Abstract
Smooth muscle cells (SMCs) possess remarkable phenotypic plasticity that allows rapid adaptation to fluctuating environmental cues, including during development and progression of vascular diseases such as atherosclerosis. Although much is known regarding factors and mechanisms that control SMC phenotypic plasticity in cultured cells, our knowledge of the mechanisms controlling SMC phenotypic switching in vivo is far from complete. Indeed, the lack of definitive SMC lineage-tracing studies in the context of atherosclerosis, and difficulties in identifying phenotypically modulated SMCs within lesions that have down-regulated typical SMC marker genes, and/or activated expression of markers of alternative cell types including macrophages, raise major questions regarding the contributions of SMCs at all stages of atherogenesis. The goal of this review is to rigorously evaluate the current state of our knowledge regarding possible phenotypes exhibited by SMCs within atherosclerotic lesions and the factors and mechanisms that may control these phenotypic transitions.
Keywords: Smooth muscle cells, Epigenetics, Plasticity, Pluripotency genes, Macrophages, KLF4
1. Smooth muscle cell exhibits an extensive plasticity
The vascular smooth muscle cell (SMC) is a highly specialized and differentiated cell in adult animals. Its principal function is contraction which permits regulation of vessel tone and diameter and thus control of blood pressure and blood flow distribution. SMCs within the adult blood vessel proliferate at an extremely low rate and exhibit a very low synthetic activity. They express a unique repertoire of contractile proteins, ion channels, and signalling molecules required for SMC contractile function that is clearly unique compared with other cell types and other muscle lineages including the skeletal muscle and cardiac muscle (reviewed in Owens et al.1,2). A number of SMC-selective or -specific genes have been identified that are used as markers of the mature-differentiated SMC. These include the smooth muscle isoforms of proteins that comprise the contractile apparatus including SM α-actin,3–5 SM myosin heavy chain (MHC),6–9 h1-calponin,10,11 SM22α,10,12 and smoothelin.13 Although this repertoire is specifically expressed in the fully differentiated SMC, most of these SMC markers are expressed, at least transiently in other cell types during development, tissue repair, or disease states.14 As such, evidence of expression of a single SMC differentiation marker gene alone is not sufficient for SMC identification and assessment of SMC maturation, with the possible exception for the SM MHC isoforms which are considered the most specific markers of SMC. Thus, the rigorous identification of mature SMC requires examination of multiple marker genes1 and if in tissues, appropriate localization within the medial layer of blood vessels or other SMC tissues.
The mature SMC is a cell type which is not terminally differentiated and that retains remarkable plasticity. SMC plasticity is likely dependent on variations in environmental cues and extracellular signals sensed by the cell. The plasticity of SMCs and SMC-like pericytes is required for vascular formation and maturation during embryogenesis and vascular remodelling.15–17 These fundamental properties discriminate SMC from the other muscle cell types including skeletal muscle and cardiac muscle cells which are terminally differentiated.18 Although different types of phenotypic states can be envisaged, SMC phenotypic switching is generally associated with markedly decreased SMC-selective marker gene expression and increased SMC proliferation and migration.
The concept of phenotypic switching is widely accepted. However, remarkably, there has not been a single study that has definitively or quantitatively assessed the contribution of SMCs to development of alternative phenotypes within atherosclerotic lesions and how these processes influence plaque stability in either experimental animal models or man. Indeed, all previous studies in this area are inconclusive due to three major limitations (Table 1): first, the lack of rigorous lineage-tracing methods that permit unambiguous identification of cells of SMC origin even if the cell has undergone major morphological changes and/or has undetectable expression of endogenous SMC differentiation markers such as SM α-actin, SM22α, and SM MHC that are typically used to identify it as an SMC. As such, many phenotypically modulated SMC within lesions may not be identified as being of SMC origin. Secondly, multiple cell types other than SMC within lesions can express SMC marker genes such as SM α-actin, a marker that has routinely been used to identify SMCs within lesions.19,20 Indeed, Caplice et al.20 presented evidence that ∼10% of cells within advanced human coronary artery atherosclerotic lesions that express SM α-actin are of myeloid and not SMC lineage. There is also evidence that macrophages can be induced to express multiple SMC markers including SM α-actin and SM22α in response to treatment with transforming growth factor-β or thrombin.21,22 As such, a subset of SMC marker-positive cells in lesions is not derived from SMCs. Thirdly, Rong et al.23 have shown that cholesterol loading of cultured SMCs resulted in marked suppression of SMC markers, but activation of multiple macrophage markers in cultured vascular SMCs raising the possibility that at least some macrophage marker-positive cells within lesions may be of SMC origin. Consistent with this possibility, Bentzon et al.24 presented evidence showing that a significant fraction of lesion cells positive for the macrophage marker Mac2 are not derived from bone marrow cells. Given these uncertainties regarding which cells in lesions are actually of SMC origin, it becomes impossible to ascertain how the cells might positively or negatively influence plaque stability, or what factors and mechanisms might regulate transitions in SMC phenotype within lesions (Table 1 and Figure 1). This review will summarize evidence regarding possible phenotypes exhibited by SMC within atherosclerotic lesions, and mechanisms that control these phenotypic transitions. Importantly, we will focus on studies in vivo in animal models and in human lesions rather than cell culture, which, unfortunately, is where most studies of SMC phenotypic switching have been done.
Table 1.
