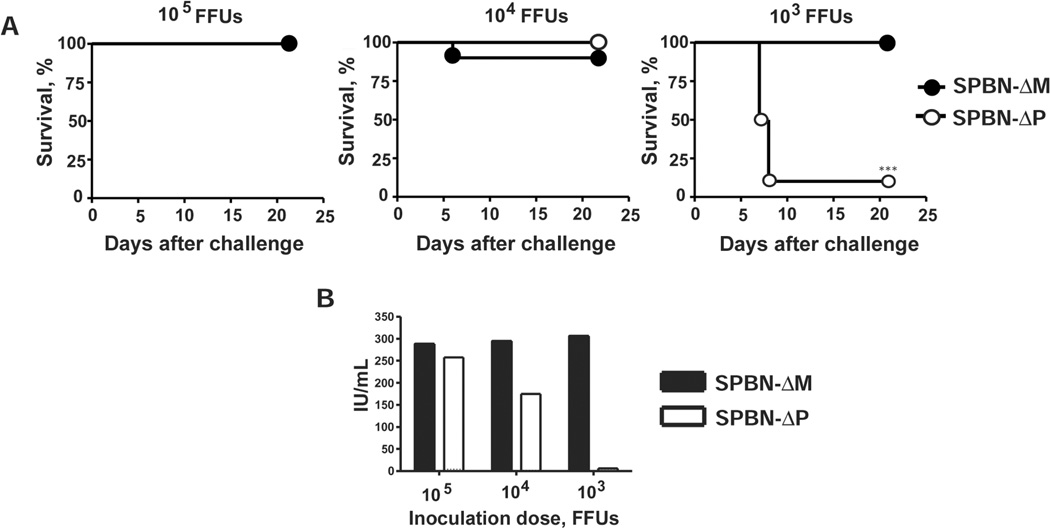
Higher antibody titers were detected in SPBN-ΔM–immunized mice than in SPBN-ΔP–immunized mice, and this difference paralleled enhanced protection against pathogenic RV challenge (Figure 3A). Mice immunized with 105, 104, and 103 FFUs of SPBN-ΔM were 100%, 90%, and 100% protected, respectively, against challenge with pathogenic CVS-N2c at 100 times the LD50 (Figure 3A). In comparison, survival after SPBN-ΔP vaccination was 100%, 100%, and 10% for 105, 104, and 103 FFUs, respectively. Antibody responses were measured 28 days after challenge in surviving mice. As shown for the prechallenge antibody responses, SPBN-ΔM–immunized mice did not show a dose-dependent decrease in VNA titers over time. VNA titers were consistently >500-fold the presumed protective level at all doses tested. On the other hand, a dose-dependent decrease was observed in mice immunized with SPBN-ΔP over the course of the experiment. It should be noted that the VNA titer from only 1 of the 10 mice that received 103 FFUs of SPBN-ΔP was reported because only 1 mouse was alive at the time of blood collection.
Figure 3.
Protection against pathogenic rabies virus (RV) challenge produced by the matrix (M) gene–deficient RV vaccine (SPBN-ΔM). Mice described in Figure 2 were challenged intramuscularly 5 weeks after immunization with 100 times the median lethal dose (LD50) of pathogenic challenge virus strain (CVS) N2c. A, Percent survival of immunized and challenged mice. B, Characterization of virus-neutralizing antibodies from mice that survived 28 days after challenge. Data are antibody levels in surviving animals from the 2 independent experiments described in Figure 2 (n = 10) except that only 1 of the mice immunized with 103 foci-forming units (FFUs) of phosphoprotein (P) gene–deficient RV vaccine (SPBN-ΔP) survived 28 days after challenge (n = 1).
