Abstract
Oxaliplatin is a platinum based cytotoxic agent commonly used to treat colorectal cancers. Despite its effectiveness, oxaliplatin administration is associated with the development of cold-induced peripheral neuropathy. This potentially permanent side effect is provoked by cold exposure and can range from mild and self limited to severe and debilitating. Even with tumor shrinkage, these painful side effects can force dose-reduction or discontinuation of treatment. Neither the mechanism of action of oxaliplatin nor that of cold-induced neuropathy is understood. Paclitaxel, an entirely different chemotherapeutic agent used to treat a variety of malignancies, also is associated with the development of peripheral neuropathy. Unlike oxaliplatin, neurotoxicity arising from paclitaxel treatment is better understood and was found to have profound effects on intracellular calcium signaling (1,2). In this study we examined the effects of oxaliplatin on calcium signaling pathways and found that acute exposure of either a neuroblastoma cell line or primary neurons with therapeutic concentrations of oxaliplatin had no effect on intracellular calcium signaling. We also found that cellular temperature sensors (TRP channels) were also not activated by oxaliplatin. Interestingly, prolonged exposure of oxaliplatin sensitized cells to subsequent stimuli and enhanced the magnitude of intracellular calcium responses. Taken together, our results suggest that acute oxaliplatin exposure will not induce abnormal calcium signaling but oxaliplatin-primed cells do exhibit enhanced sensitivity. These findings provide new insight to the mechanism behind oxaliplatin-induced neuropathy.
Keywords: inositol 1, 4, 5 trisphosphate receptor, calcium signaling, neuroblastoma cells, dorsal root ganglion cells
Introduction
Oxaliplatin is a platinum based chemotherapeutic agent which exhibits robust cytotoxic activity (3,4). When given in combination with the pyrimidine analog 5-fluorouracil (5-FU), oxaliplatin exhibits in vitro and in vivo antiproliferative activity greater than either compound alone in colon, breast and leukemia tumor models. This combination of drugs has been approved by the Food and Drug Administration for surgically resected (adjuvant) colorectal cancer and as first line therapy for patients with unresectable or metastatic disease. Despite its extensive use, the mechanism of action for oxaliplatin remains poorly understood. The drug appears to undergo non-enzymatic conversion in physiologic solutions to active derivatives via displacement of a labile oxalate ligand. Several transient reactive species are then formed which bind with macromolecules. In particular, DNA replication and transcription are inhibited by the complexes that are subsequently formed (3,4).
Oxaliplatin given with infusional 5-FU is generally well tolerated. The most common adverse events are fatigue, nausea, vomiting, diarrhea, and neuropathy. Less common side effects (occurring in <2% of all treated patients) include pulmonary fibrosis and hypersensitivity reactions which can be fatal. The incidence of peripheral sensory neuropathy is quite high and has been reported with a frequency of 92% (all grades, from Grade I, defined as loss of deep tendon reflex or paresthesias but not interfering with function to Grade IV, defined as permanent sensory loss that interferes with function) and 13% (Grade III, defined as sensory loss or paresthesias interfering with activities of daily living). These effects persisted. At the 28-day follow up after the last treatment cycle, 60% of all patients reported peripheral sensory neuropathy of at least grade I decreasing to 39% at 6 months follow-up and 21% at 18 months of follow-up (3,4).
Oxaliplatin is associated with two types of neuropathy: acute and persistent (5,6). An acute, reversible, primarily peripheral, sensory neuropathy may occur within hours or one to two days after exposure to oxaliplatin. Symptoms often resolve completely within 14 days, but may recur with additional oxaliplatin doses. The neuropathic symptoms tend to be precipitated or exacerbated by exposure to cold temperature or cold objects. These symptoms include paresthesia, dysesthesia and hypoesthesia in the hands, feet, perioral area, or throat. Jaw spasm, abnormal tongue sensation, dysarthria, eye pain, and a feeling of chest pressure have also been described. Severe (Grade III) pharyngolaryngeal dysesthesia has been reported in 1–2% of patients previously untreated for advanced colorectal cancer (5,6). Only half of patients who develop acute sensory neuropathy have full resolution of their symptoms within six months after discontinuation of the drug.
Persistent oxaliplatin associated neuropathy (>14 days) is primarily peripheral and sensory neuropathy. Common presentations of persistent neuropathy are retarded fine motor skills and/or impaired ambulation as a result of compromised proprioception. Although persistent neuropathy may occur without any previous acute neuropathic symptoms, most patients (80%) who have developed Grade III persistent neuropathy progressed from prior Grade 1 or 2 acute events. Neuropathic symptoms may improve after discontinuation of treatment, but in many cases the neuropathic symptoms are permanent(5,6).
It has been suggested that dysregulated calcium (Ca2+) homeostasis may play an important role in the pathogenesis of oxaliplatin-associated nerve damage. Intravenous Ca2+ gluconate and magnesium sulfate given one hour pre/post each oxaliplatin infusion is routinely offered at some institutions with the hopes of lessening subsequent neuropathy. Ca2+ channel blockers which limit Ca2+ influx across the plasma membrane also have been used with some success. These observations support the suggestion that dysregulated Ca2+ homeostasis plays an important role. Further support for this hypothesis comes from the observation that administration of substances modifying cellular Ca2+ homeostasis attenuates neuropathy induced by paclitaxel, vincristine and the antiretroviral agent zalcitabine (7).
