Abstract
In connective tissue diseases panniculitis can be the sole manifestation or occur along with the underlying disease process. The best described forms of connective tissue panniculitis are lupus erythematosus panniculitis (LEP) and lupus profundus, panniculitis associated with dermatomyositis, and morphea and scleroderma associated panniculitis. These processes cause significant morbidity, such as deep atrophic scars, cosmetic disfigurement and psychiatric sequelae. Due to the location of the inflammation in the subcutaneous adipose layer, topical therapies may not penetrate enough to be effective, and systemic agents are required. Despite the large number of reported cases and therapies, recommendations for treatment are based largely on case series and expert opinion due to a lack of controlled therapeutic trials. All treatments are off-label in the United States. The lack of validated clinical outcome measures makes systematic and controlled studies difficult. Nonetheless further investigation into the most effective therapies for these conditions are needed.
Keywords: lupus erythematosus panniculitis, lupus profundus, dermatomyositis, morphea, morphea profunda
INTRODUCTION
Panniculitis refers to inflammation of the subcutaneous tissue and can be seen in many disease processes including trauma, infection, neoplasm, vascular and enzymatic insufficiency, and connective tissue diseases. In connective tissue diseases panniculitis can be the sole manifestation of the disease or occur along with other findings of the underlying disease process.
The best described forms of panniculitis occurring in the setting of connective tissue disease are lupus erythematosus panniculitis (LEP) and lupus profundus, panniculitis associated with dermatomyositis, and morphea and scleroderma associated panniculitis. There are also reports of so-called “connective tissue panniculitides” that are associated with autoimmune phenomena, but are not attributable to a well defined connective tissue disease1,2.
The panniculitides associated with connective tissue disease cause significant morbidity. Deep and painful subcutaneous nodules characterize the early inflammatory phase. Once the inflammatory phase has resolved, patients are left with deep atrophic scars, cosmetic disfigurement and psychiatric sequelae3. Limb length discrepancy and joint contractures can also occur impairing mobility and daily function in morphea profunda4.
Clinical findings help correctly diagnose the connective tissue panniculitides, but histopathologic features are also essential since patients with the underlying connective tissue diseases may be at increased risk for other forms of panniculitis, such as infection and lymphoproliferative processes5. Connective tissue panniculitides share a histologic appearance of a predominantly lobular lymphocytic infiltrate in the adipose tissue, although sometimes the infiltrate has a mixed (lobular and septal) pattern. Changes in the dermis overlying the subcutaneous adipose tissue, such as vacuolar change at the dermal-epidermal junction, mucin deposition, and sclerosis, can help distinguish the clinical entities. Accurate and timely diagnosis is essential because treatment should be aimed at the early inflammatory phase, as the resultant atrophy is permanent and difficult to treat. Due to the location of the inflammation in the subcutaneous adipose layer, topical therapies may not penetrate enough to be effective, and systemic agents are required.
While there are many similarities in the clinical and histologic appearance of the connective tissue panniculitides, there are interesting differences in their presentation and response to therapies. Hydroxychloroquine, for example, is normally effective for lupus panniculitis relative to the mixed response seen in dermatomyositis. Better understanding of these differences in response might broaden the understanding of each connective tissue disease and advance the development of more targeted and efficacious therapies.
Despite the large number of reported cases and therapies, recommendations for treatment are based largely on case series and expert opinion due to a lack of controlled therapeutic trials. All treatments for panniculitides of connective tissues disease are off-label in the United States. Treatment for the sequelae of panniculitis, including surgical techniques and injection of fillers, will be briefly addressed, but remain controversial in the active inflammatory phase, as trauma itself may be an inciting factor. These techniques may be considered for stable, noninflammatory atrophic plaques, however, without a clear understanding of what drives the panniculitic process, there is always a theoretical risk of disease reactivation.
Connective tissue panniculitides are uncommon conditions, some with a waxing and waning natural history. The lack of validated clinical outcome measures makes systematic and controlled studies difficult. Nonetheless further investigation into the most effective therapies for these conditions are needed.
LUPUS PANNICULITIS/LUPUS PROFUNDUS
Clinical features
Lupus erythematosus panniculitis (LEP) was first described by Kaposi in 1883 as involvement of the subcutaneous fat in lupus erythematosus6. When the features of lupus panniculitis are seen with overlying changes of discoid lupus, such as erythema, scaling and follicular plugging, the term lupus profundus is preferred7. LEP and lupus profundus are classified as forms of chronic cutaneous lupus erythematosus and share a similar treatment algorithm to discoid lupus erythematosus (DLE)8.
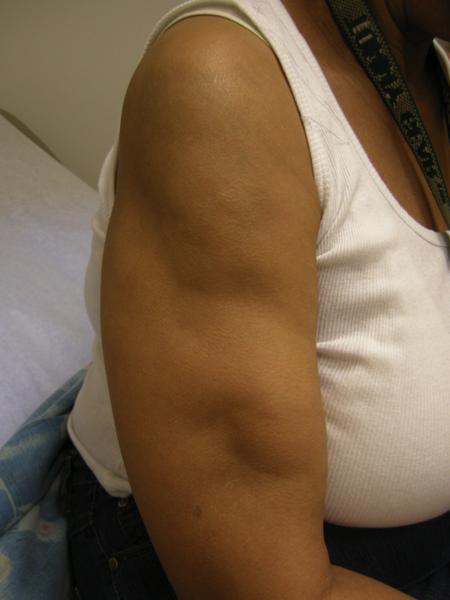
LEP and lupus profundus present as tender and deep subcutaneous nodules or plaques that may appear in crops sometimes with overlying hyperpigmentation. The nodules typically appear on proximal extremities including lateral upper arms, shoulders, buttocks, trunk, breast, face and scalp (Figure 1). Involvement of the legs is unusual and can be a helpful distinguishing feature from other forms of pannciulitis6. Unusual variants involving the breast (“lupus mastitis”), parotid gland, and periocular tissue have been described6,9. In children there is a predilection for facial involvement6,10. LEP and lupus profundus typically affect young women in their late 30's and early 40's. Like other forms of CLE, there is a female predominance with a female to male ratio of approximately 2:111–15. LEP has a waxing and waning course and lesions tend to resolve with permanent atrophic scarring and significant disfigurement. Untreated lesions of LEP and lupus profundus can ulcerate.
