Abstract
Background
High chronic exposure to inorganic arsenic may contribute to the development of hypertension. Limited information is available, however, on the association of low to moderate exposure to inorganic arsenic with blood pressure levels and hypertension. We investigated the association of exposure to inorganic arsenic (as measured in urine) with systolic and diastolic blood pressure levels and the prevalence of hypertension in U.S. adults.
Methods
We studied 4167 adults 20 years of age or older who participated in the National Health and Nutrition Examination Survey (NHANES) from 2003 through 2008 and for whom total arsenic, dimethylarsinate (DMA) and arsenobetaine had been assessed in urine.
Results
The median (inter-quartile range) urine concentrations were 8.3 μg/L (4.2– 17.1) for total arsenic, 3.6 μg/L (2.0– 6.0) for DMA and 1.4 μg/L (0.3– 6.3) for arsenobetaine. The weighted prevalence of hypertension in the study population was 36%. After multivariable adjustment, a 2-fold increase in total arsenic was associated with a hypertension odds ratio of 0.98 (95% confidence interval = 0.86 to 1.11). A doubling of total arsenic minus arsenobetaine was associated with a hypertension OR of 1.03 (0.94 to 1.14) and a doubling of DMA concentrations was associated with a hypertension OR of 1.11 (0.99 to 1.24). Total arsenic, total arsenic minus arsenobetaine, or DMA levels were not associated with systolic or diastolic blood pressure.
Conclusions
At the low to moderate levels typical of the U.S. population, total arsenic, total arsenic minus arsenobetaine, and DMA concentrations in urine were not associated with the prevalence of hypertension or with systolic or diastolic blood pressure levels. A weak association of DMA with hypertension could not be ruled out.
Arsenic is widespread in the environment. Humans are exposed to inorganic forms (arsenite, arsenate) and organic forms (arsenobetaine, arsenosugars and arsenolipids). Inorganic arsenic is toxic and carcinogenic. Organic arsenicals (found mostly in seafood) have low or very low toxicity.1 For the general population, the main source of inorganic arsenic is ingestion of arsenic-contaminated drinking water and certain foods (e.g., rice and grains). After exposure, inorganic arsenic and its methylated metabolites (methylarsonate and dimethylarsinate [DMA]) are widely distributed within the body and excreted mostly through the kidneys.2,3 Arsenic levels in urine reflect ongoing exposures and are well correlated with arsenic intake from drinking water and dietary sources.4–6
Several epidemiologic studies have found increased prevalence of hypertension or increased systolic and diastolic blood pressure levels associated with higher arsenic levels in drinking water among residents in high arsenic exposure areas in Taiwan, Bangladesh and Inner Mongolia (>100 μg/L), and also with occupational exposure.7–10 Another study in Bangladesh found no association between arsenic in drinking water and prevalence of hypertension, except perhaps among participants with low folate and vitamins B intake.11 In a cross-sectional study in Wisconsin, arsenic in drinking water ≥10 μg/L vs. <2 μg/L was associated with self-reported hypertension.12 Studies with measured blood pressure levels in low arsenic exposure areas are not yet available. The objective of this study was to investigate the association of inorganic arsenic exposure (using urine arsenic biomarkers) with systolic and diastolic blood pressure and the prevalence of hypertension in a representative sample of U.S. adults who participated in the 2003–2008 National Health and Nutrition Examination Survey (NHANES).
METHODS
Study Population
NHANES is conducted by the US National Center for Health Statistics (NCHS; Centers for Disease Control and Prevention [CDC], Atlanta, GA), using a complex multistage sampling design to obtain a representative sample of the civilian non-institutionalized US population. NHANES study protocols for the 2003–2008 survey years were approved by the National Center for Health Statistics Institutional Review Board, and oral and written informed consent was obtained from all participants. The participation rate for NHANES examinations was 76% for survey years 2003–2008. A total of 15,955 adults 20 years of age or older participated in NHANES between 2003 and 2008, and 4843 were randomly selected for urine arsenic analysis. We excluded 189 pregnant women, 258 participants whose blood pressure measures were missing or whose diastolic blood pressure levels were equal to zero, and 229 participants missing other relevant covariates, leaving 4167 participants for this study.
Urine Arsenic Assessment
Spot urine samples for arsenic analysis were collected in arsenic-free containers, shipped on dry ice, stored frozen at ≤ −70°C, and analyzed within 3 weeks of collection.13 Total arsenic and arsenic species were measured at the Environmental Health Sciences Laboratory of the National Center for Environmental Health following a standardized protocol.14–16 Total urine arsenic concentrations were determined using inductively coupled-plasma dynamic reaction cell-mass spectrometry on a Perkin-Elmer ELAN® 6100 DRCPLUS or ELAN® DRC II ICPMS (Perkin Elmer SCIEX, Concord, ON, Canada). Arsenite, arsenate, methylarsonate, DMA and arsenobetaine were measured by high performance liquid chromatography coupled to spectrometry. The limit of detection for total arsenic was 0.6 μg/L for survey years 2003–2004 and 0.74 μg/L for survey years 2005–2008. The percentage of study participants with total urine arsenic levels below the limit of detection was 0.9%. The limits of detection for arsenite (1.2 μg/L), arsenate (1.0 μg/L) and methylarsonate (0.9 μg/L), species that directly reflect inorganic arsenic exposure, were too high for use in a population exposed to low to moderate levels of inorganic arsenic.13 Indeed, the percentage of study participants with arsenite, arsenate and methylarsonate below the limit of detection were 96%, 96% and 65%, respectively; therefore, these species could not be considered in this study. The limit of detection for DMA was 1.7 μg/L for all survey years; 15% participants had urine DMA concentrations below the limit of detection. The limit of detection for arsenobetaine was 0.4 μg/L; 30% of participants had urine arsenobetaine below the limit of detection. For participants with total arsenic, DMA or arsenobetaine levels below the limit of detection, a level equal to the limit of detection divided by the square root of two was assigned. The inter-assay coefficients of variation for quality control pooled samples were 3.7% and 8.4% for lots with mean total urine arsenic of 58.6 μg/L and 3.7 μg/L, respectively,17 6.5% for lots with mean DMA 6.66 μg/L, and 10.1% for lots with mean arsenobetaine 4.87 μg/L.18
Hypertension and Blood Pressure
Blood pressure levels were measured using a standardized protocol and certified examiners.19–21 After five minutes of rest and determination of the maximum inflation level, three (and in some cases four) systolic and diastolic blood pressures were measured. Mean systolic and diastolic blood pressure levels were determined after disregarding the first reading, unless only one reading was available. Hypertension was defined as a mean systolic blood pressure ≥ 140 mmHg, a mean diastolic blood pressure ≥ 90 mmHg, a self-reported physician diagnosis, or use of antihypertensive medication.