Ambiguities regarding lesion cell origin and possible cell transition in human and animal model of atherosclerosis
| Transition/transdifferentiation | Model | Observations | Ambiguity | Reference |
|---|---|---|---|---|
| Macrophages → SMC-like cells | In vitro stimulation | TGF-β induces SM α-actin expression in macrophages | No evidence in vivo | Stewart et al.21 |
| Macrophages → SMC-like cells | In vitro stimulation | Thrombin induces SM α-actin expression in macrophages | No evidence in vivo | Martin et al.22 |
| Myeloid cells → SMC-like cells and SMCs → macrophage-like cells | Cross-gender bone marrow transplant—human coronary arteries | Approximately 10% of cells within advanced atherosclerotic lesion that express SM α-actin are of myeloid origin | Studies were focused exclusively on determining whether haematopoietic cells give rise to SMCs or SMC-like cells in lesions and did not attempt to do SMC lineage tracing | Caplice et al.20 |
| Myeloid cells → SMC-like cells | ApoE−/− transplanted with BM from eGFP+ ApoE−/− mice | SMCs in atherosclerotic plaques are derived from the local vessel and not from circulating cells. A significant fraction of Mac2+ cells are NOT of myeloid origin (GFP+) | Studies were focused exclusively on determining whether haematopoietic cells give rise to SMCs or SMC-like cells in lesions and did not attempt to do SMC lineage tracing | Bentzon et al.24 |
| Myeloid cells → SMC-like cells | ApoE−/− transplanted with BM from eGFP transgenic ApoE−/− mice | SMCs required in healing within the atherosclerotic lesion are of local but not bone marrow-derived origin | Bentzon et al.35 | |
| Myeloid cells → SMC-like cells | Vascular injury of femoral artery in WT mice transplanted with ROSA26/LacZ bone marrow. | Claim that the majority of the SM αA+ cells within the neointima are of haematopoietic origin but results were later refuted by Bentzon et al. and this same lab in Iwata et al. | Studies were focused exclusively on determining whether haematopoietic cells give rise to SMCs or SMC-like cells in lesions, and did not attempt to do SMC lineage tracing. HSC lineage-tracing studies were compromised by poor resolution of images and failure to validate markers present in individual cells | Sata et al.34 |
| Myeloid cells → SMC-like cells | ApoE−/− transplanted with bone marrow from SM α-actin-EGFP or SM-MHC+/LacZ mice | Claim that some SM αA+ cells in lesions are of myeloid origin but that cells do not differentiate into mature SMCs as evidenced by activation of definitive SMC markers like SM MHC | Studies were focused exclusively on determining whether haematopoietic cells give rise to SMCs or SMC-like cells in lesions and did not attempt to do SMC lineage tracing | Iwata et al.37 |
| SMCs → macrophage-like cells | Cholesterol loading in cultured SMCs | Cholesterol loading of cultured SMC causes reduced expression of SMC marker genes while activating expression of macrophage markers and functions including phagocytosis and antigen presentation | Results were based entirely on studies in cultured SMC. No evidence was presented to show that this phenomenon occurs in vivo within atherosclerotic lesions | Rong et al.23 |
| SMCs → macrophage-like cells | Human atherosclerotic lesions | Co-localization SMaA+/CD68+ cells within the lesion | Impossible to ascertain if dual SMaA+/CD68+ cells are derived from SMC, macrophages, or other cell type | Andreeva et al.19 |
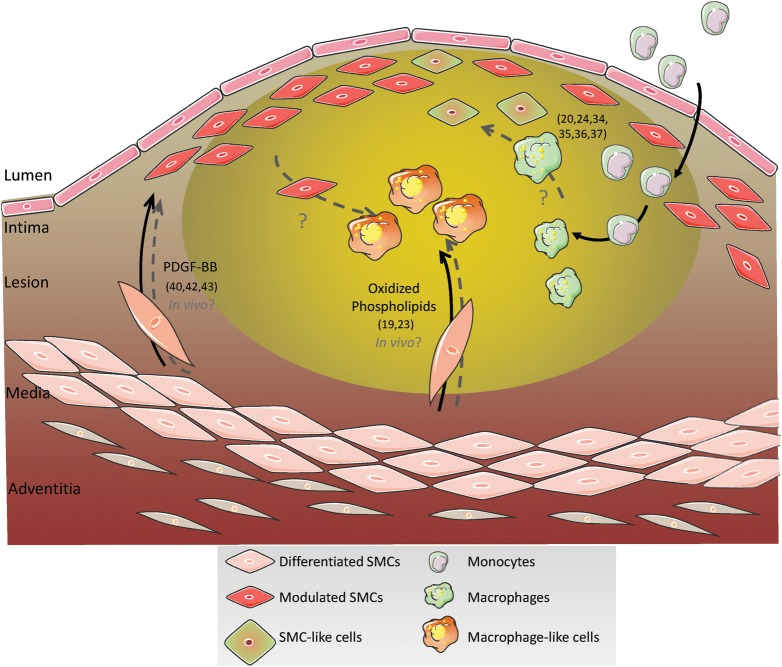
Figure 1.
Hypothetical origins of SMC- and macrophage-like cells within atherosclerotic lesions. The lack of definitive SMC lineage-tracing studies in the context of atherosclerosis, and difficulties in identifying phenotypically modulated SMCs within lesions that have down-regulated typical SMC marker genes, and/or activated expression of markers of alternative cell types including macrophages, raise major questions regarding the contributions of SMC within atherosclerotic lesions. Similarly, there is evidence showing that macrophages can activate at least some SMC markers. The net result is that there are major ambiguities regarding the origins of many of the principal cell types present within atherosclerotic lesions and their roles in plaque development and stability. For the purpose of this figure and review, we define SM-like lesion cells as being positive for at least some SMC marker genes such as SM α-actin, whereas macrophage-like cells are those expressing at least some macrophage marker genes but negative for SMC markers. Interestingly, Andreeva et al.19 reported the presence of cells that express both SM α-actin and the macrophage marker CD68 within human atherosclerotic lesions. What is unclear is whether these are SMCs that have activated macrophage markers or macrophages that have activated SMC markers? Numbers within parentheses are the references of studies summarized in this figure.