These findings are intriguing because Ca2+ is a critical intracellular messenger which is involved in the regulation of many cellular functions, including cell survival, neurotransmitter release, synaptic plasticity and cell death (reviewed by (8). By modulating the spatial and temporal pattern of Ca2+ signals it may be possible to manipulate diverse cellular signals and regulate completely dissimilar cellular processes. These processes have been linked into a large “Ca2+-signaling toolkit” (9) that has become a focus of study. However, little is known about this “Ca2+-signaling toolkit” in peripheral neurons and how the components of the toolkit could be altered in neuropathy.
Our previously published data on paclitaxel, another chemotherapy drug notorious for causing peripheral neuropathy, described a Ca2+ dependent feedback mechanism to explain paclitaxel pain syndrome. Acute paclitaxel administration activates intracellular Ca2+ oscillations which then lead to Ca2+-mediated calpain activation (1). Although the Ca2+ dependent protease μ-calpain can cleave many proteins, an important effect with respect to paclitaxel treatment is the degradation of NCS-1 (2,10). In a negative feedback loop, the loss of NCS-1 leads to the cessation of Ca2+ oscillations and to impaired phosphoinositide-mediated Ca2+ signaling, a process required to maintain normal cells. With continued paclitaxel exposure, a risk factor for paclitaxel-induced neuropathy (11), the cascade would be triggered repeatedly with mounting damage to peripheral neurons, leading to cell death (12).
Sensory neurons express thermosensitive ion channels responding to hot and cold temperature. These channels, members of the Transient Receptor Potential (TRP) family include TRPV1, the capsaicin receptor activated by noxious heat, and TRPM8, a cold and menthol activated ion channel. TRP channel expression and function are differentially regulated during inflammation and nerve injury and contribute to chronic pain and thermal hypersensitivity. These acute sensitizing effects can be recapitulated in cultured sensory neurons and heterologous expression systems following chemical stimulation of TRP channels (13). It has been suggested that oxaliplatin activates thermosensitive TRP channels thereby provoking cold sensitivity (14–16).
In this study we aimed to determine the effects of oxaliplatin on Ca2+ signaling pathways because, like paclitaxol, this chemotherapeutic agent induces neuropathy and because pretreatment with Ca2+ or with drugs that alter Ca2+ channel activity have been used with some success to ameliorate progression of peripheral neuropathy associated with oxaliplatin treatment. We found that acute exposure of neuronal cells to oxaliplatin neither effected intracellular Ca2+ signaling or the activation of thermosensitive Ca2+ permeable channels. However, after 24 hours of oxaliplatin exposure, the sensitivity of cells was significantly enhanced and phosphoinositide-mediated Ca2+ signaling was markedly altered. These results introduce a new mechanism to explain the pathogenesis of oxaliplatin-associated neuropathy.
Methods
SH-SY5Y Culture
The human neuroblastoma cell line SH-SY5Y (American Type Culture Collection, Manassas, VA) was cultured as described previously (17).
DRG Culture
Dorsal root ganglion (DRG) isolated from rat neonates (P1–3) were digested in 0.25% collagenase and separated by gentle trituration. Triturated cells were passed through a 70-μm cell strainer to remove cell clumps, and DRGs were plated on polyD-lysine/laminin coated cover slips. DRGs were maintained with Neurobasal-A media supplemented with B-27 (GIBCO- Invitrogen, Carlsbad, CA)/0.5 mM glutamine/fresh nerve growth factor (10ng/ml) and incubated overnight in a 95% air/5% CO2 humidified atmosphere in an incubator at 37°C, before treatment and imaging.
HEK-293 Culture
Human embryonic kidney (HEK-293) cells for Ca2+ imaging were cultured and transfected as described previously (22).
Ca2+ Imaging
Cells were incubated (30 min at 37°C in 5% CO2) in Hepes buffered saline (HBS) containing 6 μM Fluo-4/AM (Molecular Probes, Invitrogen, Carlsbad, CA) together with 0.1% Pluronic F-127 (Molecular Probes, Invitrogen). The HBS contained 130 mM NaCl, 4.7 mM KCl, 1.0 mM MgSO4, 1.2 mM KH2PO4, 1.25mM CaCl2, 19.7 mM Hepes, and 5 mM glucose (pH 7.4). Coverslips were mounted in a chamber (Warner Instruments, Hamden, CT) and transferred to a Zeiss LSM 510 scanning laser confocal microscope equipped with a C-Apochromat x63/1.2 water immersion objective (Zeiss, Thornwood, NY). All drugs were bath-applied. Cells were examined by using fluorescence excitation at 488 nm. Whole-cell fluorescence was measured by defining each cell as one region of interest. Cells that did not respond to stimulation or to stimulation with 1 μM thapsigargin were excluded from the evaluation. Ca2+-induced fluorescence intensity ratio Δ F/F0 was plotted as a function of time with F0 as an average of the last 10 points of the baseline before stimulation. A response is defined as a 30% rise above baseline after stimulation. Spontaneous activity is defined as 50% rise above baseline before stimulation. Data analysis was performed with Microsoft Excel. Data are expressed as mean ± SEM or as representative traces. (n/N) describes the number of cells studied (n) in N independent cultures.