Other Variables
Information on sex, age, race/ethnicity, education, smoking status, and alcohol consumption was collected by self-reported questionnaire. Race/ethnicity was subsequently categorized by NCHS as non-Hispanic white, non-Hispanic black, Mexican-American, other Hispanic, and other. Body mass index (BMI) was calculated by dividing measured weight in kilograms by measured height in meters squared. Serum cotinine was measured by an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric method. For participants with serum cotinine levels below the limit of detection (0.015 ng/mL), a level equal to the limit of detection divided by the square root of two was imputed. Urine creatinine, used to adjust for urine dilution in spot urine samples in statistical models, was determined using a Jaffe rate reaction measured with a CX3 analyzer.
Statistical Analysis
All statistical analyses were performed using the survey package22,23 (version 3.21) in R software24 (version 2.10.1) to account for the complex sampling design and to obtain appropriate estimates and standard errors using the NHANES 2003–2008 arsenic analyses sample weights. Confidence intervals were set at 95%. Urine arsenic levels were right-skewed and log-transformed for the analyses. For total arsenic, total arsenic minus arsenobetaine, and DMA, we estimated crude and multivariable adjusted odds ratios for the prevalence of hypertension, as well as mean differences in blood pressure levels (systolic and diastolic) comparing quartiles 2, 3 and 4 to the lowest quartile using logistic and linear regression, respectively. Quartile cutoffs were based on the weighted distributions of total arsenic, total arsenic minus arsenobetaine, and DMA in the study population. In addition to quartiles, log-transformed arsenic was used as a continuous variable to evaluate the association of blood pressure endpoints with a doubling in urine arsenic concentrations.
Initially we adjusted statistical models for sex, age (continuous), race/ethnicity (white/black/Mexican-American/other) and urine creatinine (log-transformed) and further adjusted for education (<high school, high school, >high school), BMI (continuous), and serum cotinine (log-transformed). Further adjustment for smoking status produced similar results (not shown). We also adjusted models of mean differences in blood pressure levels for use of anti-hypertensive medication. In the subsample of participants with serum folate and vitamin B12 measures available (n = 2591), we further adjusted for those variables with similar findings (not shown). To account for the contribution of seafood arsenicals to total arsenic, total arsenic minus arsenobetaine and DMA concentrations in the US population,25 we further adjusted the relationship of these arsenicals with blood pressure endpoints for arsenobetaine, an objective biomarker of seafood intake. In these models, total arsenic, total arsenic minus arsenobetaine and DMA reflect exposure to arsenic not derived from seafood. To evaluate the potential impact of adjustment for urine creatinine to control for urine dilution, we repeated all models without adjustment for urine creatinine as well as by dividing arsenic concentrations by urine creatinine. The results were similar using these alternative modeling strategies (not shown).
To assess the consistency of the findings across participant characteristics, we estimated the odds ratio (OR) and 95% confidence interval (CI) of hypertension for a doubling in total arsenic and DMA concentrations for subgroups defined by sex, age, race/ethnicity, education, BMI, and smoking status. P-values for the interaction of urine arsenic concentrations with participant characteristics were obtained by adding an interaction term between log-transformed arsenic (total arsenic or DMA) and the corresponding participant characteristic in the fully adjusted model.
We also conducted two sensitivity analyses. First we conducted censored regression analysis using the cnreg command in Stata26 (version 11.1) to better account for antihypertensive medication use in models for systolic and diastolic blood pressure. Second, we repeated the analysis in participants with non-detectable arsenobetaine (n = 1265) to identify participants unlikely to have been recently exposed to organic arsenicals and in whom total urine arsenic likely reflects exposure to inorganic arsenic.
RESULTS
Participant Characteristics
The weighted prevalence of hypertension in the study population was 36%. Compared with participants who did not have hypertension, participants with hypertension were more likely to be older, female, white, and less educated. They were also more likely to have a higher BMI, to be former smokers, and to have diabetes (Table 1). The median (interquartile range [IQR]) of urine concentrations of total arsenic, arsenobetaine and DMA were 8.3 (4.2– 17.1) μg/L, 1.4 (0.3–6.2) μg/L and 3.6 (2.0– 6.0) μg/L, respectively (Table 2). Total arsenic and DMA levels were higher in men, in younger participants, and in participants who were non-white. Arsenobetaine levels were higher in men, in participants with higher education, and in those who were classified as black or other race/ethnicity.
Table 1.
Participant Characteristics by Hypertension Status
| Characteristics | Hypertension | |
|---|---|---|
| Yes (n= 1761) | No (n= 2406) | |
| Age (yr); mean (SE) | 56.7 (0.6) | 41.1 (0.4) |
| Sex, male; % (SE) | 47 (1.6) | 51(1.3) |
| Race/ethnicity, White; % (SE) | 76 (2.2) | 71 (2.2) |
| Education, >high school; % (SE) | 51 (1.6) | 58(1.5) |
| Body mass index (kg/m2) | 30 (0.2) | 27 (0.2) |
| Smoking; % (SE) | ||
| Former | 32 (1.2) | 22 (1.4) |
| Current | 20 (1.4) | 27(1.4) |
| Cotinine (ng/mL)a ; mean (SE) | 0.36 (0.11 to 0.61) | 0.60 (0.31 to 0.89) |
| Serum folate (ng/mL)a,b ; mean (SE) | 13.3 (13.2 to 13.4) | 11.1 (11.1 to 11.1) |
| Vitamin B12 (pg/mL)a,b ; mean (SE) | 460(459.8 to 459.9) | 466 (466.2 to 466.3) |
| Diabetes mellitus;%(SE) | 19.0(1.0) | 5.4 (0.5) |
Geometric mean (95% CI)Other results in the table are arithmetic means or percentages (standard error)
Available only for2003–2006 (n=1106 for serum folate and n=1485 for vitamin B12)
Table 2.