Safety and spread of SPBN-ΔM in RAG2 mice
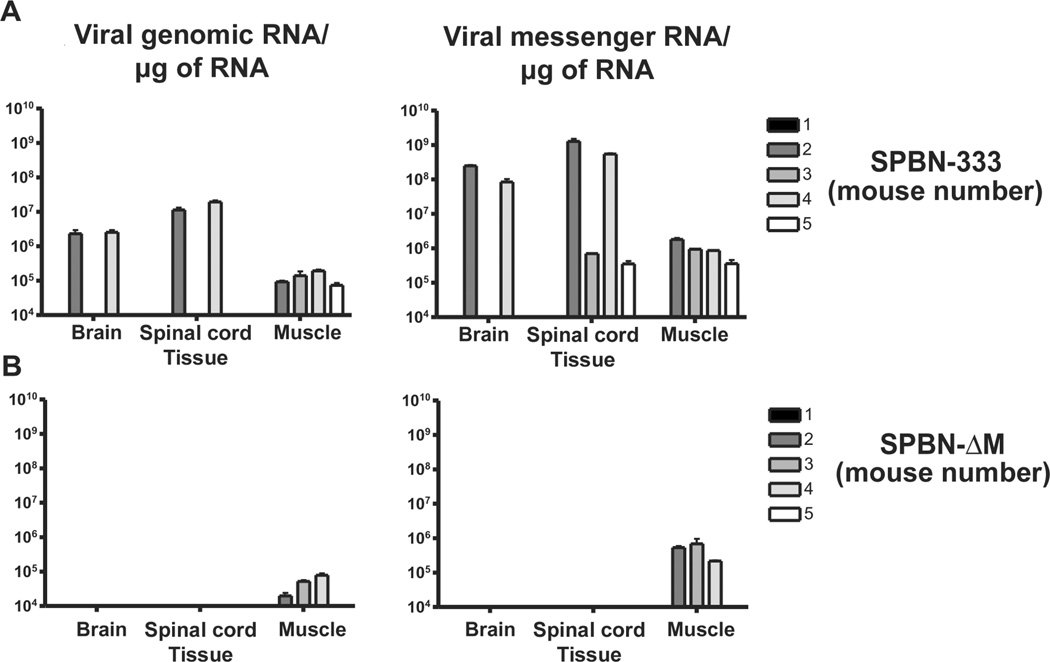
On the basis of the immune response and survival data described above, we tested SPBN-ΔM immunogenicity in nonhuman primates. However, safety was assessed before primate inoculation. We previously reported that P gene–deleted vectors did not spread into the CNS of B and T cell–deficient mice (RAG2 knockout mice) [17]. RAG2 knockout mice that were inoculated intramuscularly with SPBN-ΔM did not show signs of RV infection or lose weight during the study period (data not shown). By 24 days after infection, 2 of the 5 control mice inoculated with a highly attenuated RV vaccine vector started to lose weight, indicative of an RV infection. At this point, mice from all groups were euthanized; muscles, spinal cords, and brains were removed, and RNA was isolated. As shown in Figure 4A, genomic RNA and messenger RNA were detected in the muscle, spinal cords, and brains of the 2 mice inoculated with the attenuated replication-competent RV. Although genomic RNA and messenger RNA were detected in the muscle of SPBN-ΔM–immunized RAG2 knockout mice, viral RNA was not detected in the CNS 24 days after infection (Figure 4B). However, the effects at longer time points after inoculation may have to be examined to further assess the potential for SPBN-ΔM dissemination into the CNS.
Figure 4.
Safety of the matrix (M) gene–deficient rabies virus (RV) vaccine (SPBN-ΔM). Safety was determined by assessing dissemination to the central nervous system in immunodeficient mice. Groups of 5 immunodeficient (recombination activating gene 2 [RAG2] knockout) mice were inoculated intramuscularly with 105 foci-forming units (FFUs) of either SPBN-333 (A) or SPBN-ΔM (B). Once an animal in either group became moribund due to RV infection, all mice were euthanized. Mice inoculated with the highly attenuated SPBN-333 vector served to define the tissue-collection time points for SPBN-ΔM–immunized mice, since it was not known whether SPBN-ΔM would induce visible signs of RV infection. Total RNA was isolated from muscles, brains, and spinal cords, and viral reverse-transcription polymerase chain reaction and quantitative real-time polymerase chain reaction were used to amplify and quantify viral genomic (left) or messenger (right) RNA from individual samples.