2. SMC phenotypic switching in atherosclerosis
Atherosclerosis is a chronic disease of the arterial wall that is responsible for nearly 50% of all deaths in developed countries.25,26 The prevalence of this disease continues to rise due to adoption of a ‘Western life-style’ by an increasing fraction of the World's population and is likely to reach epidemic proportions in the next few decades. However, despite expenditure of billions of dollars and decades of research, there are still fundamental gaps in our knowledge of the underlying mechanisms that contribute to its development, progression, and end-stage clinical events including plaque rupture, myocardial infarction, and stroke. For example, whereas there is general agreement that increased SMC content of atherosclerotic lesions is associated with increased plaque stability,25,27–29 the mechanisms for this are poorly understood. Indeed, the long-standing dogma in the field is that the majority of intimal SMCs within atherosclerotic lesions are derived from resident medial SMCs that undergo phenotypic modulation and migration into the intima where they proliferate, produce extracellular matrix, and participate in fibrous cap formation.28,30 However, there is only indirect evidence in support of this hypothesis including the following. First, ultrastructural studies of human atherosclerotic lesions have routinely described cells with morphological characteristics of SMCs which appear to be in the process of migrating through the internal elastic lamina into the intima.31–33 Although these studies provide evidence that medial SMCs contribute to formation of the intima, the subsequent fate of these cells once they get into the lesion is poorly understood. Secondly, although a previous study by the Nagai lab34 claimed that the majority of SM-like cells in lesions were of haematopoietic cell origin, subsequent rigorous lineage-tracing and confocal studies by Bentzon et al.,24,35 Daniel et al.,36 and the Nagai group37 showed that the majority of SMC-like cells within atherosclerotic lesions of ApoE−/− Western diet fed mice are NOT of haematopoietic origin. Bentzon et al.24 presented results of rigorous bone marrow transfer (BMT) green fluorescent protein (GFP) lineage-tracing studies showing that a large fraction of Mac2+ cells within atherosclerotic lesions of Western diet fed ApoE−/− mice were NOT derived from haematopoietic cells. Indeed, our analyses of the co-localization between haematopoietic cells and Mac2 staining of this paper indicate that up to 30% of Mac2+ cells within lesions were of non-myeloid origin (i.e. GFP-negative), indicating that they are derived from some source other than monocytes. The design of the Bentzon studies did not permit assessing whether these cells were of SMC origin. However, results are highly consistent with the possibility that a significant fraction of macrophage-like cells within lesions are derived from SMCs not monocytes. Consistent with these findings, based on Y-chromosome lineage-tracing studies in coronary lesions from subjects who had a cross-gender bone marrow transplantation, Caplice et al.20 showed that >90% SM α-actin-expressing cells within lesions are not of haematopoietic cell origin.
Whereas the preceding studies clearly demonstrate that haematopoietic cells are not the primary source of SMC-like cells within lesions, unfortunately, none of these studies directly tested whether SMCs give rise to SMC-like cells in lesions, a critical deficiency given that intimal cells positive for at least some SMC markers could be derived from adventitial fibroblasts,38 activated macrophages,20–22 or other cell types. Moreover, the design of these studies did not permit detection of phenotypically modulated SMCs within lesions that may have lost detectible expression of known SMC marker genes and turned on markers of other cell types including macrophages. Remarkably, there are no direct SMC lineage-tracing studies that have clearly defined the roles of SMC within atherosclerotic lesions. Indeed, to our knowledge, there are no definitive SMC lineage-tracing studies at all outside of a developmental setting (reviewed by Majesky et al.39) with the exception of a study by Giachelli and co-workers (Speer et al.)40 who presented evidence suggesting that medial SMCs transdifferentiate into osteochrondrogenic precursor and chrondrogenic cells in calcified vessels of matrix Gla protein-deficient (MGP−/−) mice using an SM22α-cre×ROSA26 LacZ lineage-tracing model. However, although calcification plays a key role in atherosclerosis, this model is not atherosclerosis per se. In addition, in the light of recent studies showing that activated macrophages express SM22α,37 even these studies are ambiguous as to whether cells were derived from SMCs or myeloid cells. Taken together, we ascertain that there is complete ambiguity as to which cells within atherosclerotic lesions are indeed of SMC origin, and what role these cells might play in lesion development, progression, and end-stage disease consequences such as plaque rupture with possible myocardial infarction or stroke. Moreover, we ascertain that the dogma that SMCs undergo phenotypic transitions to states that might play a key role in atherosclerotic lesions is based nearly completely on results of studies in cultured SMCs, and extrapolation of these findings, probably inappropriately in many cases, to processes that might occur within atherosclerotic lesions. Finally, we suggest that clear and unambiguous identification of which cells within lesions are derived from SMC is a prerequisite for defining factors and mechanisms that control SMC phenotypic switching and the functional roles of these cells within lesions as well as for developing new therapeutic approaches targeting SMC phenotypic switching for purposes of promoting plaque stabilization. Indeed, consistent with this possibility, we recently demonstrated that genetic loss of interleukin (IL)-1 signalling in ApoE−/− Western diet fed mice surprisingly resulted in reduced rather than increased plaque stability and impaired beneficial outward remodelling.41 This is exactly opposite to expectation-based effects of IL-1 in enhancing activation of inflammatory cells and inducing cultured SMCs into an inflammatory phenotype.42 Results highlight the need for clearly defining which cells within lesions are SMC derived, the functional role of these cells in plaque development, progression, and stability, the factors that mediate these transitions, and how one might modulate these responses therapeutically to enhance plaque stability.
Given major ambiguities regarding phenotypes exhibited by SMC in vivo within atherosclerotic lesions, there are, of course, also major questions regarding the mechanisms and factors that might control SMC phenotypic transitions. For example, although there is compelling evidence from our lab and many other labs showing that platelet-derived growth factor-BB (PDGF-BB) can induce phenotypic switching in vitro in cultured SMCs,43,44 there is a lack of clear evidence that it does so in vivo, in spite of the attractiveness of a mechanism wherein damage to a blood vessel would result in platelet adhesion/activation and release of PDGF-BB, which in turn reprogrammes SMCs into a phenotype that is beneficial for wound repair. Indeed, PDGF-BB has been shown to be a highly efficacious in suppressing SM marker gene expression, as well as in inducing increased SMC proliferation and migration.43,45,46 However, there is no direct evidence that PDGF-BB is a potent regulator of SMC differentiation, proliferation, and migration in vivo. Conventional knockout of PDGF-B chain and PDGFR-β has been shown to result in early embryonic or perinatal lethality47,48 and as yet, there have been no studies to our knowledge testing the effects of conditional knockout of PDGF receptors in SMCs in models of atherosclerosis, vascular injury, angiogenesis, arteriolar remodelling, or other model system in which PDGF-BB signalling in SMC or pericytes is postulated to play a critical role. Administration of blocking antibodies against PDGF receptors in ApoE−/− mice on a Western diet has been shown to be associated with a 67% reduction in atherosclerotic lesion size and with reduced SMC investment of the neointima. However, results of these studies may have been due to blocking PDGF receptors in multiple cell types not just SMCs. Kozaki et al.49 generated chimeric PDGF-B chain-knockout mice by lethally irradiating wild-type mice and reconstituting with foetal liver cells (a primary site of haematopoiesis in late-stage foetuses) from PDGF-B chain-deficient E16.5-day mice. Although plaque development was reduced at early stages, SMC investment in the fibrous cap was indistinguishable from controls after 45 weeks of Western diet. Moreover, it is not possible to deduce from these studies whether PDGF deficiency directly impacted SMC phenotype. Indeed, resolution of the possible role of PDGF-BB in SMC phenotypic switching in vivo will be dependent on the development of SMC-specific conditional PDGF receptor-knockout mice in an ApoE−/− or LDL receptor−/− background. Although we do not refute the possible involvement of PDGF-BB in inducing SMC phenotypic switching and this may contribute to lesion formation as well as fibrous cap formation, at present, there is no direct evidence that this is the case. Similarly, although in vitro studies have implicated a wide range of factors in SMC phenotypic switching, including oxidized phospholipids,50,51 inflammatory cytokines,52,53 and lysophosphatidic acid,54 in no case is their corroborative direct evidence for a role of these factors in directly controlling SMC phenotype in vivo.
