Abstract
Success in breeding crops for yield and other quantitative traits depends on the use of methods to evaluate genotypes accurately under field conditions. Although many screening criteria have been suggested to distinguish between genotypes for their salt tolerance under controlled environmental conditions, there is a need to test these criteria in the field. In this study, the salt tolerance, ion concentrations, and accumulation of compatible solutes of genotypes of barley with a range of putative salt tolerance were investigated using three growing conditions (hydroponics, soil in pots, and natural saline field). Initially, 60 genotypes of barley were screened for their salt tolerance and uptake of Na+, Cl–, and K+ at 150 mM NaCl and, based on this, a subset of 15 genotypes was selected for testing in pots and in the field. Expression of salt tolerance in saline solution culture was not a reliable indicator of the differences in salt tolerance between barley plants that were evident in saline soil-based comparisons. Significant correlations were observed in the rankings of genotypes on the basis of their grain yield production at a moderately saline field site and their relative shoot growth in pots at ECe 7.2 [Spearman’s rank correlation (rs)=0.79] and ECe 15.3 (rs=0.82) and the crucial parameter of leaf Na+ (rs=0.72) and Cl– (rs=0.82) concentrations at ECe 7.2 dS m−1. This work has established screening procedures that correlated well with grain yield at sites with moderate levels of soil salinity. This study also showed that both salt exclusion and osmotic tolerance are involved in salt tolerance and that the relative importance of these traits may differ with the severity of the salt stress. In soil, ion exclusion tended to be more important at low to moderate levels of stress but osmotic stress became more important at higher stress levels. Salt exclusion coupled with a synthesis of organic solutes were shown to be important components of salt tolerance in the tolerant genotypes and further field tests of these plants under stress conditions will help to verify their potential utility in crop-improvement programmes.
Keywords: Barley, hydroponics, osmotic stress, physiological traits, salinity tolerance, screening, soil, specific ion toxicity
Introduction
Broadacre cropping in Australia is based on rainfed systems in a semi-arid environment, where the efficient uptake and use of water is the main driver of productivity. However, more than 60% of the 20 million ha of cropping soils in Australia are sodic which, together with low rainfall and high rates of evapotranspiration, have contributed to the development of transient salinity (Rengasamy, 2002). Saline subsoils adversely affect the ability of crops to use subsoil water and this imposes a significant constraint on productivity. In the last three decades, considerable effort has been directed towards gaining a better understanding of how plants respond to salinity and, in particular, the physiological and molecular bases of salinity tolerance (Munns and Tester, 2008).
A range of engineering and farm management solutions is available to control soil salinity, but their costs and slow adoption mean that substantial soil salinization is inevitable. To maintain crop production in regions with saline soils and water, a genetic approach, involving breeding cultivars with an enhanced ability to grow on salt-affected land, has been proposed in conjunction with the normal reclamation and management practices. The majority of the work on developing selection criteria for improved salt tolerance has been done using solution culture, either in hydroponic or supported hydroponic systems (Munns et al., 2002; Genc et al., 2007), or using sand-based systems (Munns et al., 2002), with the implicit assumption that differences in salinity tolerance expressed in these systems will result in improved performance in the field. Strong evidence to support this is lacking and the ability of solution culture to identify genotypes expressing salt tolerance under stressed conditions in the field needs to be evaluated critically (Gregory et al., 2009). Recently, Tavakkoli et al. (2010a) demonstrated using two genotypes of barley that solution culture may not be able to discern differences in salt tolerance between genotypes of barley that are expressed when grown in soil. However, screening for salt tolerance needs to assess large numbers of genotypes and so it is necessary to examine whether the conclusions based on an assessment of two genotypes is valid when large-scale screening occurs.
Most studies evaluating genetic variation in salt resistance in crop plants have been performed in controlled or semi-controlled environments at a single level of salt stress with no validation of the results under field conditions. Furthermore, studies under controlled conditions generally involve imposing salinization on seedlings over a relatively short period (often 1–2 d) whereas the salinity stress in the field may show a greater level of spatial and temporal variation (Richards, 1983; Flowers and Hajibagheri, 2001; Munns et al., 2002; Genc et al., 2007; James et al., 2008; Rajendran et al., 2009; Kopittke et al., 2011; Tavakkoli, 2011). The variation in salt stress in the field also means that plants can be exposed to a range of salt concentrations at different growth stages, but it is not clear which is the most appropriate salinity level for screening and what stage of development best relates to genetic differences expressed in the field. This information is necessary to develop efficient breeding and selection methods for salt tolerance in crops, and it needs to be compared with the results of studies carried out in naturally saline field environments (Richards, 1983; Richards et al., 1987; El-hendawy et al., 2005).
Efforts to enhance crop yields under salinity stress have also had a limited success because available knowledge of the mechanisms of salt tolerance has not been turned into useful selection criteria to evaluate a wide range of genotypes within and across species. Attempts have been made to evaluate salt tolerance at germination and emergence stages in wheat and barley, and large genotypic differences were reported (Munns et al., 2000; Chen et al., 2008; James et al., 2008), but this early evaluation appears to have little relation to overall performance under saline conditions (Munns et al., 2002). Though Na+ exclusion and K+/Na+ ratios have been suggested to be reliable traits for selecting salt-tolerant crops (Munns et al., 2002; Munns and James, 2003; Poustini and Siosemardeh, 2004), the value of this trait has not been used routinely in plant-breeding programmes. Therefore, there is a need to identify traits associated with salinity tolerance and to develop simple, high-throughput, repeatable screening methods to evaluate a large number of genotypes. Studies on salt tolerance among the major cereals have concentrated on Na+ transport and accumulation, while the role of Cl– in growth and yield reduction of grain crops has been neglected. It is generally considered that Cl– toxicity is not a major cause of reductions in growth of grain crops (Kingsbury and Epstein, 1986; Kinraide, 1999) but some recent work in both field and greenhouse experiments has questioned this assumption (Dang et al., 2008, Tavakkoli et al., 2010b , 2011).
The aim of this work was to examine critically the ability of hydroponic screening to identify differences in salt tolerance in soil, either in pots or in the field. The first experiment evaluated the genotypic variation for salinity tolerance and ion uptake during the early vegetative stage among 60 varieties of barley. On the basis of this initial screen a subset of genotypes was selected for evaluation in soil under controlled conditions and of yield in the field. The experiments investigated possible physiological traits that could be used as screening criteria in selected genotypes in a soil-based experiment and in the field.