Urine Arsenic Concentrations by Participant Characteristics
| Characteristics | No.(%) | Urine Arsenic Concentration (μg/L)
|
||
|---|---|---|---|---|
| Total Arsenic Median (IQR) |
Dimethylarsinate (DMA) Median (IQR) |
Arsenobetaine Median (IQR) |
||
| Overall | 4167 | 8.3 (4.2–17.1) | 3.6 (2.0–6.0) | 1.4 (0.3–6.2) |
| Sex | ||||
| Men | 2141 (49) | 9.2 (4.9–19.0) | 4.0 (2.5–6.5) | 1.5 (0.3–6.9) |
| Women | 2026 (51) | 7.2 (3.6–15.5) | 3.3 (2.0–5.5) | 1.3 (0.3–5.5) |
| Age (y) | ||||
| 20–39 | 1331 (37) | 8.5 (4.4–18.9) | 3.9 (2.2–6.3) | 1.4 (0.3–6.5) |
| 40–59 | 1327 (40) | 8.4 (4.3–16.2) | 3.7 (2.0–6.0) | 1.4 (0.3–5.7) |
| ≥60 | 1509 (23) | 7.6 (3.6–16.5) | 3.3 (2.0–5.5) | 1.4 (0.3–6.9) |
| Race/ethnicity | ||||
| White | 2127 (73) | 7.4 (3.7–15.4) | 3.3 (2.0–5.3) | 1.2 (0.3–5.4) |
| Black | 861 (11) | 10.4 (5.6–22.7) | 4.0 (2.5–6.9) | 2.5 (0.5–8.7) |
| Mexican-American | 794 (8) | 10.0 (5.3–16.8) | 5.0 (3.0–7.3) | 1.1 (0.3–4.2) |
| Other | 385 (8) | 14.4 (6.0–33.6) | 6.0 (3.3–11.6) | 2.7 (0.5–12.1) |
| Education | ||||
| <High school | 1244 (19) | 8.3 (4.3–16.8) | 3.7 (2.0–6.8) | 1.1 (0.3–4.8) |
| High school | 1020 (26) | 7.6 (3.9–15.9) | 3.4 (2.0–5.6) | 1.1 (0.3–5.6) |
| >High school | 1903 (55) | 8.6 (4.3–18.4) | 3.8 (2.1–6.0) | 1.7 (0.3–7.2) |
| BMI (kg/m2) | ||||
| <25 | 1256 (33) | 8.4 (3.9–17.1) | 3.4 (2.0–6.0) | 1.4 (0.3–6.3) |
| 25-<29.9 | 1494 (36) | 7.9 (3.9–18.3) | 3.8 (2.1–6.2) | 1.4 (0.3–6.7) |
| ≥30 | 1417 (32) | 8.5 (4.6–16.5) | 3.8 (2.2–6.0) | 1.5 (0.3–5.6) |
| Smoking | ||||
| Never | 2126 (50) | 8.5 (4.2–16.7) | 3.7 (2.1–6.0) | 1.5 (0.3–6.2) |
| Former | 1118 (26) | 8.6 (4.3–19.6) | 3.7 (2.1–6.0) | 1.5 (0.3–7.5) |
| Current | 922 (24) | 7.6 (3.8–15.9) | 3.5 (2.0–5.9) | 1.0 (0.3–4.8) |
| Serum cotinine (ng/mL) | ||||
| <0.015 | 729 (17) | 8.7 (3.7–16.2) | 3.7 (2.0–6.0) | 1.4 (0.3–5.6) |
| 0.015-<10.0 | 2365 (56) | 8.5 (4.4–18.1) | 3.6 (2.1–6.2) | 1.6 (0.3–7.1) |
| ≥10.0 | 1073 (28) | 7.6 (3.9–16.1) | 3.5 (2.0–5.8) | 0.9 (0.3–5.4) |
| Serum folate (ng/mLa) | ||||
| ≤ 11.6 | 1325 (33) | 8.4 (4.5–17.9) | 4.0 (2.1–6.0) | 1.6 (0.3–7.2) |
| > 11.6 | 1263 (33) | 8.6 (4.3–17.8) | 3.6 (2.0–6.0) | 1.6 (0.3–6.8) |
| Vitamin B12 (pg/mLa) | ||||
| ≤ 461 | 1215 (33) | 8.3 (4.5–16.2) | 3.7 (2.1–6.0) | 1.6 (0.3–6.6) |
| > 461 | 1359 (33) | 8.8 (4.3–19.2) | 3.9 (2.0–6.2) | 1.6 (0.3–7.1) |
| Diabetes | ||||
| Yes | 621 (10) | 8.4 (4.2–16.5) | 3.8 (2.1–6.0) | 1.1 (0.3–5.8) |
| No | 3546 (90) | 8.2 (4.2–17.2) | 3.6 (2.0–6.0) | 1.5 (0.3–6.3) |
| Hypertension | ||||
| Yes | 1761 (36) | 8.3 (4.2–16.5) | 3.6 (2.0–5.8) | 1.5 (0.3–6.4) |
| No | 2406 (64) | 8.3 (4.2–17.4) | 3.7 (2.1–6.0) | 1.3 (0.3–6.1) |
Available only for2003–2006
Arsenic and Blood Pressure Endpoints
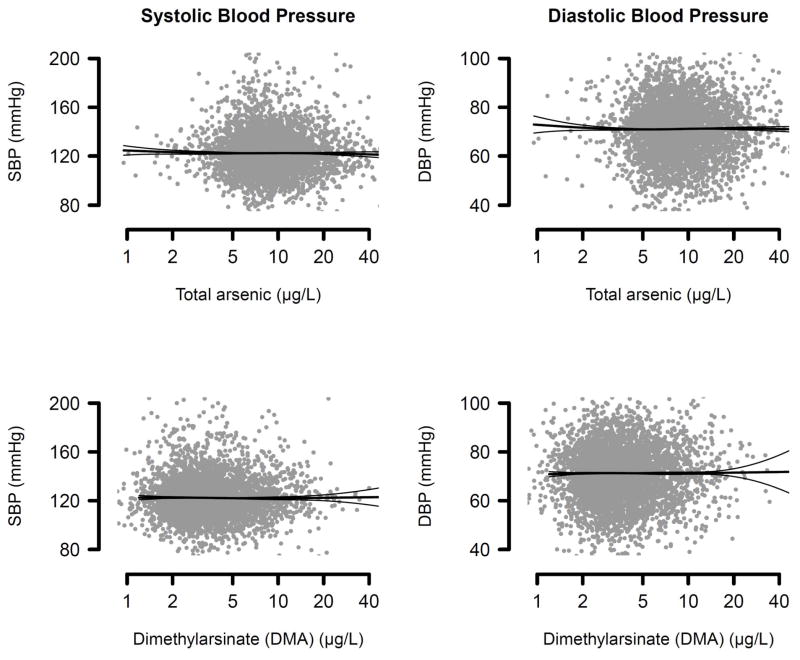
Median total arsenic and DMA in participants with hypertension were 8.3 μg/L and 3.6 μg/L, respectively, compared with 8.3 μg/L and 3.7 μg/L in those without hypertension (Table 2). After adjustment for demographics, BMI, serum cotinine, urine creatinine, and urine arsenobetaine, the OR for hypertension was 0.98 (95% CI = 0.86 to 1.11) for a 2-fold increase in total arsenic concentrations, 1.03 (0.94 to 1.14) for a 2-fold increase in total arsenic minus arsenobetaine, and 1.11 (0.99 to 1.24) for a 2-fold increase in DMA concentrations (Table 3, model 3). Without adjustment for arsenobetaine, the ORs for hypertension for a doubling in total arsenic, total arsenic minus arsenobetaine, and DMA were 1.03, 1.12 and 1.05 respectively (Table 3, model 2). Total arsenic, total arsenic minus arsenobetaine and DMA were not associated with systolic or diastolic blood pressure levels (Table 4, Figure 1).