Even more confounding is the controversy regarding the participation of SMC and myeloid (mainly monocyte/macrophage) lineages in the progression of atherosclerotic disease and end-stage clinical consequences including plaque rupture with possible myocardial infarction and stroke. We do not refute the well-established dogma that an increase in the ratio of macrophages to SMC within lesions is causally linked to plaque destabilization as stated repeatedly in major reviews in the field.26,55 However, given the uncertainties in identification of cells within lesions already discussed, we challenge the dogma regarding the possible origins of lesion cells including the general assumption in the field that SM α-actin+ lesion cells are ‘SMC derived’ and macrophage marker-positive cells are ‘macrophages’ (Figure 1). Of course, this becomes highly ambiguous given that phenotypically modulated SMCs profoundly down-regulate SMC markers,56–58 whereas macrophages turn on at least some of these SMC markers.21,22 Similarly, cholesterol-loaded SMCs, at least in culture, activate multiple macrophage markers and exhibit functional properties of macrophages including antigen presentation and phagocytosis, while simultaneously suppressing SMC markers needed to identify them as SMC.23 As such, there are many unresolved questions including the following. Are SM α-actin+ cells within the fibrous cap of SMC (the dogma) or macrophage origin? Are macrophage marker-positive intimal cells of macrophage origin or SMC origin? Do SMC-derived macrophage-like cells exhibit different functional properties than macrophages derived from myeloid cells and how do these impact plaque stability? What proportion of macrophage-like cells in lesions may not be of myeloid origin? What mechanisms and factors control the phenotypic transitions of macrophages and SMCs? What other cell types contribute to lesion development but may be mis-identified using conventional markers? We ascertain that definitive resolution of these questions is paramount to understanding mechanisms and factors that contribute to atherosclerosis development, progression, and its end-stage complications, and that the initial step in this process needs to start with much more rigorous and definitive SMC and macrophage lineage-tracing studies. Moreover, it is critical to resolve these issues for purposes of identifying novel therapeutic approaches to treating atherosclerosis and to better understand the effects of the current therapies including the widespread use of statins and other lipid-lowering agents.
3. Molecular mechanisms regulating SMC phenotypic switching following vascular injury or atherosclerosis
So given the major ambiguities regarding identification of the origins of SMC-like cells in atherosclerotic lesions, what, if anything, do we know regarding cellular and molecular mechanisms that control SMC phenotypic switching in vivo. We have previously identified sufficient regions of the SM α-actin5 and SM MHC8,59 promoters necessary to recapitulate expression patterns of these endogenous genes in transgenic mice and identified numerous cis-regulatory elements required for SMC-specific expression in vivo in transgenic mice.5,60–68 Of major significance, we previously demonstrated that mutation of a highly conserved G/C repressor element 5′ to the proximal CARG element in the SM22α promoter, and also found in the promoters of many other SMC marker genes (reviewed in Owens et al.1), nearly abolished down-regulation of this gene in vivo in response to vascular injury56 or in atherosclerotic lesions of ApoE−/− mice58 (Figure 2). Of major significance, using this mutant SM22α transgene, we were able to identify large numbers of presumptive SMC-derived cells within lesions (as well as the media underlying lesions, compare the top and bottom panels in Figure 2) that could not be identified as being of SMC origin using either a wild-type SM22α transgene or expression of endogenous SMC marker genes such as SM α-actin or SM22α. Indeed, these studies were the first, and to date, the only studies to our knowledge that have identified presumptive phenotypically modulated SMC within lesions that are unrecognizable as being SMC based on expression of endogenous marker genes. Even more importantly, however, is that we have identified a specific molecular mechanism (i.e. a G/C repressor-dependent process) critical in mediating SMC phenotypic switching within atherosclerotic lesions in vivo.
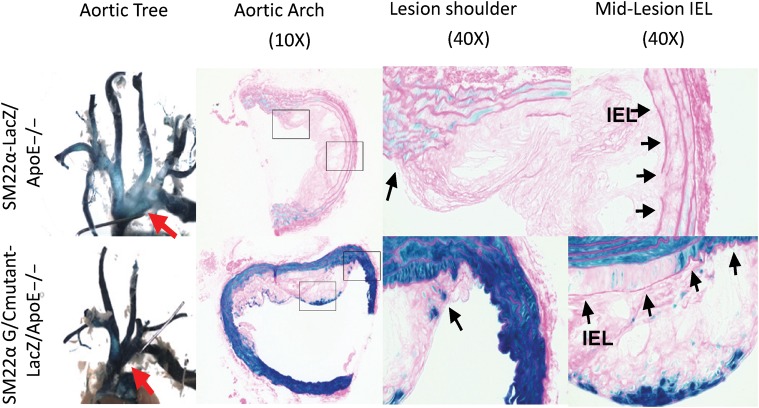
Figure 2.