Figure 1.
Atrophic plaques on the upper extremities in a patient with lupus erythematosus panniculitis.
Like other forms of cutaneous lupus erythematosus (CLE) there is clinical overlap with other CLE subtypes and with systemic lupus erythematosus (SLE). Reports in the literature suggest that approximately 70% of patients with LEP will have a prior, concomitant, or subsequent history of DLE11. Additionally, LEP is reported to occur in 2–3% of patients with SLE12,16. The literature suggests up to 35% of LEP patients will have a preceding, concurrent or subsequent diagnosis of SLE, thus patients with LEP should be followed for development of SLE, although most patients will not have systemic manifestation11,17. When patients with LEP do have SLE, they tend to have a less severe phenotype13,14,18, although reports of aggressive generalized LEP lesions in the setting of SLE have been reported and argue for prompt initiation of systemic treatment3.
Diagnosis
The histologic features of LEP have been organized into proposed criteria by Peters and Su15. Major criteria include hyaline fat necrosis, lymphocytic aggregates and lymphoid follicle formation, periseptal or lobular lymphocytic panniculitis, and calcification. Minor criteria include changes of DLE in the overlying skin, lymphocytic vascular inflammation, hyalinization of the subepidermal zone, mucin deposition, histiocytes and small granulomas, and infiltrates of plasma cells and eosinophils. Direct immunoflourescence may show granular staining of IgG, IgM and C3 at the dermal-epidermal junctional about 50% of the time, particularly in patients with concomitant DLE, and sometimes there is deposition of immune complexes in small deep vessels. These findings may aid in diagnosis1,12,15. Although these criteria have not been universally adopted, most contributors to the literature agree that LEP has a distinctive histologic appearance. One exception is the difficulty is distinguishing LEP from subcutaneous panniculitic-like T-cell lymphoma (SPTCL) and indeterminate lymphocytic lobular panniculitis, a recently described form of T-cell dyscrasia5,19. Features that may help distinguish LEP from these lymphoproliferative processes include vacuolar change at dermal-epidermal junction, periadnexal inflammation and mucin deposition10. Biopsies should ideally be reviewed by a dermatopathologist due to the expertise required to distinguish these entities. The role of lab testing is not well established in the diagnosis of lupus panniculitis, although patients with LEP may have a positive anti-nuclear antibody (ANA), low complement levels, and leucopenia5,6,20. SPTCL can have similar laboratory abnormalities and present with fever, mimicking SLE. In SPTCL T-cell receptor gene rearrangement studies may be helpful. If positive they support the diagnosis, however cases of lupus panniculitis with T-cell clonality have been reported5,21. Given the clinical and histologic overlap, repeat biopsies and close clinical follow up are warranted for lesions that do not respond to standard therapies5,19.
Pathophysiology
The cause of lupus panniculitis is poorly understood. Some have shown reduced levels of C4 suggesting an underlying genetic component20,22,23. Tuffanelli has described lupus profundus in two sisters and in a patient with four first degree family members with lupus erythematosus12. Trauma is also a suggested trigger and ulceration of a lesion at a biopsy site is not infrequently reported12. Response to cyclosporine and histologic findings of predominantly CD4+ T cells suggests a T-cell driven process24,25. Nonetheless, since the earliest English language reports in the early to mid 20th century, the mainstay therapy for LEP has been the antimalarials, whose mechanism of action remains ill-defined.
Treatment
Treatment strategies for LEP and lupus profundus are difficult to study given the low prevalence of the disease and its relapsing and remitting natural history. In addition, the lack of an appropriate outcome measures complicates study. The Cutaneous Lupus Areas and Severity Index (CLASI) has been validated for use in the most common forms of CLE, but is not appropriate for assessment of lupus panniculitis26. Reliable clinical assessment of panniculitis activity is very difficult, making development of disease severity tools a challenge for this lupus subset. Antimalarials have a long history of use with successful clearing of lesions of LEP and lupus profundus in adults and in children. They are considered first line therapy for most cases of LEP1,6,10,12,13,15–17,27. Hydroxychloroquine is typically given at a dose of < 6.5 mg/kg/day based on ideal body weight. Antimalarials are slow acting and up to three months of treatment are required to see results. Up to six months is needed in some cases, although some authors report efficacy within 3–4 weeks10,28. The mechanism of action of antimalarials is incompletely understood, but effects of antigen presentation, inhibition of prostaglandin and cytokines, photoprotection, inhibition of Toll-Like Receptor signaling and lysosomal stabilization are thought to contribute to their efficacy29.
The use of chloroquine as monotherapy at doses of 250–500 mg a day has been reported23,30,12,16. Chloroquine is also dosed by ideal body weight and dosages should not exceed 3.5 mg/kg/day. Hydroxychloroquine monotherapy is preferred over chloroquine given its improved safety profile, in particular with regards to retinal toxicity. If hydroxychloroquine or chloroquine alone does not lead to a response, the addition of quinacrine 100 mg daily can be beneficial31. Quinacrine is obtained through compounding pharmacies in the United States. It can cause a yellow discoloration of the skin, but does not increase the risk of ophthalmologic toxicity. Expert opinion suggests quinacrine monotherapy is not as effective as either hydroxychloroquine or chloroquine28. Use of hydroxychloroquine or chloroquine requires regular ophthalmologic screening, although recent literature suggests the risk of retinal toxicity does not appear until 5 years into treatment and suggest that after a baseline exam, regular screening should not begin until five years into treatment32. Other common side effects of antimalarials include gastrointestinal upset and cutaneous changes including lichenoid drug eruptions, pruritis and dyspigmentation. Extremely rare side effects include hematologic toxicity, psychosis, myopathy, and cardiomyopathy. Laboratory monitoring is not required.