Materials and methods
Experiment 1: hydroponic screening
Sixty genotypes of barley were screened for their tolerance to salinity in two individual experiments under identical environmental conditions (Table 1).The genotypes were a selection of varieties and breeding lines that have been used in barley breeding trials in South Australia and were representative of the range of genetic material that has been grown in the region. The pedigrees of these genotypes are diverse, coming from a range of genetic backgrounds. The experiment used a supported hydroponic system (Genc et al., 2007). Plants were grown in cylindrical PVC tubes (4 cm diameter×28 cm depth) filled with cylindrical black polycarbonate pellets (approximately 2–4 mm long and 1–2 mm in diameter) in a series of 50 l tubs each of which contained 42 PVC tubes. Two tubs were served by a single tank of 80 l nutrient solution. Each tub was filled and drained with 25 l of nutrient solution every 30 min. A modified Hoagland’s solution (Tavakkoli et al., 2010a ) was used, the composition of which (in mM) was: NH4NO3 (0.2); KNO3 (5); Ca NO3.2 (2); MgSO4 (2); KH2PO4 (0.1); Na2SiO3 (0.5); NaFe (III)–hydroxyethyl ethylenediamine triacetic acid (HEDTA) (0.05); H3BO3 (0.01); MnCl2 (0.005); ZnSO4 (0.005); CuSO4 (0.0005); and Na2MoO3 (0.0001). Solutions were changed every 7 d, at which time the pH was adjusted to 6.0. The experiment was conducted in a temperature-controlled growth chamber with day/night temperatures of approximately 23/19 °C. The intensity of photosynthetically active radiation was measured using a Li-Cor quantum sensor meter Model LI-1000, Li-Cor, Lincoln, NE, USA. and varied from 550–600 mmol m−2 s−1. Uniformly sized seeds of each genotype were surface-sterilized in 70% ethanol for 1 min, followed by soaking in 3% sodium hypochlorite for 5 min and three lots of rinsing with deionized water. Seeds were germinated on filter paper in Petri dishes at room temperature for 3 d. The seedlings were then transplanted into PVC tubes (one seedling per tube) filled with cylindrical black polycarbonate pellets. A NaCl concentration of 150 mM (EC ∼14.7 dS m−1) was used as the salinity stress treatment. This concentration was selected on the basis of applied salt treatment in most of the current studies on salinity tolerance of barley (Garthwaite et al., 2005; James et al., 2006; Huang et al., 2008; Britto et al., 2010; Munns et al., 2010; Shavrukov et al., 2010). At 8–10 d after transplanting, when the third leaf was beginning to appear, the salt treatment was imposed in increments of 25 mM NaCl per day until the final concentration of 150 mM NaCl was achieved. Supplementary Ca2+ (5 mM) as CaCl2 was added to the NaCl treatment to prevent Ca2+ deficiencies in plants (Munns and James, 2003;Genc et al., 2010). Plants were harvested 49 d after transplanting. The blade of the youngest fully expanded leaf was separately placed in a capped plastic vial. Fresh weight was measured and plants dried at 80 °C for 72 h and dry weights were recorded. The whole shoot moisture content was calculated from the fresh and dry weights. The salt tolerance was calculated as the percentage ratio of shoot dry matter production in salt treatment to control.
Table 1.
The genotypes of barley used in Experiment 1
| Var/line | Origin | Source/Reference |
| Albecta | – | – |
| Arivat | USA | (Aslam et al., 1984) |
| Arta | Syria | (Muehlbauer et al., 2009) |
| Arupo | CIMMYT | – |
| Barque | Australia | (McDonald, 2006) |
| Barque 73 | Australia | (Tavakkoli et al., 2011) |
| Baudin | Australia | – |
| Beecher | Australia | (Rawson, 1986) |
| Briggs | USA | (Lynch and Lauchli, 1985) |
| Buloke | Australia | (Nuttall et al., 2010) |
| California Mariout | North Africa | (Halperin et al., 1997) |
| Capstan | Australia | – |
| Chevron | USA | (Gorham et al., 1994) |
| Cl-3576 | North Africa | – |
| Clipper | Australia | (Tavakkoli et al., 2010a ) |
| Club Mariout | North Africa | (Richards et al., 1987) |
| CM67 | North Africa | (Gorham et al., 1994) |
| CM72 | North Africa | (Cramer et al., 1990) |
| CPI 71284-48 | Iran | (Shavrukov et al., 2010) |
| CPI 77146-32 | Iran | – |
| Dhow | Australia | – |
| Dobla | Spain | (Royo and Aragüés, 1999) |
| Egmont | ICARDA | (Flowers and Hajibagheri, 2001) |
| Er/Apm | Syria | (Othman et al., 2006) |
| Flagship | Australia | – |
| Fleet | Australia | (Ellis et al., 2002) |
| Franklin | Australia | (James et al., 2006) |
| Gairdner | Australia | (Tajbakhsh et al., 2006) |
| Gerbel | UK | (Royo and Aragüés, 1999) |
| H. Spont 41.1 | Syria | – |
| Halycon | UK | – |
| Harmel | Syria | (Othman et al., 2006) |
| Hindmarsh | Australia | – |
| ICARDA 382 | Syria | – |
| ICARDA 391 | Syria | – |
| Kaputar | CIMMYT | – |
| Keel | Australia | (Harris et al., 2010) |
| Maritime | CIMMYT | (Browning et al., 2006) |
| Mundah | Australia | (Harris et al., 2010) |
| O2D/20 | Australia | – |
| Parent 08 | Syria | – |
| Parent 12 | Syria | – |
| Parent 15 | Syria | – |
| Parent 16 | Syria | – |
| Parent19 | Syria | – |
| Prato | USA | (Ramagopal, 1987) |
| Ratna | USA | (Nair and Khulbe, 1990) |
| Sahara | North Africa | (Tavakkoli et al., 2010a ) |
| Schooner | Australia | (James et al., 2006) |
| Skiff | Australia | (Munns and James, 2003) |
| Sloop | Australia | (Jiang et al., 2006) |
| Tadmor | Syria | (Muehlbauer et al., 2009) |
| Vlamingh | Australia | – |
| WI 2198 | Australia | – |
| WI 3416 | Australia | – |
| WI 3788 | Australia | – |
| WI 4262 | Australia | – |
| Yarra | Australia | – |
| YU 6472 | China | (Tajbakhsh et al., 2006) |
The osmotic potential of leaf sap was measured. A disc of Whatman GF/B glass micro-fibre paper was placed in the barrel of a 2 ml plastic syringe so that it covered the outlet hole. A fresh leaf was then put in the barrel, the plunger was re-inserted, and the tip of the syringe was sealed with Blu-Tack® (pressure-sensitive adhesive putty). The syringe was frozen in liquid nitrogen and, still sealed, was thawed to ambient temperature. When temperature equilibration was complete, the plunger and Blu-Tack were removed and the barrel of the syringe was placed in a 15 ml centrifuge tube, with its tip resting inside a 1.5 ml Ependorff tube. After centrifugation at 2500 g for 10 min at 4 °C, the osmolality of a 10 μl sample was measured by a calibrated vapour pressure osmometer (Model 5520; Wescor, Inc., UTAH, USA). Values (mmol kg−1) were converted to megaPascals (MPa) by multiplying by 2.469×10−3 (Genc et al., 2010).