Statistical Analysis
Statistical analysis of the differences between multiple groups was performed by using one-way ANOVA (Dunnett multiple-comparisons test) for two groups using t test; P ≤ 0.05 was considered statistically significant (P < 0.001 is shown as ***).
Results
Effects of therapeutic oxaliplatin concentrations on intracellular Ca2+ in human neuroblastoma cells
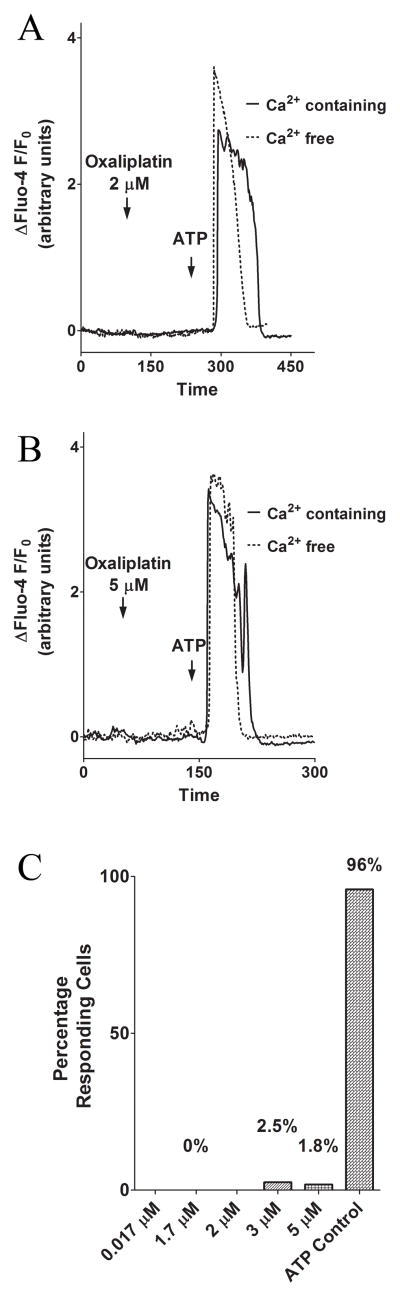
We monitored intracellular Ca2+ changes in the human neuroblastoma cell line SH-SY5Y, a cell line commonly used for studies of peripheral neurons (17), with the fluorescent dye Fluo-4/acetoxymethylester (AM). The experiments reported here used oxaliplatin concentrations comparable to steady-state concentrations observed in patients (4). Application of oxaliplatin at the therapeutic concentration of 0.814 μg/ml (2.05 μM) evoked no increase in intracellular Ca2+ within 100 seconds after bath application. However, addition of ATP, an extracellular agonist known to initiate inositol 1,4,5 trisphosphate receptor (InsP3R)-mediated Ca2+ release through activation of metabotropic P2Y receptors (18) at a concentration of 5 μM generated an increase in intracellular Ca2+, showing that the cells were viable. To avoid including a signal from stimulation of the ionotropic P2X receptor family (19), cells were treated with extracellular ATP in Ca2+ free buffer (0 Ca2+ and 100 μM EGTA). In this case, where there is no extracellular Ca2+, treatment with oxaliplatin still was unable to evoke a change in intracellular Ca2+, suggesting that acute addition of oxaliplatin does not stimulate Ca2+-signaling (Fig. 1A). Next, we tested the effect of lower and higher oxaliplatin concentrations. Oxaliplatin concentrations of 0.017 to 5.14 μM eluicited no changes in intracellular Ca2+. This result was the same whether Ca2+ was present in the extracellular solution or if Ca2+–free buffer was used (Fig. 1B). Over the range of tested oxaliplatin concentrations a maximum of 2.5% of the cells responded to stimulation by oxaliplatin within 100 seconds, whereas 96% of the cells responded to challenge by ATP (control stimulation; Fig. 1C). The lack of any effect of oxaliplatin treatment on intracellular Ca2+-signaling in SH-SY5Y cells was unexpected given what had been observed in our paclitaxol studies (1,2).
Figure 1. The effect of acute addition of oxaliplatin (2 μM and 5 μM) on SH-SY5Y cells.
Oxaliplatin at therapeutic concentrations and concentrations exceeding the therapeutic range induces no change in intracellular Ca2+ in SHSY5Y cells, independent of extracellular Ca2+. (A) Representative Ca2+ changes induced by 2.05 μM oxaliplatin and ATP (5 μM ATP in Ca2+ free and 10 μM ATP in Ca2+ containing solution (arrows)). (B) Representative Ca2+ changes induced by 5.14 μM oxaliplatin and ATP (5 μM ATP in Ca2+ free and 10 μM ATP in Ca2+ containing solution). (C) Percentage of cells responding to oxaliplatin over the concentration range of 0.017 to 5 μM compared to cells responding to the control stimulus, ATP. At least 23 cells were included in each condition.
To explain the lack of effect of oxaliplatin on intracellular Ca2+ we tested several hypotheses. First we tested whether a process other than release of intracellular Ca2+ was involved. For example, plasma membrane TRP-channels are well established as a temperature sensing pathway (20) and oxaliplatin could activate this class of channel. A second hypothesis we tested was that the SH-SY5Y cell line used was not the ideal model system and that oxaliplatin needed to be tested on primary cells. The third hypothesis tested was that only prolonged oxaliplatin exposure would provoke detectable intracellular Ca2+ signaling changes.