Table 3.
Odds Ratio (95% CI) of Hypertension by Urine Arsenic Concentrations
| Quartile
| ||||||
|---|---|---|---|---|---|---|
| Per doubling of arsenic | 1a | 2 | 3 | 4 | Test for Trendb | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Total arsenic (μg/L) | ||||||
| <4.2 | 4.2 to 8.3 | >8.3 to 17.1 | >17.1 | |||
| No. | (n=1761/2406)c | (n=418/534) c | (n=451/606) c | (n=446/644) c | (n=446/622) c | |
| Crude | 0.98 (0.94 to 1.03) | 1.00 | 0.98 (0.79 to 1.21) | 1.06 (0.85 to 1.32) | 0.94 (0.76 to 1.17) | P =0.39 |
| Model 1d | 0.99 (0.93 to 1.06) | 1.00 | 1.08 (0.84 to 1.38) | 1.18 (0.88 to 1.57) | 1.04 (0.77 to 1.40) | P =0.79 |
| Model 2e | 1.03 (0.96 to 1.10) | 1.00 | 1.09 (0.84 to 1.41) | 1.33 (0.99 to 1.80) | 1.22 (0.91 to 1.64) | P =0.45 |
| Model 3f | 0.98 (0.86 to 1.11) | 1.00 | 1.08 (0.83 to 1.40) | 1.30 (0.94 to 1.80) | 1.17 (0.75 to 1.83) | P =0.71 |
| Total arsenic minus arsenobetaine (μg/L) | ||||||
| <3.1 | 3.1 to 5.8 | >5.8 to 10.8 | >10.8 | |||
| No. | (n=1761/2406) c | (n=426/489) c | (n=428/605) c | (n=463/636) c | (n=432/670) c | |
| Crude | 0.96 (0.92 to 1.01) | 1.00 | 0.89 (0.72 to 1.10) | 0.96 (0.77 to 1.20) | 0.83 (0.68 to 1.00) | P =0.11 |
| Model 1d | 0.99 (0.92 to 1.08) | 1.00 | 0.97 (0.74 to 1.28) | 1.14 (0.83 to 1.56) | 1.04 (0.74 to 1.45) | P =0.89 |
| Model 2e | 1.05 (0.96 to 1.14) | 1.00 | 1.07 (0.81 to 1.42) | 1.34 (0.95 to 1.89) | 1.30 (0.92 to 1.83) | P =0.28 |
| Model 3f | 1.03 (0.94 to 1.14) | 1.00 | 1.07 (0.81 to 1.40) | 1.33 (0.95 to 1.85) | 1.27 (0.88 to 1.83) | P =0.53 |
| Dimethylarsinate (μg/L) | ||||||
| <2.0 | 2.0 to 3.6 | >3.6 to 6.0 | >6.0 | |||
| No. | (n=1761/2406) c | (n=415/496) c | (n=461/613) c | (n=448/631) c | (n=437/666) c | |
| Crude | 0.96 (0.90 to 1.02) | 1.00 | 0.95 (0.78 to 1.17) | 0.92 (0.73 to 1.16) | 0.88 (0.72 to 1.08) | P =0.18 |
| Model 1d | 1.03 (0.93 to 1.14) | 1.00 | 0.96 (0.73 to 1.28) | 1.04 (0.76 to 1.40) | 1.05 (0.75 to 1.46) | P =0.55 |
| Model 2e | 1.12 (1.01 to 1.23) | 1.00 | 1.06 (0.80 to 1.41) | 1.21 (0.89 to 1.64) | 1.29 (0.93 to 1.79) | P =0.03 |
| Model 3f | 1.11 (0.99 to 1.24) | 1.00 | 1.05 (0.77 to 1.42) | 1.18 (0.84 to 1.66) | 1.24 (0.84 to 1.83) | P =0.07 |
Reference category
Based on log-transformed arsenic concentrations
No. with/without hypertension
Adjusted for sex, age, race and ethnicity, and urine creatinine level (log-transformed)
further adjusted for education, body mass index, serum cotinine level (log-transformed)
further adjusted for arsenobetaine (log-transformed)
Table 4.