There have been reports of success using topical steroids, including clobetasol proprionate 0.05% ointment under occlusion, however the literature is limited on the use of topical steroids or topical calcineurin inhibitors alone. These agents are mentioned frequently as failed therapies before the use of antimalarials or other systemic agents10,33. Improvement with oral steroids has been repeatedly reported15,31. Consensus opinion is that oral steroids should be avoided, or reserved for the most severe cases associated with SLE, due to the morbidity of long-term use, including exacerbation of lesion atrophy6,12.
Although it is not clear if ultraviolet light plays a role in the development of LEP lesions, sun protection is a recommended therapeutic approach in all forms of CLE6,15,30. The clinical distribution of lesions on proximal extremities and the deep location of the inflammatory infiltrate argue against an ultraviolet trigger.
When antimalarials are not tolerated, inaccessible, or fail to produce remission, success with immunomodulators like thalidomide and dapsone have been reported. Thalidomide has been efficacious in other recalcitrant forms of CLE, although it has little effect on the systemic manifestations of lupus erythematosus. Burrows et al and Weinert et al report the successful treatment of LEP with thalidomide in patients who previously failed topical steroids, antimalarials, anti-tuberculosis treatment, and oral or intralesional steroids20,34. Thalidomide is often used at doses of 50–300 mg/day, although doses above 150 mg are typically not needed28. The use of thalidomide is limited by the common side effects, which are dose related and include drowsiness, constipation, headache, weight gain, and amenorrhea. More concerning are the serious side effects of thrombosis, peripheral neuropathy and teratogenicity. Patients should be monitored for neuropathy with nerve conduction studies at baseline and every six months. The continuation of antimalarials for their antiplatelet effect or the use of aspirin may help mitigate the thrombotic effects of thalidomide, although guidelines for thrombotic prophylaxis do not exist. Enrollment in the System for Thalidomide Education and Prescribing Safety (STEPS) program, a manufacturer based restricted drug dispensing program, is required. The use of the lowest dose possible, including every other day dosing or 50 mg daily, as a maintenance regimen is recommended.
Dapsone has been used successfully at dose of 25 to 75 mg daily. In a review of 10 cases from Japan all lesions of LEP regressed within 8 weeks of dapsone treatment. Three patients experienced mild side effects including drug eruption, headache, hypertension and anemia35. Grossberg et al also report use of dapsone at 150 mg/daily in combination with antimalarials and oral prednisone in a 23-year-old with features of SLE and lupus profundus3. Dapsone may cause more severe dose-dependent adverse effects including hemolytic anemia and methemoglobinemia. Patients should be screened for glucose-6-phosphate reductase deficiency before initiation. Idiosyncratic agranulocytosis or hypersensitivity reactions can also occur, making appropriate monitoring with regular complete blood counts and liver function tests necessary. The immunomodulatory mechanism of action for these medications remains to be elucidated.
Systemic immunosuppressive agents have also been used in cases of LEP resistant to antimalarials and immunomodulators. The patients reported in these case studies tend to have SLE and often, although not always, experience resolution of systemic symptoms along with cutaneous symptoms. Azathioprine has been reported as an adjuvant to prednisone and hydroxychloroquine in patients with concomitant SLE16,22. Methotrexate was used in three patients with LEP and SLE with control of symptoms3. Cyclosphosphamide also has also been reported effective in patients with underlying SLE7,3. Cyclosporin A was used in patients with SLE and LEP and resulted in rapid remission, within 10 days25. Saeki et al report a young female with recurrent LEP who maintained remission with cyclosporin A despite prior failures with low dose systemic corticosteroids, dapsone, azathioprine and cyclophosphamide24.
Other treatments for LEP in the literatures are limited to a few case reports. Intravenous immunoglobulin (IVIG) has been reported in a patient with SLE who could not tolerate hydroxychloroquine due to hepatic and ocular side effects and failed to improve with thalidomide 300 mg daily and azathioprine. She responded after six monthly IVIG infusions with a sustained response with repeat infusions every three months7. Photopheresis was also reported in one patient with SLE and LEP36. Finally, rituximab was used successfully in one patient with LEP with improvement in cutaneous and systemic symptoms after two infusions, allowing discontinuation of cyclosphosphamide and hydroxychloroquine. The biopsy in this case report showed a neutrophil-rich septal and lobular panniculitis, which raises concern about the actual diagnosis37.
Calcification is not uncommon in lesions of lupus panniculitis and is frequently seen in later stage lesions and is accompanied by significant pain6,38. Diltiazem, a calcium-channel blocker, has been proposed as a treatment for the calcified variants of panniculitis although the mechanism by which diltiazem causes regression of calcinosis is unclear30.
Different approaches for treatment of the permanent atrophy and disfigurement from LEP include use of lasers, tissue augmentation, autologous fat transfer and surgical excision, however there is a lack of published reports of these for use in LEP specifically. Given the risk of koebnerization and ulceration the benefit of these surgical approaches is unproven. A conservative approach, including the use of a small test area, would be prudent28. Importantly, patients should have their disease controlled prior to undertaking such treatments.
DERMATOMYOSITIS
Clinical features
Panniculitis is an usual finding in dermatomyositis and, although it was first described in 1924, there few case reports describing this entity in the subsequent century39,40. Despite the paucity of clinical reports, histopathologic studies suggest that up to 10% of dermatomyositis biopsies show focal panniculitis, suggesting subclinical panniculitis may be an under-recognized feature41. Review of the limited literature suggests that the panniculitis can occur before, concurrently or subsequent to the diagnosis of dermatomyositis, ranging from 14 months before to 5 years after initial diagnosis. The lesions of panniculitis associated with dermatomyositis typically present on the buttocks, thighs, arms and abdomen and the majority (75%) of reported cases occur in females40. Childhood cases have been reported42,43. Only one malignancy was reported in association with dermatomyositis, a rhabdomyosarcoma in a 51 year-old, suggesting that panniculitis may characterize a clinical subset of dermatomyositis with less risk of malignancy40,44,45. The panniculitis of dermatomyositis does not appear to resolve spontaneously, unlike what is seen in LEP, but it has been noted to respond to treament directed at dermatomyositis28.