Acute treatment with oxaliplatin does not alter InsP3R or TRP-channel mediated Ca2+ signaling in cells transfected with specific TRP-channels
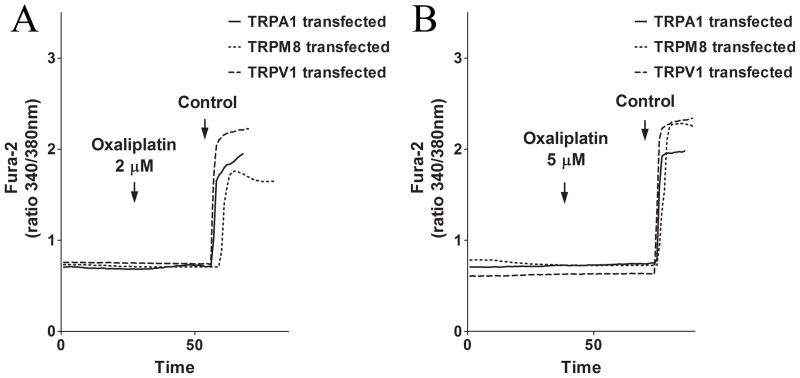
We first tested the possibility that oxaliplatin induced peripheral neuropathy is associated with an alteration in TRP-channel mediated Ca2+-signaling. It was not feasible to use SH-SY5Y cells in this series of experiments, because this cell line does not appear to express any endogenous TRP-channels. To test the cell line’s lack of TRP-channels we applied three TRP-specific agonists: mustard oil (MO) for the TRPA1-channel, menthol for the TRPM8-channel, and capsacin for the TRPV1-channel (13). After applying concentrations of the agonists spanning and above the typical concentration range used to activate these TRP channels in dorsal root ganglion (DRG) cells (13), no change in intracellular Ca2+ was recorded. These results are consistent with the lack of any reports of measurements of DRG-specific TRP channel activity in neuronal cell lines (Jordt, unpublished observations). Therefore, we transfected HEK293 cells, a cell line derived from human embryonic kidney cells known to lack sensory TRP channels, with TRPA1, TRPM8 or TRPV1. We then monitored intracellular Ca2+ changes. Addition of oxaliplatin at the therapeutic concentration (2.05 μM) or at a concentration 3-fold higher than the therapeutic concentration (6.15 μM) did not evoke a Ca2+-response in any of the transfected HEK cells when Ca2+ was present in the imaging solution. However, addition of the TRP-channel specific agonists, mustard oil (MO) for the TRPA1-channel, menthol for the TRPM8-channel and capsacin for the TRPV1-channel, lead to a strong Ca2+-response in the transfected cells. These results show that the cells were successfully transfected with functional TRP channels, yet the cells still did not respond with Ca2+ transients after addition of oxaliplatin (Fig. 2A–B).
Figure 2. The effect of acute addition of oxaliplatin (2 μM and 6 μM) on TRP channels.
Oxaliplatin at therapeutic concentrations and concentrations exceeding the therapeutic range induce no Ca2+ changes in TRP-channel transfected HEK cells. (A) Representative Ca2+ changes induced by 2.05 μM oxaliplatin and a control stimulus in TRPA1 (control: Mustard Oil), TRPM8 (control: Menthol) and TRPV1 (control: Capsacin) transfected HEK cells. (B) Representative Ca2+-changes induced by 6.146 μM oxaliplatin and a control stimulus in TRPA1 (control: Mustard Oil), TRPM8 (control: Menthol) and TRP V1 (control: Capsacin) transfected HEK cells.
Acute treatment with oxaliplatin does not alter InsP3 or TRP-channel mediated Ca2+ signaling in primary DRG cells
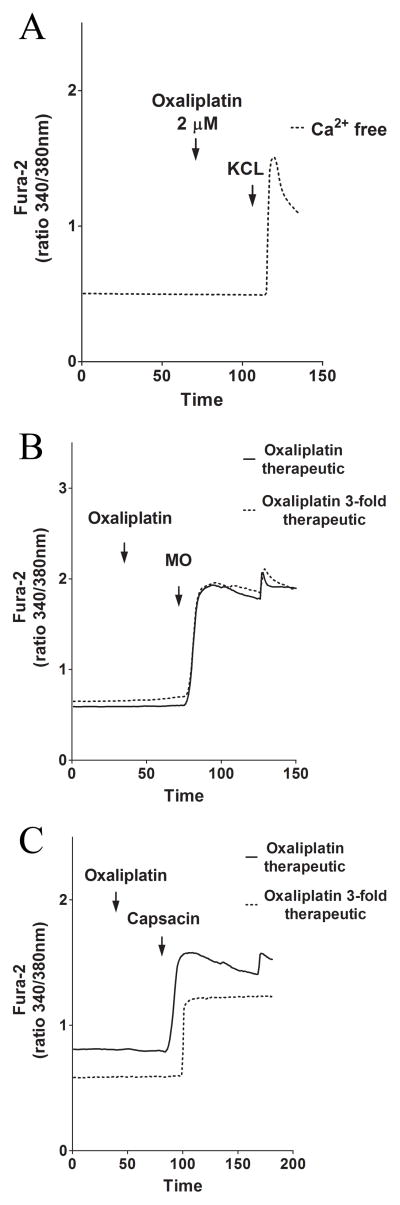
To test whether the lack of effect of oxaliplatin on SH-SY5Y cells was because the target is not present, we monitored Ca2+ changes in a primary neuronal cell line, rat DRG cells. In the absence of extracellular Ca2+, treatment with oxaliplatin had no effect on intracellular Ca2+ changes in DRG cells. To ensure that the cells were alive and excitable, we also stimulated them with extracellular KCl (Fig. 3A). To test the effects of oxaliplatin on endogenous TRP channels in the presence of extracellular Ca2+ oxaliplatin concentrations from 2.05 μM to 6.146 μM were used. Still, no Ca2+-response was evoked in the DRG cells. Subsequent administration of extracellular agonists MO or capsacin showed that the DRG cells were alive and excitable (Fig. 3B–C) suggesting that acute treatment with oxaliplatin had no effect on Ca2+-signaling in primary neuronal cells.