Difference in Systolic and Diastolic Blood Pressure (mmHg) by Urine Arsenic Concentrations
| Quartile
| ||||||
|---|---|---|---|---|---|---|
| Per doubling of arsenic | 1a | 2 | 3 | 4 | Test for Trendb | |
| Difference (95% CI) | Difference(95% CI) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | ||
| Systolic Blood Pressure | ||||||
| Total arsenic (μg/L) | ||||||
| <4.2 | 4.2 to 8.3 | >8.3 to 17.1 | >17.1 | |||
| Crude | −0.47 (−0.86 to −0.09) | 0.00 | −1.25 (−3.10 to 0.61) | 0.14 (−0.76 to 2.05) | −1.93 (−3.83 to −0.03) | P =0.02 |
| Model 1c | −0.28 (−0.73 to 0.18) | 0.00 | −0.68 (−2.43 to 1.07) | 0.88 (−0.95 to 2.72) | −0.75 (−2.82 to 1.32) | P =0.23 |
| Model 2d | −0.75 (−1.52 to 0.02) | 0.00 | −0.69 (−2.49 to 1.11) | 0.86 (−1.28 to 3.00) | −0.79 (−4.06 to 2.47) | P =0.06 |
| Total arsenic minus arsenobetaine (μg/L) | ||||||
| <3.1 | 3.1 to 5.8 | >5.8 to 10.8 | >10.8 | |||
| Crude | −0.67 (−1.09 to −0.25) | 0.00 | −1.58 (−3.35 to 0.18) | −1.51 (−3.59 to 0.57) | −3.05 (−4.92 to −1.18) | P =0.002 |
| Model 1c | −0.40 (−0.97 to 0.18) | 0.00 | −0.78 (−2.46 to 0.90) | −0.49 (−2.72 to 1.74) | −1.28 (−3.76 to 1.19) | P =0.18 |
| Model 2d | −0.50 (−1.15 to 0.15) | 0.00 | −0.81 (−2.50 to 0.88) | −0.55 (−2.82 to 1.73) | −1.40 (−4.17 to 1.38) | P =0.13 |
| Dimethylarsinate (μg/L) | ||||||
| <2.0 | 2.0 to 3.6 | >3.6 to 6.0 | >6.0 | |||
| Crude | −0.74 (−1.29 to −0.20) | 0.00 | −0.91(−2.90 to 1.08) | −1.03 (−3.38 to 1.33) | −2.12 (−4.17 to −0.08) | P =0.009 |
| Model 1c | −0.14 (−0.91 to 0.63) | 0.00 | −0.53 (−2.58 to 1.52) | 0.19 (−2.32 to 2.71) | −0.47 (−3.08 to 2.14) | P =0.72 |
| Model 2d | −0.10 (−0.96 to 0.77) | 0.00 | −0.48 (−2.56 to 1.59) | 0.28 (−2.36 to 2.92) | −0.31(−3.20 to 2.58) | P =0.83 |
| Diastolic Blood Pressure | ||||||
| Total arsenic (μg/L) | ||||||
| <4.2 | 4.2 to 8.3 | >8.3 to 17.1 | >17.1 | |||
| Crude | −0.08 (−0.29 to 0.14) | 0.00 | −0.43 (−1.90 to 1.05) | 0.29 (−0.97 to 1.55) | −0.37 (−1.40 to 0.67) | P =0.48 |
| Model 1c | 0.01 (−0.29 to 0.32) | 0.00 | −0.39 (−1.98 to 1.19) | 0.63 (−0.90 to 2.16) | 0.07 (−1.40 to 1.54) | P =0.94 |
| Model 2d | −0.36 (−1.02 to 0.29) | 0.00 | −0.56 (−2.19 to 1.07) | 0.27 (−1.50 to 2.05) | −0.65 (−2.86 to 1.56) | P =0.27 |
| Total arsenic minus arsenobetaine (μg/L) | ||||||
| <3.1 | 3.1 to 5.8 | >5.8 to 10.8 | >10.8 | |||
| Crude | −0.13 (−0.34 to 0.09) | 0.00 | −0.29 (−1.53 to 0.95) | −0.08 (−1.19 to 1.04) | −0.33 (−1.29 to 0.64) | P =0.24 |
| Model 1c | −0.02 (−0.42 to 0.39) | 0.00 | −0.05 (−1.45 to 1.36) | 0.38 (−1.08 to 1.84) | 0.39 (−1.12 to 1.91) | P =0.94 |
| Model 2d | −0.17 (−0.68 to 0.35) | 0.00 | −0.13 (−1.58 to 1.33) | 0.22 (−1.42 to 1.86) | 0.09 (−1.81 to 1.98) | P =0.52 |
| Dimethylarsinate (μg/L) | ||||||
| <2.0 | 2.0 to 3.6 | >3.6 to 6.0 | >6.0 | |||
| Crude | −0.09 (−0.41 to 0.23) | 0.00 | −0.30 (−1.64 to 1.03) | 0.29 (−0.96 to 1.55) | −0.55 (−1.76 to 0.65) | P =0.58 |
| Model 1c | 0.18 (−0.29 to 0.65) | 0.00 | −0.16 (−1.74 to 1.41) | 0.64 (−1.03 to 2.30) | 0.12 (−1.72 to 1.97) | P =0.44 |
| Model 2d | 0.11 (−0.44 to 0.65) | 0.00 | −0.23 −.84 to 1.37) | 0.49 (−1.33 to 2.31) | −0.14 (−2.23 to 1.95) | P =0.70 |
Reference category
Based on log-transformed arsenic concentrations
Adjusted for sex, age, race and ethnicity, and urine creatinine level (log-transformed), education, body mass index, serum cotinine level (log-transformed), and antihypertensive medication use
further adjusted for arsenobetaine (log-transformed)
Figure 1.
Systolic (SBP) and Diastolic Blood Pressure (DBP) by Total Urine Arsenic and Urine DMA Concentrations. Scatterplots represent fully adjusted levels of arsenic and blood pressure levels. Lines represent blood pressure levels (thinner lines represent 95% confidence intervals) based on restricted quadratic splines for log-transformed arsenic concentrations with knots at 5th, 50th and 95th percentiles. Results were adjusted for age, sex, race/ethnicity, urine creatinine (log-transformed), education, body mass index, serum cotinine (log-transformed), urine arsenobetaine (log-transformed) and antihypertensive medication use.