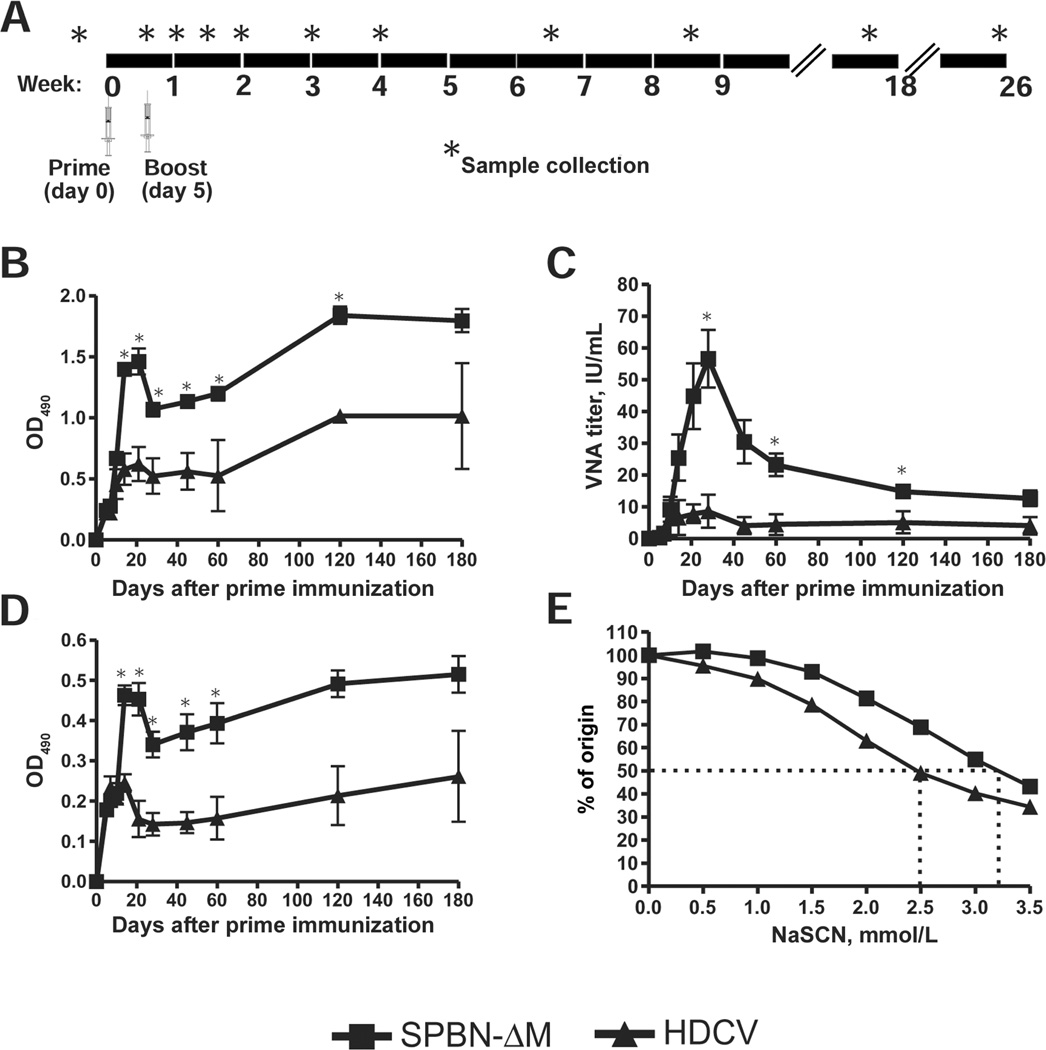
Rhesus macaque vaccination and sample collection
Current RV vaccines for humans are used in pre- and postexposure settings. Therefore, successful second-generation vaccines must induce more rapid and longer-lasting anti-RV immunity. The immunogenicity and protection data from mice described above indicated that both SPBN-ΔP and SPBN-ΔM induced potent anti-RV antibody responses, demonstrating that either could serve as potential anti-RV vaccines. However, the rapid and potent antibody response elicited by SPBN-ΔM indicates that this formulation likely holds the greatest therapeutic potential for the development of a novel anti-RV vaccine for humans. To that end, 4 rhesus macaques (DK07, DK86, DE72, and DG69) were immunized intramuscularly with live SPBN-ΔM. Two additional animals (DG78 and N432) were inoculated intramuscularly with a commercially available killed HDCV and given booster inoculations 5 days later in a similar fashion (Figure 5A). Blood was collected 3 days before immunization and at various time points after immunization.
Figure 5.