Molecular mechanisms of decreased SM marker gene expression in vivo within atherosclerotic lesions of ApoE−/− Western diet fed mice (reprinted from Wamhoff et al., Circ Res 2004;95:981–988; used with permission). Mutation of the G/C repressor virtually abolished repression of the SM22α transgene in intimal SMC in atherosclerotic lesions within the aortic arch region in ApoE−/− mice fed a Western diet for 18 weeks (compare lacZ transgene staining in the upper panels in mice containing the wild-type SM22α lacZ transgene with that in the lower panels in mice with the G/C repressor mutant SM22α lacZ transgene).58 The LacZ-positive cells within the intima in SM22α G/C mutant-lacZ mice in the lower panels represent putative phenotypically modulated SMCs unidentifiable as being SMCs based on expression of their endogenous SMC marker genes such as SM α-actin or SM22α.58 However, given that macrophages can activate expression of SMC markers including SM22α,37 we cannot unambiguously identify the G/C repressor mutant SM22α lacZ-positive cells as being of SMC origin since they could possibly be of macrophage origin. Taken together, studies illustrate why there is major ambiguity regarding identification of SMC-derived cells in lesions and their possible contributions to lesion development and plaque stability.
We subsequently demonstrated that transcriptional repression of SMC marker genes in response to treatment of cultured SMC with PDGF-BB, PDGF DD, or pro-atherogenic oxidized phospholipids such as POVPC was dependent on binding of Krüppel-Like Factor-4 (KLF4) to the G/C repressor.50,51,69,70 KLF4 is a gene known to be critical in maintenance of pluripotency in embryonic stem cells (ESC)71 and more recently was shown to be one of four factors72,73 along with Oct4, Sox2, and c-myc, shown to be capable of reprogramming a variety of somatic cells including dermal fibroblasts into ESC-like cells or induced pluripotential stem (iPS) cells. Of major importance, KLF4 is not expressed in adult-differentiated mesenchymal cells including SMCs. Nevertheless, several studies provide evidence that KLF4 is re-expressed and involved in mediating SMC phenotypic switching in vivo as described below. First, KLF4 expression is increased within lesions of ApoE−/− mice on a Western diet69 as well as following vascular injury.74 Secondly, KLF4 overexpression is associated with profound inhibition of expression of the potent SMC-selective SRF co-activator myocardin and all known SMC marker genes,75 and also induces epigenetic changes of SMC marker gene loci associated with transcriptional silencing, including HDAC recruitment and histone hypo-acetylation.51,76,77 Thirdly, conditional knockout of KLF4 was associated with a transient delay in repression of SM α-actin and SM22α following vascular injury in vivo, but with subsequent hyperproliferation of SMC and increased neointima formation, likely as the result of reduced KLF4-dependent activation of the cell cycle inhibitory gene p21.74 However, it should be noted that conditional knockout of KLF4 in the preceding studies occurred in all cell types so that effects cannot be ascribed solely to loss of KLF4 in SMCs. Fourthly, oxidized phospholipid-induced increases in extracellular matrix gene expression were shown to be KLF4-dependent. Taken together, the preceding results provide compelling evidence in support of the hypothesis that the G/C repressor is required for suppression of SMC marker genes in vivo during the development of experimental atherosclerosis and that the transcriptional suppressor activity of the G/C repressor is regulated by binding the iPS cell-ESC pluripotency factor KLF4. However, further studies are needed to directly test this hypothesis including generation of SMC-specific conditional KLF4-knockout mice and investigation of how this impacts SMC phenotypic switching, as well as overall lesion development, plaque composition, and stability. Key questions include the following. Is KLF4-dependent SMC phenotypic switching beneficial (because it augments fibrous cap formation) or detrimental (because it promotes a phenotype that may increase overall lesion size) in the context of atherosclerotic lesions? Does KLF4-dependent SMC phenotypic switching also play an important role in beneficial outward remodelling of atherosclerotic blood vessels? Are ESC and iPS cell pluripotency factors other than KLF4 also involved in mediating SMC phenotypic switching during atherogenesis? If so, what are the molecular mechanisms that result in activation of expression of KLF4 and other ESC pluripotency factors in mature SMCs given it was believed that these genes including Oct4 undergo stable epigenetic silencing in somatic cells? Is re-activation of pluripotency factors in SMC as well as other cell types a general mechanism that evolved because it optimized survival of organisms by increasing cellular plasticity and injury-repair processes? Indeed, we postulate that the reason it is feasible to reprogramme somatic cells into iPS cells is because normal cells retain at least some capacity to activate and respond to these pluripotency factors to increase cell plasticity in the face of injury, repair, and inflammation, all processes believed to be critical in the aetiology of atherosclerosis. Consistent with this possibility, Peault and co-workers (Crisan et al.)78 have presented evidence, albeit based on results in culture studies, that SMC and pericytes give rise to mesenchymal stem cells. Clearly further studies are needed to address these important questions.
4. The SMC epigenetic signature
Epigenetic mechanisms are defined as a heritable code other than the genomic sequence and include histone post-translational modifications, DNA methylation, ATP-dependent chromatin remodelling, exchange of histone and histone variants, and small RNA molecules.79–82 These epigenetic mechanisms have been implicated in the regulation of gene activation and silencing at the transcription level by regulating chromatin packaging and accessibility. Properties of epigenetic modifications, whatever their types, are the dependence towards environmental cues, reversibility, stability, and heritability through mitosis.83,84 Due to these properties, epigenetics plays a crucial role in cell differentiation and cell lineage determination. Pluripotent cells such as ESC employ several unique histone modification mechanisms for maintaining pluripotency, as well as permissiveness for activation of cell lineage upon appropriate environmental cues.85,86 Thus, acquisition of new epigenetic marks at specific genomic loci permits the selective expression of protein repertoires, acquisition of functional properties, and differentiation into particular cell lineage. It is widely assumed that epigenetic modifications and subsequent chromatin remodelling are key processes in cell differentiation and control cell-specific marker expression due to acquisition of a unique epigenetic signature. In support of this idea, our laboratory was one of the first to explore the role of epigenetic modifications and regulation of the expression of SMC-specific marker genes.87 We provided evidence that chromatin remodelling is a key step in control of the CArG-dependent genes in a retinoic acid-A404 model of early stages of SMC differentiation.88 A404 is an SMC precursor cell line established from multipotent cells (P19) which can be induced to differentiate into SMC.89 In this SMC precursor cell line (A404), none of the SMC marker genes including SM α-actin, SM22α, or SM MHC were detectable, despite the expression of SRF and myocardin.90 Treatment with retinoic acid induces A404 differentiation into SMC with expression of all known SM marker genes. Mechanistically, retinoic acid treatment was associated with hyperacetylation and H3K4 dimethylation (H3K4dime) of histones within the CArG-containing regions of the SM marker genes, and an increase in SRF binding and transcriptional activation. These results provide evidence that coordinate expression of SRF and myocardin alone is not sufficient to control the expression of the SM marker genes but that the regulation of chromatin conformation and transcription factor accessibility to cis-elements via regulation by histone modifications is crucial. However, it remains to be determined whether these mechanisms are also involved in vivo in regulating SMC differentiation and/or SMC phenotypic switching in the context of atherosclerosis or vascular injury.