Diagnosis
To diagnosis the panniculitis of dermatomyositis, clinicopathologic correlation, including incorporation of laboratory findings, is essential28. Like LEP, the panniculitis of dermatomyositis is characterized by a lobular lymphocytic infiltrate. There are reports of overlying dermal-epidermal vacuolar change and increased mucin deposition42,45. Distinguishing this entity from LEP relies on clinical information. Importantly, infection must be ruled out. Cases of misdiagnosis, where an infection was labeled as panniculitis of dermatomyositis, have been reported46,47. For this reason an adequate biopsy, including subcutaneous fat, should be performed in all patients with dermatomyositis who develop skin nodules28. Membranocystic change, findings of eosinophilic arabesque structures in areas of adipocyte necrosis and dropout, may be seen in the panniculitis of dermatomyositis, but has also been described in LEP and ischemic processes48. Magnetic resonance imaging was reported as a useful diagnostic tool in one patient49.
Pathophysiology
Like the other connective tissue panniculitides, the cause is unknown. However, descriptions of a parallel flare and remission of panniculitis and myositis point to a single underlying process40. There are no reports of panniculitis in patients with amyopathic dermatomyositis to these author's knowledge.
Treatment
Treatment strategies for the panniculitis of dermatomyositis are based on limited number of uncontrolled case reports and case series. Like in lupus, the validated disease specific outcome measures (Cutaneous Dermatomyositis Disease Area and Severity Index or CDASI) do not measure induration because of the difficulty of the assessment by physical exam. Lack of standardization of clinical assessment makes studies difficult. The primary treatments used for the panniculitis of dermatomyositis are systemic corticosteroids and immunosuppressives. Many cases are responsive to an increase in oral or intravenous corticosteroid dose40,42,48. For dermatomyositis, prednisone is typically initiated at 0.5–1.0 mg/kg/day and then tapered slowly based on clinical response. If panniculitis lesions appear in the setting of a prednisone taper, an increase in dose of corticosteroids may be beneficial39,40,48. Intravenous methylprednisilone is sometimes used in pulse dosing for the three consecutive days at 1 gram/daily for severe cases and has also been reported efficacious in oral formulation for the treatment of panniculitis in dermatomyositis45.
The long-term effects of oral corticosteroids should be reviewed with the patient and the proper prophylaxis and monitoring should occur. Importantly, prophylaxis with bisphosphonates and calcium supplementation is recommended for courses of steroids equivalent to > 5 mg/day of prednisone anticipated to last greater than 3 months50. New approaches to prevent glucocorticoid-induced osteoporosis, such as recombinant human parathyroid hormone, may also be indicated51. Blood pressure and serum glucose should also be regularly monitored. Due to the side effects of long-term steroid use, the use of steroid-sparing agents should be considered as soon as the flare in disease activity has been controlled.
When corticosteroids are ineffective in treating the panniculitis of dermatomyositis, the addition of immunosuppressives, such as methotrexate and cyclosporin A has been reported successful43,44,52. Corticosteroids, methotrexate, azathioprine and mycophenolate mofetil are considered first-line therapies for adult dermatomyositis, but there is a lack of prospective double-blind, placebo-controlled trials53. To the authors knowledge there are no reports of panniculitis due to dermatomyositis responding to either azathioprine or mycophenolate mofetil. High dose IVIG has been shown to be effective for the muscle and skin symptoms of dermatomyositis in a double-blind, placebo-controlled study and is considered a second-line agent54. IVIG has been reported once, to our knowledge, for the treatment of the panniculitis associated with dermatomyositis. Monthly treatments at 2 mg/kg resulted in improvement in skin lesions that responded incompletely to antimalarials and cyclosporin A39.
While the literature is composed of uncontrolled case series and reports, there are interesting observations to consider. For example, a case of panniculitis developing in the setting of an increase in hydroxychloroquine dose suggests an distinctive feature from lupus associated panniculitides42. On the other hand, some reported cases describe positive antinuclear antibodies40,42, cytopenias45, increased mucin deposition42,45 and positive direct immunofluorescence in vessels44 pointing towards overlap with LEP. Yoo et al exemplify the overlap, and the possibility for confusion, with their case of panniculitis occurring in the setting of a markedly elevated creatine kinase and an elevated double-stranded DNA titer, which they characterized as a combination of LEP and panniculitis of dermatomyositis55.
PANNICULITIS IN MORPHEA AND SYSTEMIC SCLEROSIS
Clinical Features
Panniculitis can also occur in morphea (formerly localized scleroderma) and systemic sclerosis (scleroderma), accounting for another clinical overlap between these fibrosing processes that have been historically and nosologically grouped. It is important to discriminate the two entities because of their distinct prognosis and clinical implications. We agree that the term localized scleroderma may be misleading and prefer the use of morphea28.
Morphea is a form of sclerosis limited to the skin, although extracutaneous symptoms of arthritis, fatigue and malaise can be seen56. In general the prognosis of morphea is favorable, with most lesions softening spontaneously within 3–5 years, however the disfigurement and functional consequences of morphea contribute to its significant morbidity4.
Morphea profunda, or deep morphea, is characterized by primary involvement of the subcutaneous fat. Patients present with hyperpigmented, ill-defined, and mildly inflamed sclerotic plaques. A female predominance is noted57. Different classification schemes for morphea have been proposed for the presentations of morphea that, while distinct, share overlapping features like panniculitis58,59. Deep morphea is part of the classification system proposed by Peterson et al, and includes variants that extend to involve the panniculus, fascia and underlying muscle. These variants are morphea profunda, eosinophilic fasciitis, and disabling linear morphea of childhood. Notably, other variants of morphea, including linear morphea and generalized morphea, can involve the panniculus as well, blurring the boundaries between these classifications57. Newer morphea classification schemes do not include a deep morphea category, but do include deep variants of circumscribed morphea and also include linear and generalized morphea variants59.