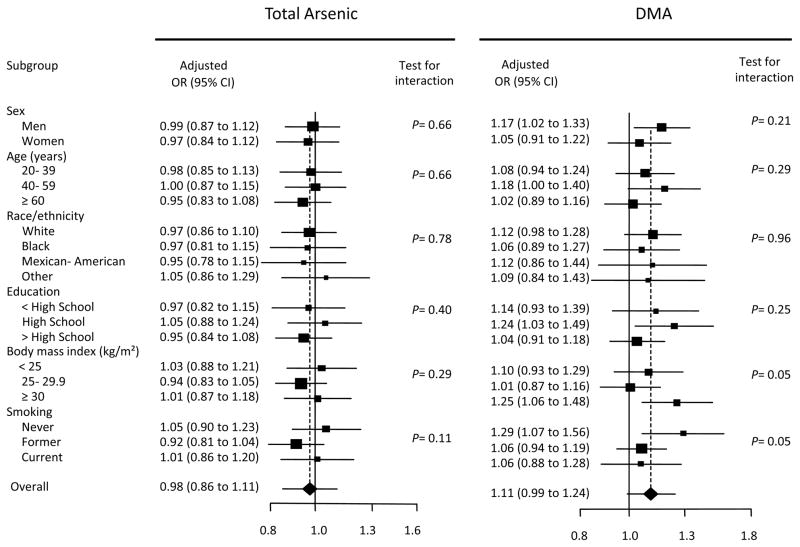
The lack of association of total arsenic with the prevalence of hypertension was similar across participant characteristics (Figure 2). For DMA, a statistically significant interaction was observed by BMI and smoking status. The ORs of hypertension for a 2-fold increase in DMA concentrations were 1.25 (1.06 to 1.48) in participants with a body mass index ≥ 30 kg/m2 and 1.29 (1.07 to 1.56) in never-smokers (Figure 2).
Figure 2.
Odds Ratio of Hypertension for a Doubling of Urine Arsenic Concentrations (μg/L) by Participant Characteristics. Boxes represent the hypertension ratio for a doubling of urine arsenic concentrations. The size of the box is weighted by the inverse of the variance. Horizontal lines represent 95% CIs. ORs were adjusted for age, sex, race/ethnicity, urine creatinine (log-transformed), education, BMI, serum cotinine (log-transformed), and urine arsenobetaine (log-transformed). P-values for interaction between log transformed arsenic and participant characteristics were computed using the Wald test, accounting for the complex design.
Sensitivity analyses using censored regression to correct for the effect of use of antihypertensive medication resulted in similar findings (not shown). Sensitivity analyses in the participants with non-detectable arsenobetaine levels (n= 1265) also resulted in similar findings (eTable, http://links.lww.com). For total urine arsenic, the OR of hypertension was 0.95 (0.75 to 1.19) for a 2-fold increase, and 0.79 (0.40 to 1.53), 0.83 (0.43 to 1.59) and 0.92 (0.48 to 1.75) for quartiles 2, 3 and 4, respectively, compared with quartile 1. For urine DMA, the OR of hypertension was 1.15 (0.92 to 1.45) for a 2-fold increase, and 0.98 (0.55 to 1.74), 0.99 (0.59 to 1.64) and 1.22 (0.71 to 2.11) for quartiles 2, 3 and 4, respectively, compared with quartile 1.
DISCUSSION
In a representative sample of U.S. adults who participated in NHANES 2003–2008, we found no association between urine concentrations of total arsenic or total arsenic minus arsenobetaine with the prevalence of hypertension or with systolic and diastolic blood pressure levels. For urine DMA, while no association was found with systolic or diastolic blood pressure, the findings for hypertension could be consistent with no association or with a small positive association, especially in certain subgroups (such as participants with BMI ≥ 30 kg/m2 or in never-smokers). Subgroup findings, however, need to be considered cautiously as we had no a priori hypothesis and these analyses were exploratory.
Most studies investigating the association between arsenic exposure and hypertension endpoints have been conducted at much higher levels of exposure than those in the general U.S. population.7,9,11,27–29 Most of these studies assessed arsenic exposure based on measures of arsenic in drinking water. Chronic exposure to high levels of inorganic arsenic in drinking water (median levels ranging from <500 to >1000 μg/L) have been associated with the prevalence of hypertension in Taiwan and Bangladesh.9,10 In a cross-sectional study of 898 residents in Southwestern Taiwan, the multivariate-adjusted OR for hypertension comparing participants with cumulative arsenic exposure >18.5 mg/L-years to <0.1 mg/L-years was 2.9 (1.1 to 7.3).9 In a cross-sectional study of 1481 residents in Bangladesh, the OR for hypertension comparing participants with cumulative arsenic exposure >10.0 mg/L-years to unexposed participants was 3.0 (95% CI = 1.5 to 5.8) after adjustment for age, sex and BMI.10 In contrast, another cross-sectional study conducted in Bangladesh (n = 10,910) found that time-weighted well arsenic concentration was not associated with hypertension,11 although increasing arsenic concentrations in drinking water were associated with systolic hypertension (systolic ≥ 140mmHg) and with high pulse pressure (≥ 55mmHg), especially among participants with low vitamin B6, B12 or folate levels.11 In our study, the results were similar after further adjustment for serum folate and vitamin B12 and in analyses stratified by serum folate and vitamin B12 levels, although folate and vitamin B levels are substantially higher in the US than in Bangladesh.
Few studies have addressed the association of low or moderate exposure to inorganic arsenic with hypertension.12,30,31 In a cross-sectional study in Wisconsin, the age, sex and BMI adjusted OR for self-reported hypertension comparing residents with arsenic levels in well water between 2 and 10 μg/L and >10 μg/L to <2 μg/L were 1.2 (95% CI = 0.8 to 1.6) and 1.7 (1.1 to 2.5), respectively.12 In 432 participants exposed to relatively low arsenic levels in drinking water in Central Taiwan, hair arsenic levels were associated with the prevalence of hypertension.31 In an occupational study of 59 workers in Denmark, mean systolic and diastolic blood pressure levels were higher in arsenic-exposed workers (median total urine arsenic = 14.8 μg/g of creatinine) compared with unexposed workers (7.9 μg/g of creatinine).8 This study was small and did not adjust for hypertension risk factors. The concentrations of total urine arsenic, although not high compared with levels in populations exposed to arsenic in drinking water in Taiwan and Bangladesh, were higher than in NHANES participants.