Figure 3. The effect of acute addition of oxaliplatin (2 μM and 6 μM) on freshly dissociated DRG.
Oxaliplatin at therapeutic concentrations and concentrations exceeding the therapeutic range induce no Ca2+ changes in freshly dissociated rat DRG cells. (A) Representative Ca2+ changes induced by 2.05 μM oxaliplatin and KCl in Ca2+ -free solution. (B) Representative Ca2+ changes induced by 2.05 μM and 6.146 μM oxaliplatin and the TRPA1 agonist mustard oil in Ca2 -containing solution. (C) Representative Ca2+ changes induced by 2.05 μM and 6.146 μM oxaliplatin and the TRP V1 agonist capsacin in Ca2+-containing solution.
Prolonged exposure to oxaliplatin alters InsP3-mediated Ca2+ signaling
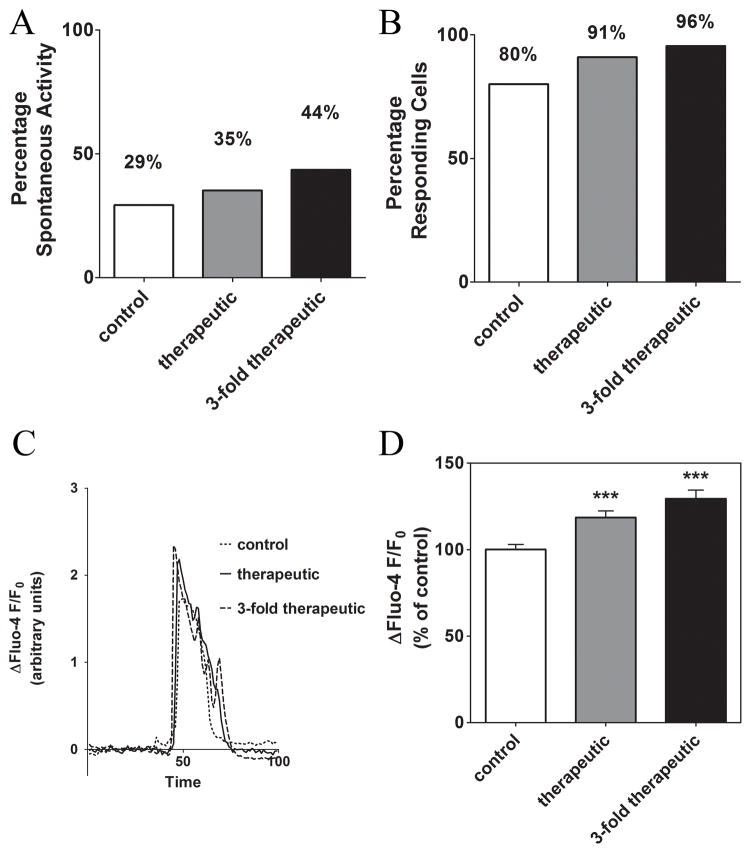
To test the effect of prolonged exposure to oxaliplatin, we monitored intracellular Ca2+ changes in SH-SY5Y incubated for 24 hours with therapeutic and 3-fold therapeutic oxaliplatin concentrations (2.05 μM and 6.146 μM). To avoid binding of oxaliplatin to serum proteins, the cells were incubated with oxaliplatin in serum-free culture media. Therefore, we also tested the effect of the absence of serum in culture media for 24 hours. After an overnight incubation in serum containing media cells usually remain quiescent, with less than 5 % of the cells showing spontaneous activity. Withholding serum made these cells more likely to produce spontaneous Ca2+ transients and this sensitivity was significantly enhanced with prolonged treatment with oxaliplatin (Fig. 4A).
Figure 4. The effect of prolonged treatment of SH-SY5Y cells with oxaliplatin on the spontaneous activity and response to ATP (0.5 μM).
Prolonged exposure to oxaliplatin alters intracellular Ca2+ signaling in neuroblastoma cells. (A) Percentage of cells showing spontaneous activity in oxaliplatin-treated cells compared with control-treated cells. (B) Percentage of oxaliplatin-treated cells responding to 0.5 μM ATP compared with control-treated cells. (C) Representative traces showing the Ca2+ changes induced by 0.5 μM ATP in oxaliplatin-treated cells compared with control-treated cells. (D) A comparison of the response amplitude to 0.5 μM ATP in cells treated with oxaliplatin within the therapeutic range, with 3-fold therapeutic concentrations, and in the control treatment. ***, P < 0.001.