Systemic sclerosis can occur in a limited or diffuse variant and is characterized by sclerosis of both cutaneous and internal connective tissues with accompanying systemic comorbidities. Panniculitis can complicate these conditions, which are sometimes aggressive and fatal60.
Diagnosis
The panniculitis seen in morphea and systemic sclerosis is characterized by an early lymphocytic lobular panniculitis, and in later stages septal sclerosis and overlying dermal sclerosis occur61. It is not possible to distinguish morphea from systemic sclerosis on histopathologic analysis. Clinical features, including the presence of Raynaud's phenomenon, nail fold capillary changes and autoantibody profiles are important in making the diagnosis of systemic sclerosis56.
Pathophysiology
The pathophysiology of these fibrosing disorders is unknown, but is thought to be a consequence of imbalanced collagen production and destruction. A possible mechanism involves changes in the vascular endothelium with resultant occlusion, injury and fibroblast activation and underlies the rationale for targeted therapies directed towards profibrotic cytokines and vascular mediators62.
Treatment
Unlike LEP and the panniculitis associated with dermatomyositis, the panniculitis of morphea and systemic sclerosis have not been reported to occur in isolation of the underlying disease process. Thus, literature focused on treatment of the panniculitic aspect does not exist, however there are reports of cases highlighting response of the panniculitic component, which are discussed below.
Fett and Zwischenberger recently reviewed morphea treatments4,63. Again, the lack of a validated outcome measure makes study difficult. The Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) is a promising outcome tool, but further validation studies in particular with the deep variants of morphea are required63. Nonetheless, available evidence supports the use of phototherapy with UVA1 or narrow band UVB (NBUVB) alone or in combination with methotrexate and systemic steroids for generalized morphea. Mycophenolate mofetil may be considered for nonresponsive cases4,63,64. For morphea involving the face or crossing a joint, methotrexate and systemic steroids in combination with phototherapy are recommended4,63. The treatment of limited plaque morphea falls outside the realm of this review on connective tissues disease panniculitides, but topical approaches with calcineurin inhibitors, calcipotriol, imiquimod and phototherapy are suggested and are likely efficacious due to the more superficial nature of the inflammatory infiltrate63. With specific regard to panniculitis, Martini et al show efficacy of mycophenolate mofetil in methotrexate-resistant juvenile deep morphea, generalized morphea, and linear morphea64. Case reports of benefit with cyclosporine A, methotrexate, and extracorporeal photopheresis in deep variants of morphea are noted65–67. A report of abatacept for treatment of morphea profunda was recently published and showed promising results in two patients. Abatacept is a recombinant fusion protein that competitively binds T-cell costimulatory receptors CD80 and CD86, inhibiting T-cell activation68. Interestingly, ceftriaxone was published as a successful treatment for subcutaneous morphea in an Austrian patient who notably had negative Borrelia burgdorferi serology69. Bosentan, an orally active endothelin receptor antagonist used for pulmonary hypertension, was effective against cutaneous ulceration and sclerosis in a child with disabling pansclerotic morphea70.
Therapies directed towards the secondary atrophy in morphea have been described, including the use of surgical excision and implantation of fillers. Recently, hyaluronic acid was used successfully for a nonactive atrophic plaque in a patient with a history of en coup de sabre. Prior UVA1 treatments had halted the inflammatory phase, but a cosmetically disfiguring defect remained. Response occurred, and was maintained for five months, after the first injection71.
In systemic sclerosis, the search for disease modifying therapies continues. There are a lack of proven disease modifying therapies in systemic sclerosis and this problem is compounded by inadequate outcome measure which at this point may overemphasize cutaneous specific findings62. This main explain why the reported success of agents in systemic sclerosis, for example cyclosporine A, bosentan and extracorporeal phototherapy, has prompted subsequent study of these agents in morphea65–67,70. Treatments for systemic sclerosis depend on the clinical manifestations, and may include immunosuppressives, such as corticosteroids, mycophenolate mofetil, cyclophosphamide, methotrexate, rapamycin, cyclosporin, IVIG and immunomodulatory agents, such as antimalarials and extracorporeal phototherapy, and antifibrotic agents, such as tyrosine kinase inhibitors and anti-transforming growth factor beta (TGF-β) antibodies62. It is difficult to determine the efficacy of an individual agent on the systemic disease and on the panniculitic specific process. Jinnin et al report sclerosing panniculitis in 8% of patients with systemic sclerosis and note an association with pulmonary hypertension. They propose panniculitis may be marker of isolated pulmonary hypertension, highlighting the importance of identifying the panniculitis process in these patients72.
CONNECTIVE TISSUE PANNICULITIS
In 1980, Peters and Winklemann introduced the concept of connective tissue panniculitides as processes that have the histologic appearance of a lymphocytic lobular panniculitis and are associated with autoimmune phenomena, but are not attributable to a specific disease73. There have been improvements in the classification of panniculitides with time, for example, cases that were once called Weber-Christian disease (a term for a relapsing febrile non-suppurative panniculitis with lipophages) have been retrospectively reclassified into specific forms on panniculitis74. However, there continue to be case reports of connective tissue panniculitides that lack specific features of lupus, dermatomyositis or morphea, but are associated with other autoimmune diseases. For example there is a report of a hydroxychloroquine-responsive connective tissue panniculitis in a patient with Hashimoto's disease, insulin dependent diabetes mellitus and juvenile rheumatoid arthritis2, a report of lupus-like panniculitis in association with the autoimmune polyendocrinopathy, candidiasis, and ectodermal dystrophy (APECED) syndrome that improved with oral steroids75, and reports of lipomembranous panniculitis with mixed connective tissue disease76,77. Cases with overlapping features that blur the boundaries between definitive diagnoses are also present, such as the LEP and dermatomyositis overlap described by Yoo et al and an overlap between systemic sclerosis and LEP described by Oka et al55,78.
Other diseases, such as annular lipoatrophy of the ankles and lipoatrophic panniculitis of childhood, are uncommon childhood conditions characterized by striking circumferential atrophy and a lymphocytic lobular panniculitis sometimes accompanied by lipophagocytosis. These may also be variants of connective tissue panniculitides73,79,80. Response to therapies such as prednisone, hydroxychloroquine, dapsone, azathioprine and methotrexate support this possible relatinship81.