The high performance liquid chromatography HPLC. Dionex DX 500 system consisting of an AS40 Autosampler, GP40 gradient pump, AD20 UV/Visible absorbance detector, ED40 electrochemical detector, and LC20 chromatography enclosure was used to quantify levels of compatible solutes in plants. Immediately following harvest, the leaf sap was extracted as described for osmotic potential measurement. One ml of methanol:chloroform:water (60:25:15 by vol) was added to each sample and the samples were vortexed for 1 min before centrifugation for 10 min at 10 000 g at 4 °C. The supernatant was removed and the samples were freeze-dried. The samples were resuspended in 200 μl of milliQ water prior to injection into the HPLC. A mixture of standards (glycine betaine, sucrose, glucose, fructose, mannitol, trigonelline, and sorbitol), was prepared in methanol:water (50:50, v:v) at 0.5 μg μl−1 for glycine betaine and 2.5 μg μl−1 for the remaining solutes. Ten μl of the standard solution was injected into the HPLC while running each batch of samples. The contribution of organic and inorganic ions to leaf osmotic potential was determined using the van’t Hoff equation, where the calculated contribution of individual solutes to measured Ψs, was based on solute concentration on a molar basis (Marigo and Peltier, 1996).
The dried samples of the youngest fully expanded leaf were digested in 40 ml of 4% nitric acid HNO3. at 95 °C for 4 h in a 54-well HotBlock (Environmental Express, Mt Pleasant, SC, USA). The concentration of Na+ and K+ in the digested samples was determined using a flame photometer (Model 420, Sherwood, Cambridge, UK). Chloride concentrations of the digested extracts were determined using a chloride analyser (Model 926, Sherwood Scientific, Cambridge, UK). Plant standards (Australasian Soil and Plant Analysis Council) were included in every batch of analysis and the recovery of Na+, Cl–, and K+ from these were 95%, 91%, and 92%, respectively.
Experiment 2: responses in growth and ion concentration of 15 genotypes of barley to different soil salinity levels
Based on the results of Experiment 1 as well as previous screening work for salt tolerance (E Tavakkoli, unpublished data; S Coventry, personal communication) a subset of 15 barley genotypes showing different levels of ion exclusion and salt tolerance was selected for further study in soil. The 15 barley genotypes were Fleet, Flagship, Buloke, Hindmarsh, WI4263, Schooner, Parent 19, Gairdner, ODZ/20, Yara, Sloop, Maritime, Capstan, Keel, and Baudin.
The soil of the A horizon topsoil of a non-saline sandy loam red Chromosol (Isbell, 1996). was collected from Roseworthy (34°51′ S, 138°68′ E), South Australia. Following collection, the soil was air-dried and ground to pass through a 5 mm sieve. A soil–water characteristic curve was determined using the pressure plate method (Klute, 1986) and the soil moisture content at field capacity (–10 kPa, equivalent to 37% w/w) was estimated. Basal fertilizer was thoroughly mixed through the soil at the following concentrations (in mg pot−1): NH4NO3 (380), KH2PO4 (229), CaCl2 (131), MgCl2 (332), CuCl2 (10.7), ZnCl2 (11), Na2MoO4 (6.84), and H3BO3 (15). Two salt treatments: moderately saline (ECe ∼7.2 dS m−1) and highly saline (ECe ∼15.3 dS m−1) and a control treatment (ECe ∼1.2 dS m−1) were compared in this experiment. The amounts of NaCl required to achieve the nominal treatments were determined in an assay using 0–2000 mM NaCl and the actual soil. The saline soils were prepared by dissolving NaCl salt in milliQ H2O and spraying the solution on a 2 cm layer of soil to reach field capacity moisture content. Each soil was covered with plastic to control evaporation and left for 3 d at 25 °C to reach equilibrium, then mixed thoroughly and air-dried (Tavakkoli et al., 2010b ). Samples of the saline-synthesized soils were moistened to field capacity (water potential at –10 kPa) and centrifuged at 4000 g for 30 min to extract the soil solution which was passed through 0.25 μm filter paper. Electrical conductivity, ΨO and ion concentrations of the solutions were measured.
The plants were grown in pots, 10.4 cm in diameter and 32 cm deep in which there were two layers of soil; 2200 g of air dry soil (subsoil) which contains the salt treatment and 800 g of untreated soil above (topsoil). Each layer was packed to a bulk density of 1.35 Mg m−3. The subsoil and topsoil were separated by a 3 cm layer of plastic beads 120 g. to prevent salt rising to the topsoil through capillarity action. The top 3 cm of the pot was also covered by plastic beads to minimize the water evaporation from the soil surface. A polypropylene tube (14 cm long, 2 cm internal diameter) was inserted into the upper 10 cm zone of each pot for watering the subsoil and referred to subsequently as a subsoil watering tube. During the first 3 weeks, plants were watered with reverse osmosis (RO) water from the top, but from 20 d after planting, watering was done only through the subsoil watering tube and the topsoil was allowed to dry. This watering method was used to simulate the topsoil drying that occurs in the field.
Uniformly-sized seeds of each genotype were surface sterilized in 70% ethanol for 1 min, followed by soaking in 3% sodium hypochlorite for 5 min, then rinsed three times with deionized water. Five barley seeds were sown in each pot and thinned to three per pot after 5 d. The experiment was conducted under the same growth conditions as described in experiment 1. The pots were weighed and watered to 90% (weeks 1–4) and 65% (weeks 5–10) of field capacity regularly and daily water use calculated. Plants were grown for 10 weeks after germination. Three harvests were taken at 30, 50, and 70 d after germination, respectively. At each harvest, the fully expanded youngest leaf blade and the whole shoot were sampled for measurements of biomass, ion concentration, osmotic potential, and organic solutes as explained in Experiment 1. The experimental design was a factorial, completely randomized design comprised of three treatment×15 barley genotypes with three replicates, giving a total of 135 pots.