Inducement of rapid and potent immunity in nonhuman primates by the matrix (M) gene–deficient rabies virus (RV) vaccine (SPBN-ΔM) exceeding that induced by the human diploid cell vaccine (HDCV). A, Immunization and sample collection protocol. Four male rhesus macaques were immunized intramuscularly with SPBN-ΔM, and 2 macaques were immunized with HDCV. Five days later, animals were similarly given booster immunizations. Blood was collected 3 days before inoculation and on days 5, 7, 10, 14, 21, 28, 45, 60, 120, and 180 after prime inoculation. B, Immunoglobulin (IgG) antibody titers. Serum samples from individual animals tested for total anti–RV glycoprotein (G) IgG antibody titers by enzyme-linked immunosorbent assay (ELISA) at a dilution of 1:50. C, Virus-neutralizing antibody (VNA) titers. VNA titers were determined in individual macaques by use of the rapid fluorescent focus inhibition test. D, Anti–RV G IgG1 subclass-specific antibody titers. IgG1-specific titers were determined by ELISA at a 1:50 dilution for each animal. E, Antibody avidity. Serum samples from individual macaques collected 180 days after prime inoculation were measured for antibody avidity to RV G using the sodium thiocyanate (NaSCN) ELISA method. The dotted lines indicate the concentrations of NaSCN needed to dissociate 50% of antibody-antigen interactions. Data are the mean and standard error of the mean for each group. Data were analyzed using the unpaired Student t test, and differences with P < .05 were considered statistically significant. *P < .05.
Kinetic analysis of SPBN-ΔM–induced immunity in rhesus macaques
A kinetics analysis of the anti-RV antibody response was conducted. Higher anti–RV G IgG antibody titers were detected in SPBN-ΔM–immunized animals at all time points examined beginning 7 days after immunization, compared with those in HDCV-immunized animals (Figure 5B). Significantly higher anti–RV G IgG antibody titers were detected 14 days after primary immunization in SPBN-ΔM–immunized macaques than in HDCV-immunized animals. Similarly, VNA titers were higher in SPBN-ΔM–immunized animals at all time points examined beginning 10 days after primary inoculation (Figure 5C). By day 14 after primary immunization, SPBN-ΔM–immunized macaques showed a ~4-fold increase in VNA titers compared with HDCV-immunized animals. VNA titers peaked at ~28 days after primary inoculation and increased 6-fold in SPBN-ΔM–immunized animals compared to HDCV-immunized animals. In addition, VNA titers from SPBN-ΔM–immunized animals were >50- and >110-fold higher than the presumed protective levels of 0.5 IU/mL [33] 14 and 28 days after primary inoculation, respectively. VNA titers decreased thereafter in both groups, but SPBN-ΔM–immunized animals had higher VNA titers than did HDCV-immunized animals by the end of the 180-day study period.
Qualitative antibody differences were also compared between the 2 vaccine groups. Rhesus macaque IgG1 antibodies (like mouse IgG2a) possess ideal antiviral properties, that is, antibody-dependent cellular cytotoxicity, complement activation, and high Fc receptor binding efficiency [34]. SPBN-ΔM immunization induced higher IgG1 antibodies 10 days after primary inoculation (Figure 5D). In addition, antibodies from SPBN-ΔM–immunized macaques had increased anti–RV G avidity compared with antibodies from HDCV-immunized mice (Figure 5E). In sum, the kinetics analyses indicate that SPBN-ΔM is a promising vaccine candidate for use in both pre- and postexposure settings.
DISCUSSION
Vaccination regimens that can be universally administered and that are simple, inexpensive, effective, and safe would greatly benefit prevention of rabies in humans. To this end, replication-deficient RV-based vaccines are being evaluated as low-cost alternatives to current inactivated vaccines [17, 18, 21]. P or M gene–deleted vectors were selected for the present study on the basis of specific molecular attributes that make them attractive candidates for RV PEP [17, 26]. We have shown that both SPBN-ΔM and SPBN-ΔP are immunogenic and provide protection against pathogenic RV challenge in a mouse model for preexposure. However, SPBN-ΔM induced a more potent humoral anti–RV G response 7 days after immunization, compared with the response observed in SPBN-ΔP–immunized mice. VNA titers at this time point were ~20-fold higher than the defined protective level. Considering that <10% of people receiving PEP also receive rabies immunoglobulin, it is likely that higher circulating antibody titers would be beneficial for viral clearance. Regardless, an early and rapid immune response is critical to prevent RV from entering the CNS and producing disease onset. Vaccination with only 103 FFUs conferred 100% protection in mice after RV challenge. SPBN-ΔM vaccination also elicited a type 1 T helper response, defined in this system by the dominant IgG2a response observed.