No previous epidemiologic study has evaluated the relationship of urine arsenic with blood pressure endpoints, even though total urine arsenic and DMA are well-established biomarkers of arsenic exposure.3,32,33 We used total urine arsenic, total arsenic minus arsenobetaine, and DMA in models with and without adjustment for urine arsenobetaine, all with similar findings. Adjustment for arsenobetaine, a biomarker of seafood intake, was important to remove the contribution of organic arsenicals in seafood, including arsenosugars and arsenolipids (forms that are difficult to measure in epidemiologic studies but that co-occur with arsenobetaine in seafood), to total arsenic, total arsenic minus arsenobetaine, and DMA.25,34 To further confirm that the lack of association between total arsenic, total arsenic minus arsenobetaine, and DMA with hypertension was not related to organic arsenicals, we conducted sensitivity analyses in participants with undetectable arsenobetaine. In this subsample, total urine arsenic and urine DMA are likely to reflect exposure to inorganic arsenic from water and food other than seafood.25 The results were similar and consistent with no association between exposure to inorganic arsenic and hypertension endpoints at low to moderate levels of exposure.
Strengths and limitations
This study, characterized by rigorous quality control measures, was conducted in a representative sample of the U.S. population. To date, this is the largest study of arsenic exposure and hypertension endpoints at low to moderate levels, and among the few using urine arsenic biomarkers. The cross-sectional design does not allow for the establishment of temporality between arsenic exposure and changes in blood pressure levels. In addition, we were limited by the use of one single spot urine sample to measure arsenic. Inorganic arsenic has a relatively short half-life (approximately 2–38 days for the different inorganic arsenic species and its methylated metabolites) and arsenic concentrations measured in urine samples may not reflect chronic arsenic exposure. In the absence of interventions, however, arsenic concentrations in drinking water (a major source for the general population) are relatively stable over time.35–37 Urine arsenic concentrations in the U.S. have been shown to remain relatively constant over many years.38 In Taiwan, a single urine arsenic measure was also well correlated with cumulative arsenic exposure in drinking water.29
We could not evaluate the role of geographic region in arsenic exposure levels. Certain regions of the US, particularly rural areas in the Western, Midwestern and Northeastern states, experience substantial arsenic exposure due to elevated concentrations of inorganic arsenic in groundwater. While the study sample is representative of the U.S. population, the findings cannot be applied to US populations living in areas with high arsenic in drinking water, as these areas were likely underrepresented in NHANES. Finally, several studies have suggested that arsenic methylation patterns (including a higher proportion of methylarsonate in urine) and genetic susceptibility to arsenic health effects might be important to consider in the evaluation of arsenic related health effects.28,29 Because most participants had undetectable urine concentrations for arsenite, arsenate and methylarsonate, we were unable to evaluate the role of arsenic methylation patterns.
Conclusions
At the low to moderate levels typical of the U.S. population, total arsenic and total arsenic minus arsenobetaine were not associated with hypertension or with systolic and diastolic blood pressure. For urine DMA, we cannot rule out the possibility of a small association with hypertension, an association that could be stronger among certain subgroups. There is some epidemiologic and experimental evidence supporting an association between high-chronic arsenic exposure and hypertension. Experimental mechanisms include arsenic promotion of inflammatory activity, oxidative stress and endothelial dysfunction.11,39,40,41–43 There also is prior evidence that low to moderate exposure to inorganic arsenic could be related to increased cardiovascular disease mortality and to the prevalence of diabetes.19,44,45,34,46 Additional studies, especially prospective studies in populations with a wide range of arsenic exposure levels, are needed to evaluate the dose-response relationship between inorganic arsenic exposure and hypertension risk and to confirm the lack of an association at low levels.
Supplementary Material
Acknowledgments
Financial Support: Supported by grants R01HL090863 from the National Heart, Lung and Blood Institute (NHLBI) and R01ES015597 from the National Institute of Environmental Health Sciences
References
- 1.Agency for Toxic Substances and Disease Registry. Arsenic. Agency for Toxic Substances and Disease Registry; 2009. Feb 1, [Accessed October 20, 2009]. Available at: URL: http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=3. [Google Scholar]
- 2.Lauwerys RR, Arsenic Hoet P. Industrial Chemical Exposure: Guidelines for Biological Monitoring. 3. Boca Raton: Lewis Publishers; 2001. pp. 36–7. [Google Scholar]
- 3.National Research Council (NRC) Arsenic in drinking water- 2001 update. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 4.Ahsan H, Perrin M, Rahman A, et al. Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med. 2000 Dec;42(12):1195–201. doi: 10.1097/00043764-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect. 1999 Aug;107(8):663–7. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellizzari ED, Clayton CA. Assessing the measurement precision of various arsenic forms and arsenic exposure in the National Human Exposure Assessment Survey (NHEXAS) Environ Health Perspect. 2006 Feb;114(2):220–7. doi: 10.1289/ehp.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwok RK, Mendola P, Liu ZY, et al. Drinking water arsenic exposure and blood pressure in healthy women of reproductive age in Inner Mongolia, China. Toxicol Appl Pharmacol. 2007 Aug 1;222(3):337–43. doi: 10.1016/j.taap.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Jensen GE, Hansen ML. Occupational arsenic exposure and glycosylated haemoglobin. Analyst. 1998;123(1):77–80. doi: 10.1039/a705699k. [DOI] [PubMed] [Google Scholar]
- 9.Chen CJ, Hsueh YM, Lai MS, et al. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension. 1995 Jan;25(1):53–60. [PubMed] [Google Scholar]
- 10.Rahman M, Tondel M, Ahmad SA, Chowdhury IA, Faruquee MH, Axelson O. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999 Jan;33(1):74–8. doi: 10.1161/01.hyp.33.1.74. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Factor-Litvak P, Howe GR, et al. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007 Mar 1;165(5):541–52. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- 12.Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health. 2004 Nov;94(11):1936–7. doi: 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD. Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003–2004. J Expo Sci Environ Epidemiol. 2009 Jan;19(1):59–68. doi: 10.1038/jes.2008.32. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Environmental Health. NHANES 2003–2004: Laboratory Component: Total Arsenic and Speciated Arsenics. Centers for Disease Control and Prevention Website; 2007. Nov, [Accessed October 16, 2009]. Available at: URL: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06uas_c.pdf. [Google Scholar]