CONCLUSIONS
Connective tissue panniculitides are difficult to treat. They are difficult to study as well because they are uncommon, have unclear pathogenesis, we lack useful outcome measures, and the classification of these panniculitides remains a concept in evolution. Nonetheless, these are fascinating processes and further study will help us understand the disease spectrum in lupus, dermatomyositis and fibrosing disorders, and other connective tissue diseases. Despite the lack of controlled studies, there are many agents to be tried in these patients. Consideration of the consequences of the panniculitic processes, including cosmetic disfigurement, functional impairment and psychological sequelae, are imperative and early diagnosis and treatment is warranted.
Acknowledgements
This material is based upon work supported by the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development) and by the National Institutes of Health (NIH K24-AR 02207) to VPW.
Footnotes
No conflict of interest to report.
References
- 1.Winkelmann RK. Panniculitis in connective tissue disease. Arch Dermatol. 1983;119:336–44. [PubMed] [Google Scholar]
- 2.Mirza B, Muir J, Peake J, et al. Connective tissue panniculitis in a child with vitiligo and Hashimoto's thyroiditis. Australas J Dermatol. 2006;47:49–52. doi: 10.1111/j.1440-0960.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 3.Grossberg E, Scherschun L, Fivenson DP. Lupus profundus: not a benign disease. Lupus. 2001;10:514–6. doi: 10.1191/096120301678416105. [DOI] [PubMed] [Google Scholar]
- 4.Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65:925–41. doi: 10.1016/j.jaad.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001;28:235–47. doi: 10.1034/j.1600-0560.2001.028005235.x. [DOI] [PubMed] [Google Scholar]
- 6.Fraga J, Garcia-Diez A. Lupus erythematosus panniculitis. Dermatol Clin. 2008;26:453–63. vi. doi: 10.1016/j.det.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Espirito Santo J, Gomes MF, Gomes MJ, et al. Intravenous immunoglobulin in lupus panniculitis. Clin Rev Allergy Immunol. 2010;38:307–18. doi: 10.1007/s12016-009-8162-x. [DOI] [PubMed] [Google Scholar]
- 8.Tuffanelli DL. Management of cutaneous lupus erythematosus. Clin Dermatol. 1985;3:123–30. doi: 10.1016/0738-081x(85)90085-9. [DOI] [PubMed] [Google Scholar]
- 9.Nowinski T, Bernardino V, Naidoff M, et al. Ocular involvement in lupus erythematosus profundus (panniculitis) Ophthalmology. 1982;89:1149–54. doi: 10.1016/s0161-6420(82)34661-8. [DOI] [PubMed] [Google Scholar]
- 10.Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus Erythematosus Panniculitis in Children: Report of Three Cases and Review of Previously Reported Cases. Pediatr Dermatol. 2011 doi: 10.1111/j.1525-1470.2011.01544.x. [DOI] [PubMed] [Google Scholar]
- 11.Ng PP, Tan SH, Tan T. Lupus erythematosus panniculitis: a clinicopathologic study. Int J Dermatol. 2002;41:488–90. doi: 10.1046/j.1365-4362.2002.01510.x. [DOI] [PubMed] [Google Scholar]
- 12.Tuffanelli DL. Lupus erythematosus panniculitis (profundus) Arch Dermatol. 1971;103:231–42. [PubMed] [Google Scholar]
- 13.Martens PB, Moder KG, Ahmed I. Lupus panniculitis: clinical perspectives from a case series. J Rheumatol. 1999;26:68–72. [PubMed] [Google Scholar]
- 14.Kundig TM, Trueb RM, Krasovec M. Lupus profundus/panniculitis. Dermatology. 1997;195:99–101. doi: 10.1159/000245706. [DOI] [PubMed] [Google Scholar]
- 15.Peters MS, Su WP. Lupus erythematosus panniculitis. Med Clin North Am. 1989;73:1113–26. doi: 10.1016/s0025-7125(16)30622-8. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Jouanen E, DeHoratius RJ, Alarcon-Segovia D, et al. Systemic lupus erythematosus presenting as panniculitis (lupus profundus) Ann Intern Med. 1975;82:376–9. doi: 10.7326/0003-4819-82-3-376. [DOI] [PubMed] [Google Scholar]
- 17.Izumi AK, Takiguchi P. Lupus erythematosus panniculitis. Arch Dermatol. 1983;119:61–4. [PubMed] [Google Scholar]
- 18.Watanabe T, Tsuchida T. Lupus erythematosus profundus: a cutaneous marker for a distinct clinical subset? Br J Dermatol. 1996;134:123–5. [PubMed] [Google Scholar]
- 19.Cassis TB, Fearneyhough PK, Callen JP. Subcutaneous panniculitis-like T-cell lymphoma with vacuolar interface dermatitis resembling lupus erythematosus panniculitis. J Am Acad Dermatol. 2004;50:465–9. doi: 10.1016/s0190-9622(03)02784-1. [DOI] [PubMed] [Google Scholar]
- 20.Burrows NP, Walport MJ, Hammond AH, et al. Lupus erythematosus profundus with partial C4 deficiency responding to thalidomide. Br J Dermatol. 1991;125:62–7. doi: 10.1111/j.1365-2133.1991.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 21.Massone C, Kodama K, Salmhofer W, et al. Lupus erythematosus panniculitis (lupus profundus): clinical, histopathological, and molecular analysis of nine cases. J Cutan Pathol. 2005;32:396–404. doi: 10.1111/j.0303-6987.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- 22.Nousari HC, Kimyai-Asadi A, Provost TT. Generalized lupus erythematosus profundus in a patient with genetic partial deficiency of C4. J Am Acad Dermatol. 1999;41:362–4. doi: 10.1016/s0190-9622(99)70389-0. [DOI] [PubMed] [Google Scholar]
- 23.Taieb A, Hehunstre JP, Goetz J, et al. Lupus erythematosus panniculitis with partial genetic deficiency of C2 and C4 in a child. Arch Dermatol. 1986;122:576–82. doi: 10.1001/archderm.1986.01660170106030. [DOI] [PubMed] [Google Scholar]