Experiment 3: field study
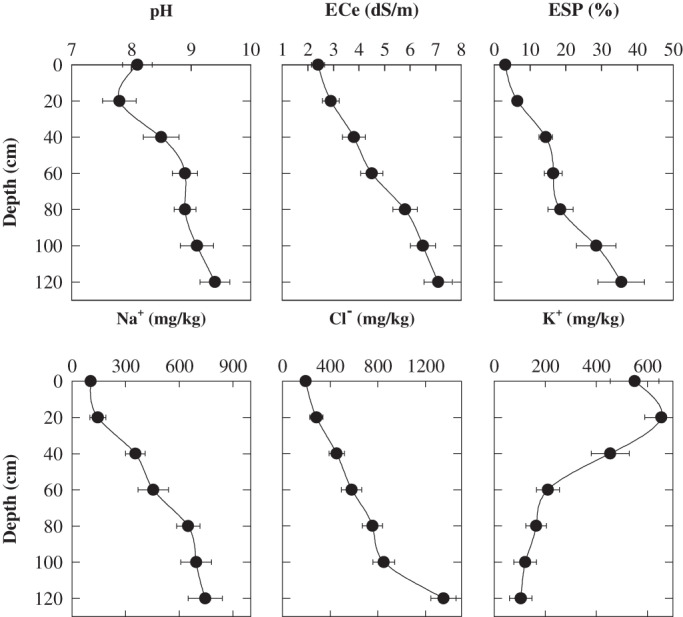
A field trial was conducted to assess the genotypic variation among 13 barley genotypes (selected from Experiment 2) in response to salinity stress at Hart, South Australia (latitude 33o75′ S and longitude 138o41' E). The region has a Mediterranean-type climate and received 404 mm of rainfall in 2009, compared to the long-term average of 460 mm. The soil at Hart is a calcareous gradational clay loam, classified as Vertic, Pedal, Hypercalcic Calcarosol (Isbell, 1996). and is the most extensive soil of the region (Hall et al., 2009). The topsoil is alkaline, non-saline, and non-sodic but the subsoil is strongly alkaline (pH ≥ 9), saline (ECe ∼7.7 dS m−1), and sodic exchangeable Na+ percentage ∼35%. (Fig. 1). A randomized, complete block design with four replications was used. The trial was sown using a custom-built cone seeder using a sowing depth of 30 mm. Basal fertilizer was applied with the seed as 12 kg P ha−1 of triple superphosphate (N:P:K:S=0:17:0:0). Granular urea (46:0:0:0) was applied by hand immediately prior to sowing and as a post-emergent application. Sowing rate was adjusted based on individual seed weight and germination percentage with the aim of establishing 180 plants m−2. The plots were 6 rows×20 m with an inter-row width of 22.5 cm and an inter plot width of 25 cm. Weeds and disease, when present, were controlled by a range of herbicides and fungicides.
Fig. 1.
The selected physical and chemical characteristics of soil at Hart site. All the analyses were made on soil solution extracted from saturated paste extract. The bars are standard errors of the means (n=10).
At Zadoks growth stages (ZGS) (45 booting), 65 (50% anthesis), and 92 (grain ripe), five randomly-selected plants from each plot were sampled (Zadoks et al., 1974). The plants were washed and separated into the upper and lower leaves of the main stem for dry weight measurements, ionic analysis, leaf osmotic potential, and organic solutes as explained in Experiment 1.
At ZGS65, ten soil cores were randomly taken from a soil depth of 0–100 cm. Electrical conductivity (ECe), pH, soluble Na+, Ca2+, and Mg2+ were determined in a saturated paste extract. ESP was calculated from the values of soluble Na+, Ca2+, and Mg2+ according to Rowell (1994). Chloride concentration was measured using a chloride analyser (Model 926, Sherwood Scientific, Cambridge, UK). The plots were machine harvested using a Wintersteiger plot harvester to determine grain yield.
Statistical analysis
Statistical analyses were performed in R 2.10.1 (R Development Core Team, 2006). Data for growth, ion content, and moisture content were analysed using two-way ANOVA to determine if significant differences were present among means. Variances were checked by plotting residual versus fitted values to confirm the homogeneity of the data. Differences among the mean values were assessed by Least Significant Differences LSD). Relationships between individual variables were examined using simple linear correlations and regressions which were performed using SigmaPlot version 12.1). Spearman’s rank correlation test (rs) was used to examine consistency in the rankings of genotypes for salt tolerance and grain yield between the three experiments. The heritability of salt-tolerant traits were estimated by using of the residual maximum likelihood (REML) statistical method to obtain unbiased estimates of the variance components and , and the best linear unbiased predictions (BLUPs) of the performance of the 60 genotypes (replicated in two identical experiments) in the first experiment and 15 genotypes in the second experiment. Broad sense heritability was estimated as h 2=/( +). The significance of genetic variability among genotypes was assessed from the standard error of the estimate of genetic variance , assuming the ratio /SE () to be normally distributed (Krishnamurthy et al., 2007).