The humoral response after SPBN-ΔM challenge was further defined by conducting a kinetics analysis of the anti-RV response in rhesus macaques. Quantitative and qualitative measures of vaccine efficiency, including defining VNA titers, IgG subclass-specific antibody profiles, and antibody avidities, were compared in SPBN-ΔM– or HDCV-immunized macaques. VNA titers (a key indicator of RV vaccine efficiency) were almost 4-, 6-, and 7-fold higher in SPBN-ΔM–immunized animals than in HDCV-immunized animals 10, 21, and 28 days after immunization, respectively. This early response confirmed our observations in mice that SPBN-ΔM might make an excellent vaccine for PEP. VNA titers 180 days after infection indicated that SPBN-ΔM vaccination induced long-lasting humoral immunity that may confer protection if administered before exposure. Qualitative differences in the humoral response were also observed—that is, IgG1 antibody levels (thought to be functionally equivalent to IgG2a antibodies in mice) were statistically higher in SPBN-ΔM–immunized mice than in HDCV-immunized mice at most time points tested. This also confirmed our observations that in mice SPBN-ΔM immunization induced antibodies suggestive of a type 1 T helper response. In addition, SPBN-ΔM vaccination elicited antibodies with higher RV G avidity than did immunization with HDCV. Taken together, the nature, speed, and magnitude of the SPBN-ΔM–induced anti-RV immune response in mice and nonhuman primates indicated the potential of this formulation to replace current vaccine regimens used before and after RV exposure.
One concern for the development of any new vaccine is the translatability of research efforts to scaled manufacturing. Methods used to produce our vaccine would be similar to those currently used to manufacture inactivated RV vaccines. In addition, significant effort by others has led to the generation of high-titer RV vaccines in Vero cells using serum-free conditions [35, 36]. Vero cells, an African green monkey kidney cell line, are well characterized, have no oncogenic properties, and can be used for large-scale vaccine production [35]. High-titer production of live RV vaccines for animal use has also been described in BSR cells by means of bioreactor technology [37]. This information indicates that the high-titer production of replication-deficient vectors is feasible.
Larger-scale studies will have to be conducted to further define differences in vaccine efficacy. In addition, this initial study in nonhuman primates focused on immunogenicity because correlates of immunity for RV are known. However, protection against pathogenic challenge after a prime-only or a prime-boost regimen needs to be verified experimentally. Finally, we previously showed that a P gene–deleted vector expressing 2 RV G copies induced very rapid and potent anti-RV immunity [17]. Future studies will need to be conducted to determine which vector induces an optimal response and provides the highest levels of protection.
Acknowledgments
We thank Andrew A. Lackner (Tulane National Primate Research Center [TNPRC]) for helpful discussions and support and the Division of Veterinary Medicine of the TNPRC for animal care.