- 15.National Center for Environmental Health. NHANES 2005–2006 Laboratory Component: Total Arsenic and Speciated Arsenics. Centers for Disease Control and Prevention Website; 2009. Jan, [Accessed October 16 2009]. Available at: URL: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/uas_d.pdf. [Google Scholar]
- 16.National Center for Environmental Health. NHANES 2007–2008: Laboratory Component: Urinary Total Arsenic and Speciated Arsenics. Centers for Disease Control and Prevention Website; 2009. Sep, [Accessed October 16, 2009]. Available at: URL: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/UAS_E.htm. [Google Scholar]
- 17.National Center for Environmental Health. Laboratory Procedure Manual: Total Arsenic. Centers for Disease Control and Prevention Website; 2006. Feb 16, [Accessed October 16, 2009]. Available at: URL: http://cdc.gov/NCHS/data/nhanes/nhanes_05_06/uas_d_met.pdf. [Google Scholar]
- 18.National Center for Environmental Health. Laboratory Procedure Manual: Arsenobetaine, Arsenocholine Trimethylarsine Oxide, Monomethylarsonic Acid, Dimethylarsininc Acid, Arsenous (III) Acid, Arsenic (V) Acid: Urine Arsenic Speciation. Centers for Disease Control and Prevention Website; 2004. Sep 14, [Accessed October 16, 2009]. Available at: URL: http://www.cdc.gov/NCHS/data/nhanes/nhanes_03_04/l06uas_c_met_arsenic_speciated.pdf. [Google Scholar]
- 19.National Center for Health Statistics. NHANES 2003–2004: Physicians Examination-Blood Pressure. Centers for Disease Control and Prevention Website; 2003. Jan, [Accessed October 16, 2009]. Available at: URL: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/PE.pdf. [Google Scholar]
- 20.National Center for Health Statistics. NHANES 2005–2006: Physicians Examination-Blood Pressure. Centers for Disease Control and Prevention Website; 2004. Jan, [Accessed October 16, 2009]. Available at: URL: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/PE.pdf. [Google Scholar]
- 21.National Center for Health Statistics. NHANES 2007–2008: Physicians Examination-Blood Pressure. Centers for Disease Control and Prevention Website; 2009. Jul, [Accessed October 16, 2009]. Available at: URL: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/BPX_E.htm. [Google Scholar]
- 22.Lumley T. Analysis of complex survey samples. Journal of Statistical Software. 2004;9(1):1–19. [Google Scholar]
- 23.Lumley T. R package version 3.21. University of Washington; Seattle: 2010. Survey: analysis of complex survey samples. [Google Scholar]
- 24.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. Available at: URL: http://www.R-project.org. [Google Scholar]
- 25.Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environmental Research. doi: 10.1016/j.envres.2010.10.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.StataCorp. Stata Statistical Software: Release 11.0. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 27.Guo JX, Hu L, Yand PZ, Tanabe K, Miyatalre M, Chen Y. Chronic arsenic poisoning in drinking water in Inner Mongolia and its associated health effects. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007 Oct;42(12):1853–8. doi: 10.1080/10934520701566918. [DOI] [PubMed] [Google Scholar]
- 28.Hsueh YM, Lin P, Chen HW, et al. Genetic polymorphisms of oxidative and antioxidant enzymes and arsenic-related hypertension. J Toxicol Environ Health A. 2005 Sep;68(17–18):1471–84. doi: 10.1080/15287390590967414. [DOI] [PubMed] [Google Scholar]
- 29.Huang YK, Tseng CH, Huang YL, Yang MH, Chen CJ, Hsueh YM. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol. 2007 Jan 15;218(2):135–42. doi: 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: A cohort mortality study. Environmental Health Perspectives. 1999;107(5):359–65. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SL, Chang FH, Liou SH, Wang HJ, Li WF, Hsieh DP. Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ Int. 2007 Aug;33(6):805–11. doi: 10.1016/j.envint.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council. Arsenic in drinking water. Washington, DC: National Academy Press; 1999. [Google Scholar]
- 33.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006 Nov;114(11):1790–6. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Rejoinder: Arsenic exposure and prevalence of type 2 diabetes: updated findings from the National Health Nutrition and Examination Survey, 2003–2006. Epidemiology. 2009 Nov;20(6):816–20. doi: 10.1097/EDE.0b013e3181afef88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karagas MR, Le CX, Morris S, et al. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int J Occup Med Environ Health. 2001;14(2):171–5. [PubMed] [Google Scholar]
- 36.Ryan PB, Huet N, MacIntosh DL. Longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environ Health Perspect. 2000 Aug;108(8):731–5. doi: 10.1289/ehp.00108731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinmaus CM, Yuan Y, Smith AH. The temporal stability of arsenic concentrations in well water in western Nevada. Environ Res. 2005 Oct;99(2):164–8. doi: 10.1016/j.envres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Navas-Acien A, Umans JG, Howard BV, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect. 2009 Sep;117(9):1428–33. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmignani M, Boscolo P, Castellino N. Metabolic Fate And Cardiovascular Effects Of Arsenic In Rats And Rabbits Chronically Exposed To Trivalent And Pentavalent Arsenic. Archives of Toxicology. 1985;(Supplement 8):452–455. doi: 10.1007/978-3-642-69928-3_103. 6 references, %19851985. [DOI] [PubMed] [Google Scholar]
- 40.Pi J, Kumagai Y, Sun G, et al. Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radic Biol Med. 2000 Apr 1;28(7):1137–42. doi: 10.1016/s0891-5849(00)00209-4. [DOI] [PubMed] [Google Scholar]
- 41.Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996;21(6):783–90. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 42.Barchowsky A, Roussel RR, Klei LR, et al. Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol Appl Pharmacol. 1999 Aug 15;159(1):65–75. doi: 10.1006/taap.1999.8723. [DOI] [PubMed] [Google Scholar]
- 43.Lee MY, Jung BI, Chung SM, et al. Arsenic-induced dysfunction in relaxation of blood vessels. Environ Health Perspect. 2003 Apr;111(4):513–7. doi: 10.1289/ehp.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medrano MA, Boix R, Pastor-Barriuso R, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res. 2009 Oct 30; doi: 10.1016/j.envres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Sohel N, Persson LA, Rahman M, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009 Nov;20(6):824–30. doi: 10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- 46.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008 Aug 20;300(7):814–22. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.