Results
Hydroponics
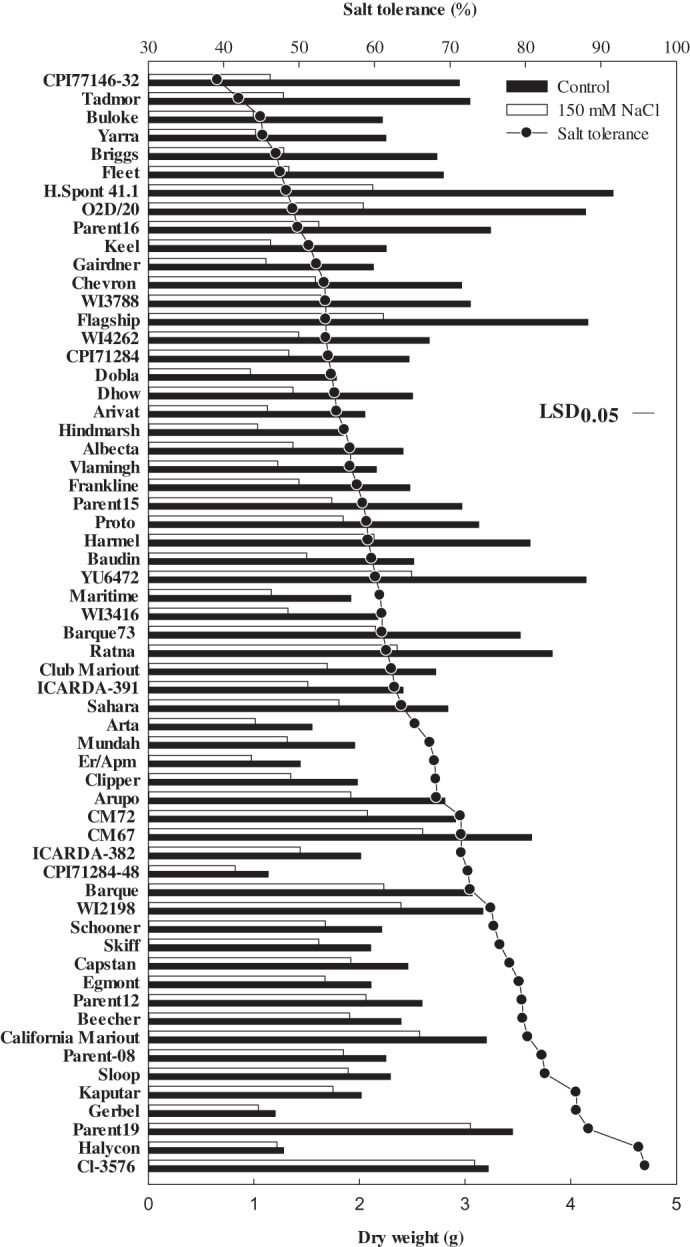
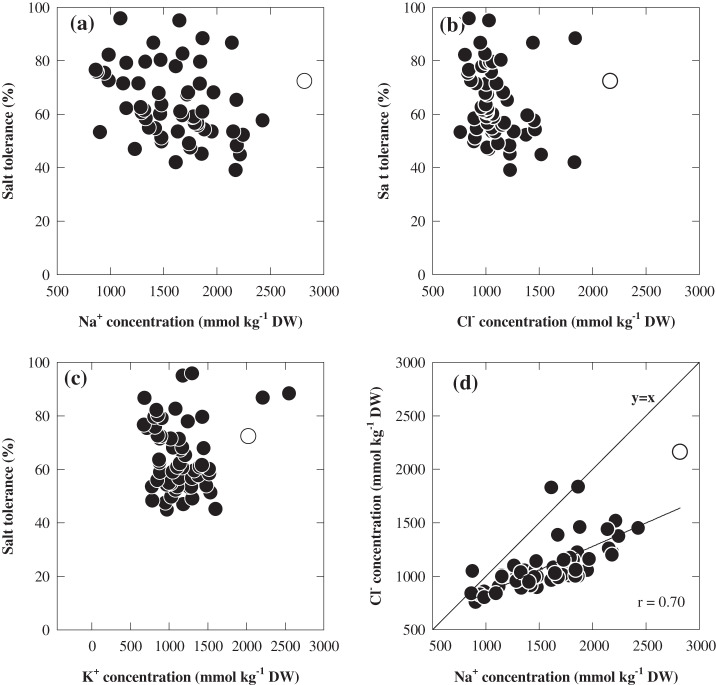
Large genotypic variation in salt tolerance was evident (Fig. 2) and it ranged from 39% in CPI77146-32 to 95% in Halycon and Cl-3576 (Fig. 2). The Na+ concentration in the youngest fully expanded leaf varied over 3.5-fold among the 60 genotypes (Fig. 3a; see Supplementary Table 1S at JXB online), ranging from 862 mmol kg−1 DW in Skiff to 2818 mmol kg−1 DW in CPI71284-48. There was also more than a 2.8-fold variation in the concentrations of Cl–, ranging from 759 mmol kg−1 DW in Chevron to 2162 mmol kg−1 DW in CPI71284-48 (Fig. 3b; see Supplementary Table 1S at JXB online). The heritability of salt tolerance (relative shoot biomass) was 0.46 (Table 2). The salinity tolerance of barley genotypes was not associated with their ability to exclude Na+ and/or Cl– (Fig. 3a, b) or with variation in K+ concentrations (Fig. 3c; see Supplementary Table 1S at JXB online). The concentrations of Na+ and Cl– were significantly related (P <0.01), with Cl- concentrations being lower than Na+ concentrations except in two genotypes (Parent 19 and Tadmor) which had similar concentrations of Na+ and Cl– (Fig. 3d).
Fig. 2.
The range in dry matter production vertical bars and salinity tolerance line-scatter plot of 60 genotypes of barley grown in supported hydroponic system for 7 weeks. The salt tolerance was calculated as the ratio of dry matter production under 150 mM NaCl treatment white bars to control condition black bars). The coefficient of variation of experiment was 4.15%. Values are means (n=4).
Fig. 3.
The relationship between whole plant salt tolerance and shoot concentration of (a) Na+ (mmol kg−1 DW), (b) Cl– (mmol kg−1 DW),(c). K+ (mmol kg−1 DW), and (d) relationship between shoot Na+ and Cl– concentration of 60 barley genotypes grown at 150 mM NaCl for 7 weeks in a supported hydroponic system. The open circle is a genotype of the wild barley (Hordeum spontaneum) and the closed circles are domesticated genotypes of barley (Hordeum vulgare). Values are means (n=4).
Table 2.