- 24.Saeki Y, Ohshima S, Kurimoto I, et al. Maintaining remission of lupus erythematosus profundus (LEP) with cyclosporin A. Lupus. 2000;9:390–2. doi: 10.1191/096120300678828406. [DOI] [PubMed] [Google Scholar]
- 25.Wozniacka A, Salamon M, Lesiak A, et al. The dynamism of cutaneous lupus erythematosus: mild discoid lupus erythematosus evolving into SLE with SCLE and treatment-resistant lupus panniculitis. Clin Rheumatol. 2007;26:1176–9. doi: 10.1007/s10067-006-0310-6. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125:889–94. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thurston CS, Curtis AC. Lupus erythematosus profundus (Kaposi-Irgang). Clinical response to hydroxychloroquine sulfate. Arch Dermatol. 1966;93:577–82. [PubMed] [Google Scholar]
- 28.Hansen CB, Callen JP. Connective tissue panniculitis: lupus panniculitis, dermatomyositis, morphea/scleroderma. Dermatologic Therapy. 2010;23:341–9. doi: 10.1111/j.1529-8019.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang AY, Werth VP. Treatment of cutaneous lupus. Curr Rheumatol Rep. 2011;13:300–7. doi: 10.1007/s11926-011-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan KW, Callen JP. Calcifying lupus panniculitis in a patient with subacute cutaneous lupus erythematosus: response to diltiazem and chloroquine. J Rheumatol. 2001;28:2129–32. [PubMed] [Google Scholar]
- 31.Chung HS, Hann SK. Lupus panniculitis treated by a combination therapy of hydroxychloroquine and quinacrine. J Dermatol. 1997;24:569–72. doi: 10.1111/j.1346-8138.1997.tb02294.x. [DOI] [PubMed] [Google Scholar]
- 32.Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415–22. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Yell JA, Burge SM. Lupus erythematosus profundus treated with clobetasol propionate under a hydrocolloid dressing. Br J Dermatol. 1993;128:103. doi: 10.1111/j.1365-2133.1993.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 34.Wienert S, Gadola S, Hunziker T. Facets of lupus erythematosus: panniculitis responding to thalidomide. J Dtsch Dermatol Ges. 2008;6:214–6. doi: 10.1111/j.1610-0387.2007.06525.x. [DOI] [PubMed] [Google Scholar]
- 35.Ujiie H, Shimizu T, Ito M, et al. Lupus erythematosus profundus successfully treated with dapsone: review of the literature. Arch Dermatol. 2006;142:399–401. doi: 10.1001/archderm.142.3.399. [DOI] [PubMed] [Google Scholar]
- 36.Morruzzi C, Liu V, Bohbot A, et al. Four cases of photopheresis treatment for cutaneous lupus erythematosus refractory to standard therapy. Ann Dermatol Venereol. 2009;136:861–7. doi: 10.1016/j.annder.2009.10.183. [DOI] [PubMed] [Google Scholar]
- 37.McArdle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab [case report] Clin Rheumatol. 2009;28:745–6. doi: 10.1007/s10067-009-1158-3. [DOI] [PubMed] [Google Scholar]
- 38.Balin SJ, Wetter DA, Andersen LK, et al. Calcinosis Cutis Occurring in Association With Autoimmune Connective Tissue Disease: The Mayo Clinic Experience With 78 Patients, 1996–2009. Arch Dermatol. 2011 doi: 10.1001/archdermatol.2011.2052. [DOI] [PubMed] [Google Scholar]
- 39.Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164–7. doi: 10.1111/j.1365-2230.1995.tb02675.x. [DOI] [PubMed] [Google Scholar]
- 40.Solans R, Cortes J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148–50. doi: 10.1067/mjd.2002.107491. [DOI] [PubMed] [Google Scholar]
- 41.Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis. A histopathologic study of 55 cases. Arch Dermatol. 1968;97:640–50. [PubMed] [Google Scholar]
- 42.Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270–2. doi: 10.1046/j.1525-1470.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 43.Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305–7. doi: 10.1016/0190-9622(95)90266-x. [DOI] [PubMed] [Google Scholar]
- 44.Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161–3. doi: 10.1046/j.1365-2133.1998.02343.x. [DOI] [PubMed] [Google Scholar]
- 45.Arias M, Hernandez MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146–8. doi: 10.1590/s0365-05962011000100023. [DOI] [PubMed] [Google Scholar]
- 46.Douvoyiannis M, Litman N, Dulau A, et al. Panniculitis, infection, and dermatomyositis: case and literature review. Clin Rheumatol. 2009;28(Suppl 1):S57–63. doi: 10.1007/s10067-009-1160-9. [DOI] [PubMed] [Google Scholar]
- 47.Leung YY, Choi KW, Ho KM, et al. Disseminated cutaneous infection with Mycobacterium chelonae mimicking panniculitis in a patient with dermatomyositis. Hong Kong Med J. 2005;11:515–9. [PubMed] [Google Scholar]
- 48.Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773–6. [PubMed] [Google Scholar]
- 49.Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151–3. doi: 10.1002/mus.21537. [DOI] [PubMed] [Google Scholar]
- 50.Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Arthritis Rheum. 2001;44:1496–503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 52.Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141–4. doi: 10.1046/j.1365-4362.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- 53.Robinson AB, Reed AM. Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nat Rev Rheumatol. 2011;7:664–75. doi: 10.1038/nrrheum.2011.139. [DOI] [PubMed] [Google Scholar]
- 54.Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329:1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- 55.Yoo JY, Jo SJ, Cho KH. Lupus panniculitis with combined features of dermatomyositis resulting in severe lipoatrophy. J Dermatol. 2004;31:552–5. doi: 10.1111/j.1346-8138.2004.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 56.Fett N, Werth VP. Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217–28. doi: 10.1016/j.jaad.2010.05.045. quiz 29-30. [DOI] [PubMed] [Google Scholar]