To test whether the InsP3 signaling pathway functioned properly after prolonged exposure to oxaliplatin, Ca2+ responses were induced by addition of extracellular ATP. As described above, cells were exposed to 0.5 μM ATP in Ca2+ free buffer (0 Ca2+ and 100 μM EGTA), to avoid contributions from activation of the ionotropic P2X receptor family (5). The percentage of cells responding to ATP was significantly increased after 24 hours of incubation with oxaliplatin (Fig. 4C–D) and the amplitude of the response to ATP also was increased significantly (Fig. 4E). Cells treated for 24 hours with therapeutic concentrations of oxaliplatin before loading with the Ca2+ indicator showed a significant increase in the amplitude of the response to 0.5 μM ATP (18.5±3.7% ΔF/F0 (253/13)) compared to control treated cells (333/17). The average normalized fluorescence after addition of 0.5 μM ATP in cells treated with oxaliplatin concentrations 3-fold higher than therapeutic levels was increased by 29.4±4.8% ΔF/F0 (171/9) in comparison to control treated cells (Fig. 4E–F). Both increases in response amplitude are statistically significant (p<0.001).
The effect of Ca2+ load of the endoplasmic reticulum (ER) on the oxaliplatin-induced changes in the Ca2+ response
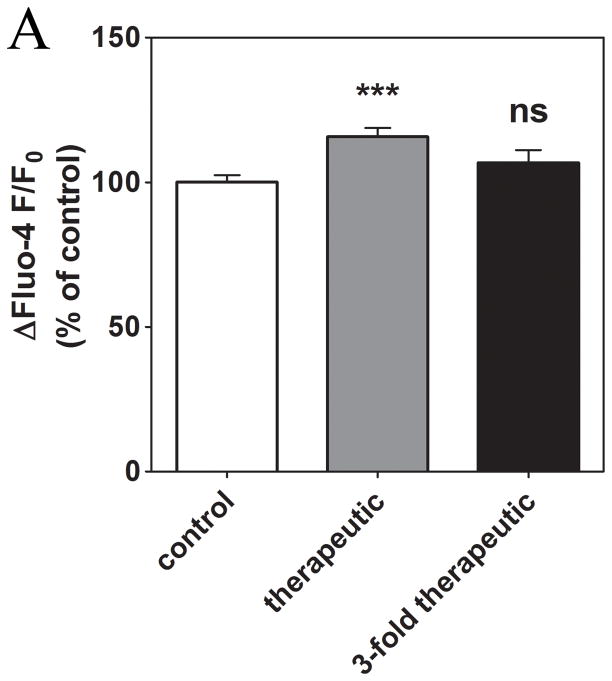
To determine the mechanism of the observed oxaliplatin-induced changes in Ca2+ signaling, the effect of oxaliplatin on the amount of Ca2+ stored in the ER was measured by depleting Ca2+ stores with thapsigargin, an inhibitor of the sarcoplasmic-ER Ca2+ ATPase (SERCA). Application of 1 μM thapsigargin to cells treated for 24 hours with therapeutic oxaliplatin concentrations resulted in significantly increased Ca2+ response amplitude (15.7±3.0% ΔF/F0 (273/13)) compared to control cells (418/18). Cells treated with a concentration of oxaliplatin 3-fold higher than therapeutic levels (146/6) showed a similar trend, but the increase was smaller and the result did not achieve significance (Fig. 5). Therefore, the Ca2+ load of the ER is likely to contribute to the alterations observed, but this parameter cannot fully explain the enhanced Ca2+ signaling observed after prolonged treatment with oxaliplatin.
Figure 5. The effect of prolonged treatment with oxaliplatin on the ER Ca2+ load.
Prolonged exposure to therapeutic oxaliplatin concentrations alters Ca2+ load of the ER in neuroblastoma cells as measured from the response to the addition of thapsigargin (1 μM). ***, P < 0.001; ns, not significant.
The effect of spontaneous activity on the oxaliplatin-induced changes in the Ca2+ response
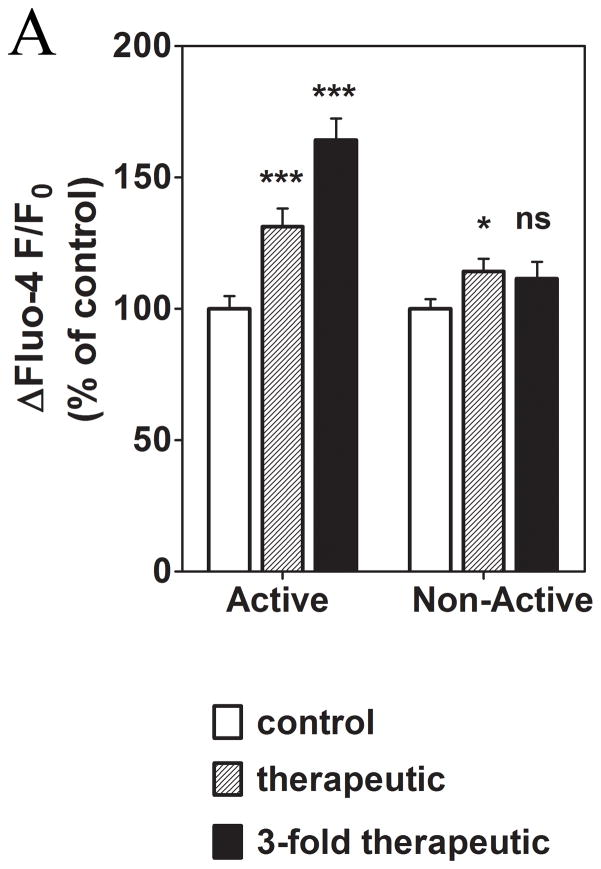
To determine whether the enhanced spontaneous activity that is induced by incubation in serum free media is a cofounder of the oxaliplatin-induced effects, we divided the cells into two groups: cells that showed spontaneous activity and those that appeared quiescent. Cells that were quiescent after preincubation with serum-free media and then oxaliplatin treatment were still able to respond to stimulation by addition of extracellular ATP. However, cells which were spontaneously active after pre-incubation with serum-free media and then oxaliplatin treatment were much more sensitive to stimulation by addition of extracellular ATP. This result was highly significant (p < 0.001) (Fig. 6A) and was seen at both therapeutic and 3-fold higher concentrations of oxaliplatin. These results suggest that the most excitable cells have the greatest response to treatment with oxaliplatin.