The values of heritability (h 2) for salt tolerance shoot biomass under salinity/shoot biomass under control., shoot concentration of Na+, Cl–, K+, and Leaf osmotic potential for plants grown in hydroponic, pot and in the field
| ST | [Na+] | [Cl–] | [K+] | Leaf osmotic potential | |
| Hydroponic | 0.46 | 0.35 | 0.33 | 0.22 | 0.35 |
| Pot experiment EC 7.2 | 0.66 | 0.75 | 0.79 | 0.35 | 0.51 |
| (Harvest 3) | |||||
| Pot experiment EC 15.3 | 0.61 | 0.71 | 0.75 | 0.28 | 0.65 |
| (Harvest 3) | |||||
| Field | – | 0.68 | 0.75 | 0.21 | 0.55 |
Pot experiment
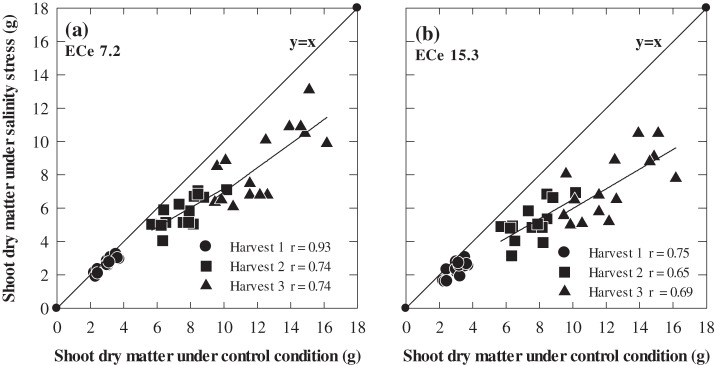
A significant linear relationship was found between biomass production of 15 genotypes under salinity and under non-saline conditions at all three harvests, however, the range of variation and ranking of genotypes varied significantly. The largest variation in salt tolerance was found at 70 d after sowing (Harvest 3) and it varied from 56% in Flagship and Schooner to 89% in WI4262 at ECe 7.2, and 43% in Schooner to 84% in WI4262 at ECe 15.3, respectively (Fig 4; see Supplementary Table 2S at JXB online).
Fig. 4.
The relationship between shoot dry matter of 15 genotypes of barley under non-saline stress and two levels of soil salinity (a) ECe 7.2 and (b) ECe 15.1 dS m−1 at 30 d (Harvest 1), 50 d (Harvest 2), and 70 d (Harvest 3) after sowing. Values are means (n=3).
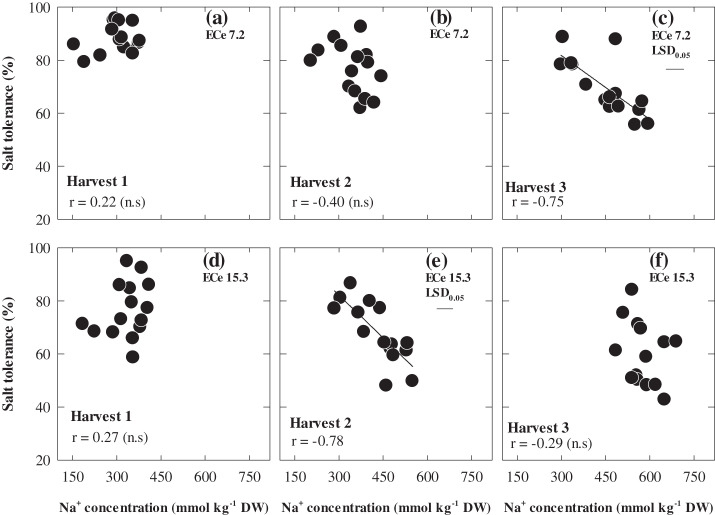
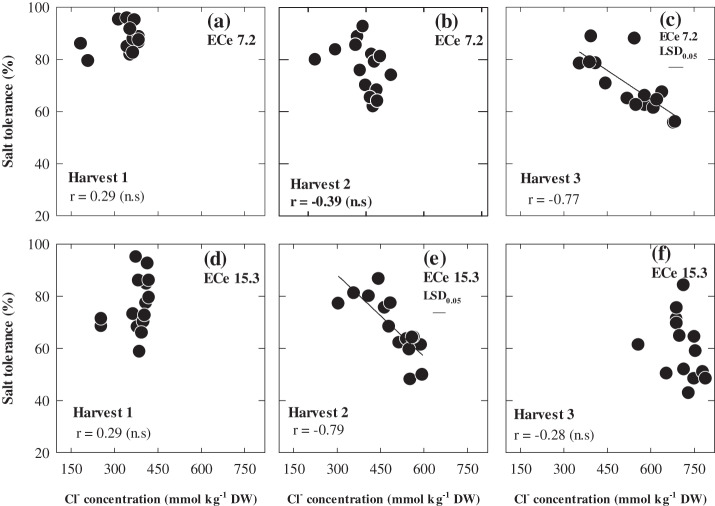
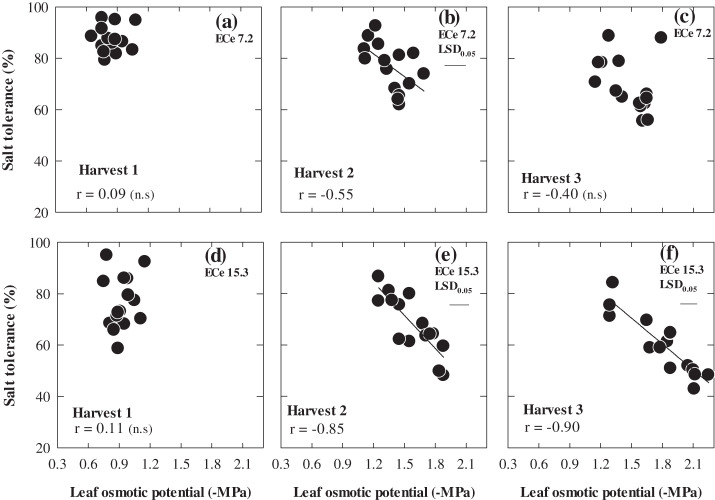
The concentrations of Na+ and Cl– in the youngest fully expanded leaf increased with successive harvests, but at each harvest there was up to 2-fold variation in Na+ and a 1.7-fold variation in Cl– concentrations among the 15 genotypes (Figs 5, 6). Plant Na+ and Cl– concentrations under saline conditions were significantly and negatively correlated with salt tolerance only at 70 d after sowing at ECe 7.2 and at 50 d after sowing at ECe 15.3 dS m−1. In both cases, the mean Na+ and Cl– was ∼450 mM. WI4262, Hindmarsh, and Capstan which were the most tolerant varieties also had the lowest leaf Na+ and Cl– concentrations. Plant Na+ and Cl– concentrations were low in the control treatment and did not show any relationships either with the shoot biomass ratio or actual shoot biomass under control. Leaf osmotic potential varied significantly among genotypes and it was significantly related to salt tolerance only at the second harvest at ECe 7.2, albeit weakly, and more strongly at the second and third harvest at ECe 15.3 (Fig. 7; P ≤0.001). Shoot K+ concentration and K+:Na+ ratios at the third harvest under ECe 7.1 were significantly related to salt tolerance r=0.70, P <0.05 whereas it was not related to salt tolerance at ECe 15.1 (data not shown). The heritability of salt tolerance (relative shoot biomass) increased with each successive harvest, with values of 0.36, 0.47, and 0.66.