- 57.Bielsa I, Ariza A. Deep morphea. Semin Cutan Med Surg. 2007;26:90–5. doi: 10.1016/j.sder.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma) Mayo Clin Proc. 1995;70:1068–76. doi: 10.4065/70.11.1068. [DOI] [PubMed] [Google Scholar]
- 59.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18:606–13. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 60.Almeida MS, Lima SC, Carvalho LL, et al. Panniculitis-an unusual cutaneous manifestation of systemic sclerosis. J Cutan Pathol. 2010;37:1170–3. doi: 10.1111/j.1600-0560.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 61.Su WP, Person JR. Morphea profunda. A new concept and a histopathologic study of 23 cases. Am J Dermatopathol. 1981;3:251–60. doi: 10.1097/00000372-198123000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Derk CT. Disease-modifying drugs for systemic sclerosis: why have we not found them yet? Expert Rev Clin Immunol. 2011;7:399–401. doi: 10.1586/eci.11.29. [DOI] [PubMed] [Google Scholar]
- 63.Fett N, Werth VP. Update on morphea: part II. Outcome measures and treatment. J Am Acad Dermatol. 2011;64:231–42. doi: 10.1016/j.jaad.2010.05.046. quiz 43-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martini G, Ramanan AV, Falcini F, et al. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology (Oxford) 2009;48:1410–3. doi: 10.1093/rheumatology/kep244. [DOI] [PubMed] [Google Scholar]
- 65.Strauss RM, Bhushan M, Goodfield MJ. Good response of linear scleroderma in a child to ciclosporin. Br J Dermatol. 2004;150:790–2. doi: 10.1111/j.0007-0963.2004.05901.x. [DOI] [PubMed] [Google Scholar]
- 66.Neustadter JH, Samarin F, Carlson KR, et al. Extracorporeal photochemotherapy for generalized deep morphea. Arch Dermatol. 2009;145:127–30. doi: 10.1001/archdermatol.2008.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crespo MP, Mas IB, Diaz JM, et al. Rapid response to cyclosporine and maintenance with methotrexate in linear scleroderma in a young girl. Pediatr Dermatol. 2009;26:118–20. doi: 10.1111/j.1525-1470.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- 68.Stausbol-Gron B, Olesen AB, Deleuran B, et al. Abatacept is a promising treatment for patients with disseminated morphea profunda: presentation of two cases. Acta Derm Venereol. 2011;91:686–8. doi: 10.2340/00015555-1136. [DOI] [PubMed] [Google Scholar]
- 69.Reiter N, El-Shabrawi L, Leinweber B, et al. Subcutaneous morphea with dystrophic calcification with response to ceftriaxone treatment. J Am Acad Dermatol. 2010;63:e53–5. doi: 10.1016/j.jaad.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Roldan R, Morote G, Castro Mdel C, et al. Efficacy of bosentan in treatment of unresponsive cutaneous ulceration in disabling pansclerotic morphea in children. J Rheumatol. 2006;33:2538–40. [PubMed] [Google Scholar]
- 71.Choksi AN, Orringer JS. Linear morphea-induced atrophy treated with hyaluronic acid filler injections. Dermatol Surg. 2011;37:880–3. doi: 10.1111/j.1524-4725.2011.02030..x. [DOI] [PubMed] [Google Scholar]
- 72.Jinnin M, Ihn H, Asano Y, et al. Sclerosing panniculitis is associated with pulmonary hypertension in systemic sclerosis. Br J Dermatol. 2005;153:579–83. doi: 10.1111/j.1365-2133.2005.06680.x. [DOI] [PubMed] [Google Scholar]
- 73.Peters MS, Winkelmann RK. Localized lipoatrophy (atrophic connective tissue disease panniculitis) Arch Dermatol. 1980;116:1363–8. [PubMed] [Google Scholar]
- 74.White JW, Jr., Winkelmann RK. Weber-Christian panniculitis: a review of 30 cases with this diagnosis. J Am Acad Dermatol. 1998;39:56–62. doi: 10.1016/s0190-9622(98)70402-5. [DOI] [PubMed] [Google Scholar]
- 75.Fuchtenbusch M, Vogel A, Achenbach P, et al. Lupus-like panniculitis in a patient with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) Exp Clin Endocrinol Diabetes. 2003;111:288–93. doi: 10.1055/s-2003-41287. [DOI] [PubMed] [Google Scholar]
- 76.Halvorson CR, Kwon SY, Kao GF, et al. Lipomembranous fat necrosis in a patient with mixed connective tissue disease. J Am Acad Dermatol. 2011;64:1010–1. doi: 10.1016/j.jaad.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 77.Nakashima M, Suzuki K, Okada M, et al. Panniculitis in a patient with mixed connective tissue disease. Mod Rheumatol. 2004;14:250–3. doi: 10.1007/s10165-004-0301-x. [DOI] [PubMed] [Google Scholar]
- 78.Oka H, Tanikawa A, Matsuda F, et al. Systemic sclerosis with unusual panniculitis and overlying discoid lupus erythematosus-like lesions. J Dtsch Dermatol Ges. 2005;3:627–9. doi: 10.1111/j.1610-0387.2005.05727.x. [DOI] [PubMed] [Google Scholar]
- 79.Handfield-Jones SE, Stephens CJ, Mayou BJ, et al. The clinical spectrum of lipoatrophic panniculitis encompasses connective tissue panniculitis. Br J Dermatol. 1993;129:619–24. doi: 10.1111/j.1365-2133.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 80.Marque M, Guillot B, Bessis D. Lipoatrophic connective tissue panniculitis. Pediatr Dermatol. 2010;27:53–7. doi: 10.1111/j.1525-1470.2009.01048.x. [DOI] [PubMed] [Google Scholar]
- 81.Shen LY, Edmonson MB, Williams GP, et al. Lipoatrophic panniculitis: case report and review of the literature. Arch Dermatol. 2010;146:877–81. doi: 10.1001/archdermatol.2010.180. [DOI] [PubMed] [Google Scholar]