Of major interest, McDonald et al.76 went on to show that H3K4dime of SMC CArG-containing promoter regions was specific to SMCs and that this epigenetic mark persists even when cultured SMCs are induced to undergo phenotypic switching (Figure 3). Indeed, PDGF-BB-induced phenotypic switching was associated with decreased SM marker gene expression, as well as decreased H4 acetylation, and SRF binding to chromatin. In contrast, PDGF-BB treatment had no effect on H3K4dime of SMC promoter regions. These results provide compelling evidence that H3K4dime of the SM MHC CArG gene locus is an epigenetic histone mark that is a specific marker of SMC lineage identity, at least in cell culture model systems. The challenge is to develop a new methodology and tools permitting the identification of cells bearing the H3K4dime modification of the SM MHC gene in intact tissues and to determine whether this marker is exclusive for SMCs and whether it persists even when SMCs have undergone phenotypic switching.
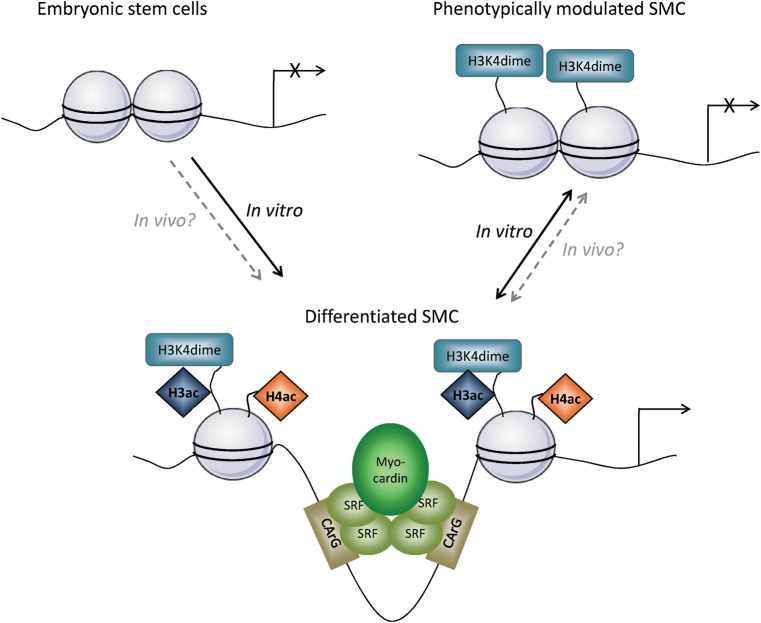
Figure 3.
Epigenetic mechanisms play a key role in SMC differentiation and phenotypic switching. During SMC differentiation, epigenetic modifications including histone acetylation and H3K4dime appear on promoters of SM marker genes such as SM α-actin and SM MHC.76,88 These modifications are thought to induce chromatin relaxation making CArG box regions accessible for binding of SRF/myocardin and other transcriptional activators. SMC phenotypic switching, in response to treatment of cultured SMCs with PDGF-BB, was associated with profound repression of expression of SMC marker genes and loss of H4 acetylation. In contrast, phenotypically modulated SMCs did not show loss of H3K4dime at SMC gene loci, suggesting that this particular epigenetic modification at SMC gene loci is a stable epigenetic signature of the SMC lineage. Although these mechanisms have been well described in vitro, there is so far no definitive evidence that similar processes occur in vivo during phenotypic switching of SMC in the context of atherosclerosis or vascular injury in which there are far more prolonged states of SMC phenotypic modulation when compared with PDGF-BB treatment models in vitro.
5. Conclusions and future directions
We conclude that there are major ambiguities regarding identification of the origins of many of the major cell types within atherosclerotic lesions including which intimal lesion cells are derived from SMC vs. monocytes–macrophages due to the lack of definitive lineage-tracing studies and the fact that the markers routinely used to identify these cell types are not exclusive to either cell type. Whereas BMT experiments have clearly shown that many of the macrophage marker-positive cells within lesions are of haematopoietic origin,20 there is also compelling evidence that other cell types including SMC may also give rise to at least a proportion of these macrophage-like lesion cells. Likewise, there is also evidence that at least some SMC-like cells in lesions are of macrophage and not SMC origin. Evidence of SMC transition into alternative states raises major questions related to the potential participation of SMC in several aspects of human atherogenesis and atherosclerotic lesion progression, including fibrous cap formation but also neovascularization and haemorrhagic events within the advanced lesion.91,92
Finally, we ascertain that in the absence of definitive and rigorous lineage tracing of both cell types, it will be impossible to clearly define the mechanisms and factors that regulate phenotype transitions of these cells within lesions, and their functional roles in plaque development, progression, and end-stage events leading to plaque rupture with possible myocardial infarction or stroke.
Conflict of interest: none declared.