Fig. 5.
The relationship between whole plant salt tolerance and leaf Na+ concentrations of 15 genotypes of barley at 30 d (Harvest 1), 50 d (Harvest 2), and 70 d (Harvest 3) after sowing under two ECe levels (7.2 and 15.3 dS m−1). Values are means (n=3).
Fig. 6.
The relationship between whole plant salt tolerance and leaf Cl– concentrations of 15 genotypes of barley at 30 d (Harvest 1), 50 d (Harvest 2), and 70 d (Harvest 3) after sowing under two ECe levels (7.2 and 15.3 dS m−1). Values are means (n=3).
Fig. 7.
The relationship between whole plant salt tolerance and leaf osmotic potential of 15 genotypes of barley at 30 d (Harvest 1), 50 d (Harvest 2), and 70 d (Harvest 3) after sowing under two ECe levels (7.2 and 15.3 dS m−1). Values are means (n=3).
Field study
Genotypic variation in ion concentration and leaf osmotic potential in relation to grain yield
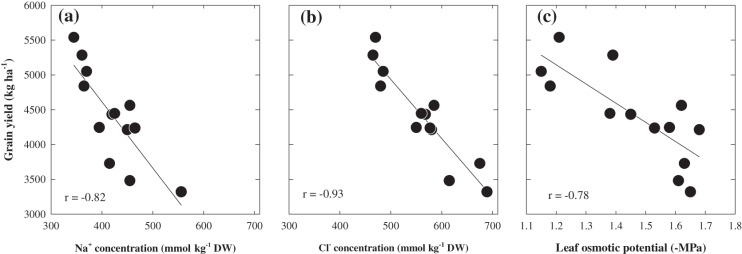
There was a wide range in plant grain yield and Na+ and Cl– concentrations among the 13 genotypes (Fig. 8; see Supplementary Table 3S at JXB online). Grain yield production ranged from 3320 kg ha−1 in Maritime to 5538 kg ha−1 in Capstan. Significant genotypic variation occurred in Na+ and Cl– concentrations as well as osmotic potential of the flag leaf blade (Fig. 8). Sodium concentrations varied widely, ranging from 345 to 556 mmol kg−1 DW. Cl– concentration also varied about 1.5-fold ranging from 415 to 670 mmol kg−1 DW. As in Experiment 2, leaf osmotic potential varied significantly ranging from –1.2 to –1.65 MPa. Leaf Na+ and Cl– concentrations and osmotic potential were negatively related to the grain yield (Fig. 8; P ≤0.001). The heritability values of the Na+ and Cl– concentrations of the flag leaf blade were 0.68 and 0.75 (Table 2).
Fig. 8.
The relationship between grain yield and leaf concentration of (a) Na+ concentration (mmol kg−1 DW), (b) Cl– concentration (mmol kg−1 DW), and (c) leaf Ψs –MPa of 13 barley genotypes grown at Hart site in 2009. The results are from youngest emerged leaves at ZGS 65. Fitted curves are derived from linear regression. The horizontal and vertical bars are LSD at 95% for the ion explanatory and dependent variable respectively. Values are averages (n=4).
Contribution of organic and inorganic solutes to leaf osmotic potential under salt stress
Analysis of the sap of leaf tissue showed that the concentrations of sucrose, glucose, fructose, betaine, and proline increased markedly in response to salinity treatments. The contribution of organic and inorganic solutes to leaf osmotic potential Ψs. was assessed for all genotypes but just the results from three tolerant (WI4262, Capstan, and Fleet) and three sensitive (Flagship, Schooner, and Maritime) varieties identified in Experiment 2 are reported (Table 3). The mean leaf Ψπ was less in the hydroponic experiment (–2.74 MPa) compared with the pot experiment (–1.45 at ECe 7.2 dS m−1, and –1.75 MPa at 15.3 dS m−1) and the field experiment (–1.38 MPa). In the hydroponic experiment, inorganic solutes accounted for 88–95% of the measured total solute potential in all genotypes whereas organic ion contribution to measured Ψs was only 3–8%. In contrast, the contribution of organic solutes to leaf Ψs in both ECe levels of Experiment 2 and in the field was significantly higher and ranged from 4-40%. The ranges in the contribution from organic osmolytes in Experiment 2 and the field experiment were similar. Three tolerant and high-yielding varieties Capstan, WI4262, and Fleet which had a better ability to maintain lower leaf Na+ and Cl– concentration also showed greater contribution from organic solutes to contribute to total Ψs. However, in Flagship, Schooner, and Maritime, the major contribution to Ψs was from the high concentrations of Na+ and Cl– (Table 3).
Table 3.