Figure 6. The effect of prolonged treatment with oxaliplatin on the spontaneous activity and its correlation to the response to ATP (0.5 μM).
Prolonged exposure to oxaliplatin alters intracellular Ca2+ signaling depending upon the degree of spontaneous activity before addition of ATP. ***, P < 0.001; ns, not significant.
Prolonged treatment with oxaliplatin may have several effects on cellular Ca2+-signaling. First, the baseline sensitivity to agonists is enhanced. Second, the amplitude of response to ATP is increased. And third, the ER load of Ca2+ may be elevated. Taken together, our results suggest that addition of oxaliplatin alone to cells will not induce abnormal Ca2+ signaling. However, if the cells are already in a state of enhanced sensitivity, then the addition of oxaliplatin will exacerbate that altered state.
Discussion
The aim of this study was to determine whether the Ca2+ homeostasis of neuronal cells was altered after exposure to therapeutic concentrations of oxaliplatin. We hypothesized that oxaliplatin treatment associated peripheral neuropathy and cold sensitivity could be explained by changes in Ca2+ homeostasis. Although acute exposure to oxaliplatin had no effect on neuronal cells, we found that 24 hours after oxaliplatin treatment, intracellular Ca2+ signaling was significantly altered. Prolonged exposure to oxaliplatin had two main effects. First, cells in the absence of agonists were observed to produce spontaneous changes in intracellular Ca2+ more frequently in treated versus untreated cells. Second, the amplitude of phosphoinositide-mediated Ca2+ responses was increased. These alterations can be partially explained partially by differences in the ER Ca2+ load, but additional pathways must also be involved. The present data suggest that oxliplatin has a sensitizing effect on intracellular Ca2+ signaling thereby providing a new mechanism for the pathogenesis of peripheral neuropathy and cold sensitivity.
The effects of oxaliplantin on intracellular Ca2+ homeostasis was assumed to be similar to that of paclitaxel (1,2,10). In these previous studies, peripheral pain in patients undergoing paclitaxel treatment was linked to changes in intracellular Ca2+ signaling and increases in Ca2+ dependent protease activity. These correlations suggested a molecular pathway for paclitaxel induced neuropathy. In contrast, oxaliplatin does not appear to activate Ca2+ dependent protease activity, but rather appears to target primarily the sensitivity of the Ca2+ release pathway, in part by altering the Ca2+ content of the ER.
TRP channel expression and function are differentially regulated during inflammation and nerve injury and they contribute to chronic pain and thermal hypersensitivity. Administration of noxious chemicals known to be TRP channel agonists such as capsaicin or mustard oil induce profound thermal hyperalgesia both in animals and humans. Using a sensory neuron cell line, we did not observe any immediate effects of oxaliplatin on intracellular Ca2+-levels, a response we would have expected were oxaliplatin to activate TRP channels. This unexpected lack of an effect of oxaliplatin on cold sensing channel proteins was difficult to measure because SH-SY5Y cells do not express TRP channel proteins (see Fig. 2). In fact, it appears that many cell lines do not express TRP’s (unpublished observations), the primary temperature sensors in most cell types (2).
Unlike the cell lines used in this study, sensory neurons generally express thermosensitive ion channels which respond to hot and cold temperature. These channels include TRPV1, the capsaicin receptor activated by noxious heat, and TRPM8, a cold- and menthol activated ion channel. Mice deficient in TRPM8 failed to discriminate between thermal stimuli in the innocuous cool range (15°C–25°C). TRPM8 is also essential for behavioral responses to evaporative cooling. TRPM8-deficient mice maintained normal responsiveness to cold temperature below 15°C, the thermal range considered to induce acute painful sensations. It is currently unclear whether TRPM8 activation contributes to acute or inflammatory cold-evoked pain perception. Recent experiments identified the ion channel TRPA1, the receptor for the pungent agent mustard oil and other reactive irritants, as an additional candidate for a sensory cold receptor (21). However, its role in cold nociception remains controversial.