Funding
This work was supported by NIH grants R01 HL57353, R01 HL087867, and R01 HL098538 to G.K.O. D.G. was supported by AHA Postdoctoral Grant 11POST7760009.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 3.Gabbiani G, Schmid E, Winter S, Chaponnier C, de Ckhastonay C, Vandekerckhove J, et al. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci USA. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hungerford JE, Owens GK, Argraves WS, Little CD. Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev Biol. 1996;178:375–392. doi: 10.1006/dbio.1996.0225. [DOI] [PubMed] [Google Scholar]
- 5.Mack CP, Owens GK. Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5′ and first intron promoter regions. Circ Res. 1999;84:852–861. doi: 10.1161/01.res.84.7.852. [DOI] [PubMed] [Google Scholar]
- 6.Arimura C, Suzuki T, Yanagisawa M, Imamura M, Hamada Y, Masaki T. Primary structure of chicken skeletal muscle and fibroblast alpha-actinins deduced from cDNA sequences. Eur J Biochem. 1988;177:649–655. doi: 10.1111/j.1432-1033.1988.tb14419.x. [DOI] [PubMed] [Google Scholar]
- 7.Babij P, Kelly C, Periasamy M. Characterization of a mammalian smooth muscle myosin heavy-chain gene: complete nucleotide and protein coding sequence and analysis of the 5′ end of the gene. Proc Natl Acad Sci USA. 1991;88:10676–10680. doi: 10.1073/pnas.88.23.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen CS, Regan CP, Hungerford JE, White SL, Manabe I, Owens GK. Smooth muscle-specific expression of the smooth muscle myosin heavy chain gene in transgenic mice requires 5′-flanking and first intronic DNA sequence. Circ Res. 1998;82:908–917. doi: 10.1161/01.res.82.8.908. [DOI] [PubMed] [Google Scholar]
- 9.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803–812. doi: 10.1161/01.res.75.5.803. [DOI] [PubMed] [Google Scholar]
- 10.Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1–11. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 11.Miano JM, Carlson MJ, Spencer JA, Misra RP. Serum response factor-dependent regulation of the smooth muscle calponin gene. J Biol Chem. 2000;275:9814–9822. doi: 10.1074/jbc.275.13.9814. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Ip HS, Lu MM, Clendenin C, Parmacek MS. A serum response factor-dependent transcriptional regulatory program identifies distinct smooth muscle cell sublineages. Mol Cell Biol. 1997;17:2266–2278. doi: 10.1128/mcb.17.4.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Loop FT, Schaart G, Timmer ED, Ramaekers FC, van Eys GJ. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol. 1996;134:401–411. doi: 10.1083/jcb.134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78:188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 17.Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36:2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- 18.Perry RL, Rudnick MA. Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci. 2000;5:D750–D767. doi: 10.2741/perry. [DOI] [PubMed] [Google Scholar]
- 19.Andreeva ER, Pugach IM, Orekhov AN. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis. 1997;135:19–27. doi: 10.1016/s0021-9150(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 20.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart HJ, Guildford AL, Lawrence-Watt DJ, Santin M. Substrate-induced phenotypical change of monocytes/macrophages into myofibroblast-like cells: a new insight into the mechanism of in-stent restenosis. J Biomed Mater Res A. 2009;90:465–471. doi: 10.1002/jbm.a.32100. [DOI] [PubMed] [Google Scholar]
- 22.Martin K, Weiss S, Metharom P, Schmeckpeper J, Hynes B, O'Sullivan J, et al. Thrombin stimulates smooth muscle cell differentiation from peripheral blood mononuclear cells via protease-activated receptor-1, RhoA, and myocardin. Circ Res. 2009;105:214–218. doi: 10.1161/CIRCRESAHA.109.199984. [DOI] [PubMed] [Google Scholar]
- 23.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA. 2003;100:13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 25.Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17:1859–1867. doi: 10.1161/01.atv.17.10.1859. [DOI] [PubMed] [Google Scholar]
- 26.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 27.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe M, Sangawa A, Sasaki Y, Yamashita M, Tanaka-Shintani M, Shintaku M, et al. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J Atheroscler Thromb. 2007;14:325–331. doi: 10.5551/jat.e489. [DOI] [PubMed] [Google Scholar]
- 30.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 31.Thomas WA, Florentin RA, Reiner JM, Lee WM, Lee KT. Alterations in population dynamics of arterial smooth muscle cells during atherogenesis. IV. Evidence for a polyclonal origin of hypercholesterolemic diet-induced atherosclerotic lesions in young swine . Exp Mol Pathol. 1976;24:244–260. doi: 10.1016/0014-4800(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 32.Grunwald J, Chobanian AV, Haudenschild CC. Smooth muscle cell migration and proliferation: atherogenic mechanisms in hypertension. Atherosclerosis. 1987;67:215–221. doi: 10.1016/0021-9150(87)90281-4. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz SM, Virmani R, Rosenfeld ME. The good smooth muscle cells in atherosclerosis. Curr Atheroscler Rep. 2000;2:422–429. doi: 10.1007/s11883-000-0081-5. [DOI] [PubMed] [Google Scholar]
- 34.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 35.Bentzon JF, Sondergaard CS, Kassem M, Falk E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circ. 2007;116:2053–2061. doi: 10.1161/CIRCULATIONAHA.107.722355. [DOI] [PubMed] [Google Scholar]
- 36.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 37.Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, et al. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circ. 2010;122:2048–2057. doi: 10.1161/CIRCULATIONAHA.110.965202. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 39.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1beta modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-kappaB-dependent mechanisms. Physiol Genomics. 2012 doi: 10.1152/physiolgenomics.00160.2011. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res. 1992;71:1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 45.Thyberg J, Palmberg L, Nilsson J, Ksiazek T, Sjolund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25:156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 46.Corjay MH, Blank RS, Owens GK. Platelet-derived growth factor-induced destabilization of smooth muscle alpha-actin mRNA. J Cell Physiol. 1990;145:391–397. doi: 10.1002/jcp.1041450302. [DOI] [PubMed] [Google Scholar]