Financial support: National Institute of Allergy and Infectious Diseases (grant AI070252 to J.P.M.); Tulane National Primate Research Center (Pilot Research Project Award to J.P.M.); Infectious Disease Society of America, National Foundation for Infectious Disease, and Wyeth Research Laboratories (Wyeth Young Investigator Award for Vaccine Development to J.P.M.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.World Health Organization (WHO) Geneva: WHO; 2001. Rabies: fact sheet 99, revised June 2001. [Google Scholar]
- 2.Cleaveland S, Fevre EM, Kaare M, Coleman PG. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ. 2002;80:304–310. [PMC free article] [PubMed] [Google Scholar]
- 3.Knobel DL, Cleaveland S, Coleman PG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson AC. Rabies. Neurol Clin. 2008;26:717–726. doi: 10.1016/j.ncl.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Dodet B, Adjogoua EV, et al. Africa Rabies Expert Bureau (AfroREB) Fighting rabies in Africa: the Africa Rabies Expert Bureau (AfroREB) Vaccine. 2008;26:6295–6298. doi: 10.1016/j.vaccine.2008.04.087. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Geneva: WHO; 2007. Rabies and envenomings: a neglected public health issue: report of a consultative meeting, 10 January 2007. [Google Scholar]
- 7.Denduangboripant J, Wacharapluesadee S, Lumlertdacha B, et al. Transmission dynamics of rabies virus in Thailand: implications for disease control. BMC Infect Dis. 2005;5:52. doi: 10.1186/1471-2334-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57:1–28. [PubMed] [Google Scholar]
- 9.O’Bell SA, McQuiston J, Bell LJ, Ferguson SC, Williams LA. Human rabies exposures and postexposure prophylaxis in South Carolina, 1993–2002. Public Health Rep. 2006;121:197–202. doi: 10.1177/003335490612100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanton JD, Palmer D, Christian KA, Rupprecht CE. Rabies surveillance in the United States during 2007. J Am Vet Med Assoc. 2008;233:884–897. doi: 10.2460/javma.233.6.884. [DOI] [PubMed] [Google Scholar]
- 11.Lodmell DL, Ewalt LC. Post-exposure DNA vaccination protects mice against rabies virus. Vaccine. 2001;19:2468–2473. doi: 10.1016/s0264-410x(00)00475-8. [DOI] [PubMed] [Google Scholar]
- 12.Lodmell DL, Parnell MJ, Bailey JR, Ewalt LC, Hanlon CA. One-time gene gun or intramuscular rabies DNA vaccination of nonhuman primates: comparison of neutralizing antibody responses and protection against rabies virus 1 year after vaccination. Vaccine. 2001;20:838–844. doi: 10.1016/s0264-410x(01)00392-9. [DOI] [PubMed] [Google Scholar]
- 13.Lodmell DL, Parnell MJ, Bailey JR, Ewalt LC, Hanlon CA. Rabies DNA vaccination of nonhuman primates: post-exposure studies using gene gun methodology that accelerates induction of neutralizing antibody and enhances neutralizing antibody titers. Vaccine. 2002;20:2221–2228. doi: 10.1016/s0264-410x(02)00143-3. [DOI] [PubMed] [Google Scholar]
- 14.Lodmell DL, Ray NB, Ewalt LC. Gene gun particle-mediated vaccination with plasmid DNA confers protective immunity against rabies virus infection. Vaccine. 1998;16:115–118. doi: 10.1016/s0264-410x(97)88325-9. [DOI] [PubMed] [Google Scholar]
- 15.Lodmell DL, Ray NB, Parnell MJ, et al. DNA immunization protects nonhuman primates against rabies virus. Nat Med. 1998;4:949–952. doi: 10.1038/nm0898-949. [DOI] [PubMed] [Google Scholar]
- 16.Lodmell DL, Ray NB, Ulrich JT, Ewalt LC. DNA vaccination of mice against rabies virus: effects of the route of vaccination and the adjuvant monophosphoryl lipid A (MPL) Vaccine. 2000;18:1059–1066. doi: 10.1016/s0264-410x(99)00352-7. [DOI] [PubMed] [Google Scholar]
- 17.Cenna J, Tan GS, Papaneri A, Dietzschold B, Schnell MJ, McGettigan JP. Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoproteins. Vaccine. 2008;26:6405–6414. doi: 10.1016/j.vaccine.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito N, Sugiyama M, Yamada K, et al. Characterization of M gene-deficient rabies virus with advantages of effective immunization and safety as a vaccine strain. Microbiol Immunol. 2005;49:971–979. doi: 10.1111/j.1348-0421.2005.tb03692.x. [DOI] [PubMed] [Google Scholar]
- 19.Lees CY, Briggs DJ, Wu X, et al. Induction of protective immunity by topic application of a recombinant adenovirus expressing rabies virus glycoprotein. Vet Microbiol. 2002;85:295–303. doi: 10.1016/s0378-1135(01)00523-5. [DOI] [PubMed] [Google Scholar]