In our experiments acute administration of oxaliplatin also failed to activate Ca2+-influx in cultured DRG neurons, indicating that cold allodynia may not result from direct effects on excitatory Ca2+-permeable thermoreceptors. Cold allodynia may arise through indirect effects, following induction of chemical stress. Indeed, a recent study investigating the role of TRPM8 in oxaliplatin-induced cold allodynia in mice observed that TRPM8 mRNA expression in DRG was significantly increased within 3 days following injection of therapeutic dosages of oxaliplatin (15). Injection of capsazepine, a TRP channel antagonist, suppressed cold allodynia in the TRPM8 expressing mice. The transcriptional level of TRPM8 mRNA returned to normal levels when measured on the tenth day following oxaliplatin treatment. However, a substantial degree of cold allodynia remained even after 25 days, suggesting that TRPM8-independent neuronal mechanisms maintain hypersensitivity to cold. These could involve other capsazepine-sensitive receptors and ion channels, because capsazepine has been shown to exert non-specific effects on other receptor classes besides TRP channels. A similar pathway may be involved in the mechanism of oxaliplatin-associated neurotoxicity.
The lack of thermosensitive TRP channel dependent pathway involvement in oxaliplatin neurotoxicity was confirmed by our observation that Ca2+ signaling changed after prolonged exposure to oxaliplatin. Pretreatment of SH-SY5Y cells with oxaliplatin increased the sensitivity to stimulation in the absence of confirmed expression of TRPV1, TRPA1 or TRPM8, suggesting that prolonged oxaliplatin exposure was the same before and after heterologous expression of these thermosensitive channels.
In summary, oxaliplatin containing chemotherapy regimens are currently considered to be a standard of care in the management of adjuvant and metastatic colorectal cancers. For those with metastatic disease, oxaliplatin-based chemotherapy is offered until disease progression or the development of dose-limiting toxicity requires suspension of treatment. Neurotoxicity can be severe and painful. Standard of cares suggests that only when a person’s quality of life is compromised does the risk of chemotherapy, despite oxaliplatin-associated neurotoxicity, outweigh the benefit. Elucidating the etiology of oxaliplatin-associated neurotoxicity may lead to the development of effective preventative interventions. Thus far, Ca2+ gluconate and magnesium sulfate infusions as well as gabapentin, amifostine and a variety of other agents have been used to mitigate oxaliplatin associated neurotoxicity with limited impact. In this context, the most common Ca2+ channels identified for maintaining Ca2+ homeostasis do not appear to play a role in oxaliplatin induced peripheral pain. More needs to be learned about the molecular mechanisms involved with this debilitating complication of an otherwise useful chemotherapeutic regimen.
Acknowledgments
Supported by grants from the NIH (DK57751, DK61747) and a scholarship from the German National Merit Foundation (CS). We thank Michael Sivula, Patricia Bimboese, Brenda DeGray, Stefan Schmidt, and Edward Chu for discussions and comments on the manuscript.
References
- 1.Boehmerle W, Splittgerber U, Lazarus MB, McKenzie KM, Johnston DG, Austin DJ, Ehrlich BE. Proc Natl Acad Sci U S A. 2006;103:18356–18361. doi: 10.1073/pnas.0607240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehmerle W, Zhang K, Sivula M, Heidrich FM, Lee Y, Jordt SE, Ehrlich BE. Proc Natl Acad Sci U S A. 2007;104:11103–11108. doi: 10.1073/pnas.0701546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 4.Graham MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E. Clin Cancer Res. 2000;6:1205–1218. [PubMed] [Google Scholar]
- 5.Cersosimo RJ. Ann Pharmacother. 2005;39:128–135. doi: 10.1345/aph.1E319. [DOI] [PubMed] [Google Scholar]
- 6.Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. Cancer Treat Rev. 2008;34:368–377. doi: 10.1016/j.ctrv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Siau C, Bennett GJ. Anesth Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge MJ. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ, Bootman MD, Roderick HL. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 10.Blachford C, Celic A, Petri ET, Ehrlich BE. Cell Calcium. 2009 doi: 10.1016/j.ceca.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielke S, Sparreboom A, Steinberg SM, Gelderblom H, Unger C, Behringer D, Mross K. Clin Cancer Res. 2005;11:4843–4850. doi: 10.1158/1078-0432.CCR-05-0298. [DOI] [PubMed] [Google Scholar]
- 12.Harwood SM, Yaqoob MM, Allen DA. Ann Clin Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- 13.Jordt SE, Ehrlich BE. Subcell Biochem. 2007;45:253–271. doi: 10.1007/978-1-4020-6191-2_9. [DOI] [PubMed] [Google Scholar]
- 14.Wu SN, Chen BS, Wu YH, Peng H, Chen LT. Neurotoxicology. 2009;30:677–685. doi: 10.1016/j.neuro.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Gauchan P, Andoh T, Kato A, Kuraishi Y. Neurosci Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Benoit E, Brienza S, Dubois JM. Gen Physiol Biophys. 2006;25:263–276. [PubMed] [Google Scholar]
- 17.Estrada M, Uhlen P, Ehrlich BE. J Cell Sci. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- 18.Vaziri C, Downes CP. Biochem J. 1992;284 (Pt 3):917–922. doi: 10.1042/bj2840917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benham CD. Ann N Y Acad Sci. 1990;603:275–285. doi: 10.1111/j.1749-6632.1990.tb37679.x. discussion 285–276. [DOI] [PubMed] [Google Scholar]
- 20.Jordt SE, McKemy DD, Julius D. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 21.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordt SE, et al. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]