- 47.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 48.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 49.Kozaki K, Kaminski WE, Tang J, Hollenbach S, Lindahl P, Sullivan C, et al. Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice. Am J Pathol. 2002;161:1395–1407. doi: 10.1016/S0002-9440(10)64415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, et al. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida T, Gan Q, Owens GK. Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol. 2008;295:C1175–C1182. doi: 10.1152/ajpcell.00288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clement N, Gueguen M, Glorian M, Blaise R, Andreani M, Brou C, et al. Notch3 and IL-1beta exert opposing effects on a vascular smooth muscle cell inflammatory pathway in which NF-kappaB drives crosstalk. J Cell Sci. 2007;120:3352–3361. doi: 10.1242/jcs.007872. [DOI] [PubMed] [Google Scholar]
- 53.Chen CN, Li YS, Yeh YT, Lee PL, Usami S, Chien S, et al. Synergistic roles of platelet-derived growth factor-BB and interleukin-1beta in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:2665–2670. doi: 10.1073/pnas.0510973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, et al. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 55.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 56.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawai-Kowase K, Owens GK. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C59–C69. doi: 10.1152/ajpcell.00394.2006. [DOI] [PubMed] [Google Scholar]
- 58.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res. 2004;95:981–988. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- 59.Manabe I, Owens GK. CArG elements control smooth muscle subtype-specific expression of smooth muscle myosin in vivo. J Clin Invest. 2001;107:823–834. doi: 10.1172/JCI11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blank RS, McQuinn TC, Yin KC, Thompson MM, Takeyasu K, Schwartz RJ, et al. Elements of the smooth muscle alpha-actin promoter required in cis for transcriptional activation in smooth muscle. Evidence for cell type-specific regulation. J Biol Chem. 1992;267:984–989. [PubMed] [Google Scholar]
- 61.Hautmann M, Madsen CS, Owens GK. A transforming growth factor beta (TGF) control element drives TGF-induced stimulation of SM alpha-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948–10956. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- 62.Hautmann M, Thompson MM, Swartz EA, Olson EN, Owens GK. Angiotensin II-induced stimulation of smooth muscle alpha-actin expression by serum response factor and the homeodomain transcription factor MHox. Circ Res. 1997;81:600–610. doi: 10.1161/01.res.81.4.600. [DOI] [PubMed] [Google Scholar]
- 63.Hautmann MB, Madsen CS, Owens GK. Substitution of the degenerate SM (smooth muscle) alpha-actin CArG elements with c-fos SREs results in increased basal expression but relaxed specificity and reduced angiotensin II inducibility. J Biol Chem. 1998;273:8398–8406. doi: 10.1074/jbc.273.14.8398. [DOI] [PubMed] [Google Scholar]
- 64.Hautmann M, Adam PJ, Owens GK. Similarities and differences in smooth muscle alpha-actin induction by transforming growth factor beta in smooth muscle versus non-muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2049–2058. doi: 10.1161/01.atv.19.9.2049. [DOI] [PubMed] [Google Scholar]
- 65.Mack CP, Thompson MM, Lawrenz-Smith S, Owens GK. Smooth muscle alpha-actin CArG elements coordinate formation of a smooth muscle cell-selective, serum response factor-containing activation complex. Circ Res. 2000;86:221–232. doi: 10.1161/01.res.86.2.221. [DOI] [PubMed] [Google Scholar]
- 66.Swartz EA, Johnson D, Owens GK. Two M-CAT elements of the smooth muscle alpha-actin promoter function differentially in smooth muscle versus non-muscle cells. Am J Physiol. 1998;275:C608–C618. doi: 10.1152/ajpcell.1998.275.2.C608. [DOI] [PubMed] [Google Scholar]
- 67.Johnson AD, Owens GK. Differential activation of the smooth muscle alpha actin promoter in smooth versus skeletal muscle cells by basic helix-loop-helix factors. Am J Physiol. 1998;276:C1420–C1431. doi: 10.1152/ajpcell.1999.276.6.C1420. [DOI] [PubMed] [Google Scholar]
- 68.Jung F, Johnson AD, Kumar MS, Wei BY, Hautmann M, Owens GK, et al. Characterization of an E-box-dependent cis element in the smooth muscle alpha-actin promoter. Arterioscler Thromb Vasc Biol. 1999;19:2591–2599. doi: 10.1161/01.atv.19.11.2591. [DOI] [PubMed] [Google Scholar]
- 69.Cherepanova OA, Pidkovka NA, Yoshida T, Gan Q, Adiguzel E, Bendeck MP, et al. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circ Res. 2009;104:609–618. doi: 10.1161/CIRCRESAHA.108.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas JA, Deaton RA, Hastings NE, Shang Y, Moehle CW, Eriksson UJ, et al. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerotic-prone flow patterns. Am J Physiol Heart Circ Physiol. 2009;296:H442–H452. doi: 10.1152/ajpheart.00165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 76.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshida T, Gan Q, Shang Y, Owens GK. Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am J Physiol Cell Physiol. 2007;292:C886–C895. doi: 10.1152/ajpcell.00449.2006. [DOI] [PubMed] [Google Scholar]
- 78.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 80.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheardown SA, Duthie SM, Johnston CM, Newall AE, Formstone EJ, Arkell RM, et al. Stabilization of Xist RNA mediates initiation of X chromosome inactivation. Cell. 1997;91:99–107. doi: 10.1016/s0092-8674(01)80012-x. [DOI] [PubMed] [Google Scholar]
- 83.Hall IM, Shankaranarayana GD, Noma K, Ayoub N, Cohen A, Grewal SI. Establishment and maintenance of a heterochromatin domain. Science. 2002;297:2232–2237. doi: 10.1126/science.1076466. [DOI] [PubMed] [Google Scholar]
- 84.Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- 85.Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 86.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 87.McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res. 2007;100:1428–1441. doi: 10.1161/01.RES.0000266448.30370.a0. [DOI] [PubMed] [Google Scholar]
- 88.Manabe I, Owens GK. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle-specific regulatory regions during differentiation of a novel P19-derived in vitro smooth muscle differentiation system. Circ Res. 2001;88:1127–1134. doi: 10.1161/hh1101.091339. [DOI] [PubMed] [Google Scholar]
- 89.Blank RS, Swartz EA, Thompson MM, Olson EN, Owens GK. A retinoic acid-induced clonal cell line derived from multipotential P19 embryonal carcinoma cells expresses smooth muscle characteristics. Circ Res. 1995;76:742–749. doi: 10.1161/01.res.76.5.742. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 91.Ho-Tin-Noe B, Le DJ, Gomez D, Louedec L, Vranckx R, El-Bouchtaoui M, et al. Early atheroma-derived agonists of peroxisome proliferator-activated receptor-{gamma} trigger intramedial angiogenesis in a smooth muscle cell-dependent manner. Circ Res. 2011;109:1003–1014. doi: 10.1161/CIRCRESAHA.110.235390. [DOI] [PubMed] [Google Scholar]
- 92.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]