- 20.Li WH, Zhang Y, Wang SH, Liu L, Yang F. [Recombinant replication-defective adenovirus based rabies vaccine] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:650–654. [PubMed] [Google Scholar]
- 21.Morimoto K, Shoji Y, Inoue K. Characterization of P gene–deficient rabies virus: propagation, pathogenicity, and antigenicity. Virus Res. 2005;111:61–67. doi: 10.1016/j.virusres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Vos A, Neubert A, Pommerening E, et al. Immunogenicity of an E1-deleted recombinant human adenovirus against rabies by different routes of administration. J Gen Virol. 2001;82:2191–2197. doi: 10.1099/0022-1317-82-9-2191. [DOI] [PubMed] [Google Scholar]
- 23.Lodmell DL, Ewalt LC. Rabies vaccination: comparison of neutralizing antibody responses after priming and boosting with different combinations of DNA, inactivated virus, or recombinant vaccinia virus vaccines. Vaccine. 2000;18:2394–2398. doi: 10.1016/s0264-410x(00)00005-0. [DOI] [PubMed] [Google Scholar]
- 24.McGettigan JP, Sarma S, Orenstein JM, Pomerantz RJ, Schnell MJ. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J Virol. 2001;75:8724–8732. doi: 10.1128/JVI.75.18.8724-8732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnell MJ, Foley HD, Siler CA, McGettigan JP, Dietzschold B, Pomerantz RJ. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc Natl Acad Sci U S A. 2000;97:3544–3549. doi: 10.1073/pnas.050589197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mebatsion T, Weiland F, Conzelmann KK. Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. J Virol. 1999;73:242–250. doi: 10.1128/jvi.73.1.242-250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGettigan JP, Naper K, Orenstein J, Koser M, McKenna PM, Schnell MJ. Functional human immunodeficiency virus type 1 (HIV-1) Gag-Pol or HIV-1 Gag-Pol and env expressed from a single rhabdovirus-based vaccine vector genome. J Virol. 2003;77:10889–10899. doi: 10.1128/JVI.77.20.10889-10899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morimoto K, Hooper DC, Carbaugh H, Fu ZF, Koprowski H, Dietzschold B. Rabies virus quasispecies: implications for pathogenesis. Proc Natl Acad Sci U S A. 1998;95:3152–3156. doi: 10.1073/pnas.95.6.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGettigan JP, Koser ML, McKenna PM, et al. Enhanced humoral HIV-1–specific immune responses generated from recombinant rhabdoviral-based vaccine vectors co-expressing HIV-1 proteins and IL-2. Virology. 2006;344:363–377. doi: 10.1016/j.virol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Ross TM, Xu Y, Green TD, Montefiori DC, Robinson HL. Enhanced avidity maturation of antibody to human immunodeficiency virus envelope: DNA vaccination with gp120-C3d fusion proteins. AIDS Res Hum Retroviruses. 2001;17:829–835. doi: 10.1089/088922201750252025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGettigan JP, Pomerantz RJ, Siler CA, et al. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. J Virol. 2003;77:237–244. doi: 10.1128/JVI.77.1.237-244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan GS, Preuss MA, Williams JC, Schnell MJ. The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc Natl Acad Sci U S A. 2007;104:7229–7234. doi: 10.1073/pnas.0701397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO) Geneva: WHO; 2007. Recommendations for inactivated rabies vaccine for human use produced in cell substrates and embryonic eggs. WHO Technical Report Series No. 941. [Google Scholar]
- 34.Shearer MH, Dark RD, Chodosh J, Kennedy RC. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin Diagn Lab Immunol. 1999;6:953–958. doi: 10.1128/cdli.6.6.953-958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frazatti-Gallina NM, Mourao-Fuches RM, Paoli RL, et al. Vero-cell rabies vaccine produced using serum-free medium. Vaccine. 2004;23:511–517. doi: 10.1016/j.vaccine.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Frazzati-Gallina NM, Paoli RL, Mourao-Fuches RM, Jorge SA, Pereira CA. Higher production of rabies virus in serum-free medium cell cultures on microcarriers. J Biotechnol. 2001;92:67–72. doi: 10.1016/s0168-1656(01)00362-5. [DOI] [PubMed] [Google Scholar]
- 37.Dietzschold ML, Faber M, Mattis JA, Pak KY, Schnell MJ, Dietzschold B. In vitro growth and stability of recombinant rabies viruses designed for vaccination of wildlife. Vaccine. 2004;23:518–524. doi: 10.1016/j.vaccine.2004.06.031. [DOI] [PubMed] [Google Scholar]