Estimated contribution of organic and inorganic ions to leaf osmotic potential (Ψs ±SEM)
| Leaf OP | Na+ | Cl– | K+ | Sucrose (MPa) | Glucose | Fructose | Betaine | Proline | Inorganic contribution | Organic contribution ( %) | Total | |
| Hydroponic experiment (49 d after germination) | ||||||||||||
| WI4262 | –2.88±0.23 | –0.81 | –0.88 | –0.85 | –0.02 | –0.01 | –0.01 | –0.01 | –0.05 | 88 | 3 | 92 |
| Capstan | –2.65±0.21 | –0.78 | –0.81 | –0.75 | –0.03 | –0.05 | –0.01 | –0.02 | –0.06 | 88 | 6 | 95 |
| Fleet | –2.85±0.20 | –0.85 | –0.7 | –0.81 | –0.05 | –0.03 | –0.04 | –0.008 | –0.11 | 83 | 8 | 91 |
| Flagship | –2.87±0.21 | –0.86 | –0.85 | –0.71 | –0.05 | –0.06 | –0.04 | –0.02 | –0.05 | 84 | 8 | 92 |
| Schooner | –2.57±0.25 | –0.83 | –0.85 | –0.75 | –0.02 | –0.02 | –0.01 | –0.005 | –0.04 | 95 | 4 | 98 |
| Maritime | –2.61±0.26 | –0.85 | –0.79 | –0.75 | –0.05 | –0.02 | –0.01 | –0.008 | –0.05 | 92 | 5 | 97 |
| Pot experiment EC 7.2 Harvest 3 (70 d after germination) | ||||||||||||
| WI4262 | –1.28±0.03 | –0.18 | –0.22 | –0.38 | –0.09 | –0.06 | –0.09 | –0.11 | –0.03 | 61 | 30 | 91 |
| Capstan | –1.38±0.05 | –0.15 | –0.19 | –0.44 | –0.11 | –0.09 | –0.08 | –0.12 | –0.05 | 57 | 33 | 89 |
| Fleet | –1.21±0.04 | –0.16 | –0.21 | –0.40 | –0.12 | –0.10 | –0.11 | –0.05 | –0.02 | 64 | 33 | 97 |
| Flagship | –1.59±0.06 | –0.35 | –0.42 | –0.31 | –0.08 | –0.05 | –0.04 | –0.01 | –0.06 | 68 | 15 | 83 |
| Schooner | –1.58±0.08 | –0.48 | –0.50 | –0.31 | –0.02 | –0.05 | –0.02 | –0.02 | –0.02 | 82 | 8 | 90 |
| Maritime | –1.66±0.09 | –0.52 | –0.65 | –0.32 | –0.05 | –0.02 | –0.01 | –0.002 | –0.01 | 90 | 6 | 95 |
| Pot experiment EC 15.3 Harvest 3 (70 d after germination) | ||||||||||||
| WI4262 | –1.32±0.03 | –0.18 | –0.31 | –0.29 | –0.07 | –0.09 | –0.11 | –0.15 | –0.05 | 59 | 36 | 95 |
| Capstan | –1.65±0.05 | –0.21 | –0.29 | –0.35 | –0.11 | –0.09 | –0.10 | –0.18 | –0.11 | 52 | 36 | 87 |
| Fleet | –1.29±0.06 | –0.21 | –0.28 | –0.25 | –0.15 | –0.08 | –0.09 | –0.15 | –0.05 | 57 | 40 | 98 |
| Flagship | –2.25±0.08 | –0.49 | –0.59 | –0.35 | –0.11 | –0.13 | –0.15 | –0.01 | –0.02 | 64 | 19 | 82 |
| Schooner | –1.88±0.09 | –0.48 | –0.51 | –0.45 | –0.05 | –0.08 | –0.05 | –0.04 | –0.05 | 77 | 14 | 91 |
| Maritime | –2.11±0.08 | –0.60 | –0.71 | –0.35 | –0.04 | –0.05 | –0.04 | –0.05 | –0.05 | 79 | 11 | 90 |
| Field experiment (ZGS 65) | ||||||||||||
| WI4262 | –1.15±0.05 | –0.25 | –0.21 | –0.28 | –0.05 | –0.08 | –0.09 | –0.05 | –0.09 | 64 | 31 | 96 |
| Capstan | –1.21±0.09 | –0.22 | –0.25 | –0.31 | –0.08 | –0.05 | –0.05 | –0.02 | –0.11 | 64 | 26 | 90 |
| Fleet | –1.18±0.11 | –0.15 | –0.23 | –0.25 | –0.11 | –0.07 | –0.07 | –0.05 | –0.12 | 53 | 36 | 89 |
| Flagship | –1.45±0.10 | –0.33 | –0.4 | –0.25 | –0.05 | –0.08 | –0.05 | –0.07 | –0.08 | 68 | 23 | 90 |
| Schooner | –1.62±0.15 | –0.45 | –0.51 | –0.25 | –0.02 | –0.05 | –0.05 | –0.09 | –0.08 | 75 | 18 | 93 |
| Maritime | –1.65±0.13 | –0.49 | –0.55 | –0.22 | –0.05 | –0.08 | –0.05 | –0.08 | –0.05 | 76 | 19 | 95 |
The contribution of individual solutes to measured Ψs was determined using the van’t Hoff equation, where the calculated Ψs, was based on solute concentration on a fresh weight basis. The percentage value is based on the measured value of leaf Ψs.
Consistencies and discrepancies between the three systems
Despite the imposition of ionic stress to approximately the same degree, based on the EC of the respective solutions in hydroponics and soil culture, the genotypic variation in salt tolerance was much greater in soil as evidenced by the much greater salt×genotype interaction. Screening in the pot experiment identified three salt-tolerant genotypes Capstan, Fleet, and WI4262. which were also identified as high-yielding genotypes at a saline site in the field. However, there was also a large discrepancy between hydroponic-based ranking of seedlings and soil-culture-based ranking of seedling when salt tolerance was expressed as relative growth. For example, the cultivars Fleet and WI4262 were two of the sensitive genotypes in hydroponics, but overall were the most tolerant and high-yielding varieties in soil and in the field.
To quantify further the relation between salt tolerance of seedlings in hydroponics and plants in pot screening with grain yield in the field the phenotypic correlation of genotypic means among the three techniques were examined (Table 4). Shoot Na+ and Cl– concentrations and leaf osmotic potential of plants grown in the field and soil-culture were significantly correlated to grain yield production and salt tolerance (Table 4). By contrast, there were no significant correlations between those traits from plants grown in hydroponics and grown in soil-culture or in the field. There was no significant correlation between leaf K+ concentration and grain yield of plants grown in the field (Table 4). The ranking of genotypes for their salt tolerance was evaluated by Spearman’s rank test (Table 5). The ranking of 13 genotypes grown in all three experiments on the basis of grain yield in the field was significantly correlated with their ranking on the basis of their salt tolerance at two different ECe levels in pot experiment. By contrast, the ranking of the 13 genotypes in hydroponic experiment differed completely from soil-culture and field screening (Table 5).
Table 4.
Correlation coefficients (r) between pairs of physiological attributes of salt-stressed barley plants grown in different cultures
| Experiment | Field | Pot ECe 7.2 | |||||||||||
| Grain yield | Leaf OP | Na+ | Cl– | K+ | K+:Na+ | ST | Na+ | Cl– | K+ | K+:Na+ | Leaf OP | ||
| Field | Gain yield | ||||||||||||
| Leaf OP | –0.78** | ||||||||||||
| Na+ | –0.82** | 0.74** | |||||||||||
| Cl– | –0.93*** | 0.82** | 0.84** | ||||||||||
| K+ | –0.31 | 0.15 | 0.11 | 0.34 | |||||||||
| K+:Na+ | 0.75** | –0.73** | –0.94*** | –0.74** | 0.20 | ||||||||
| Pot ECe 7.2 | ST | 0.78** | –0.88** | –0.71** | –0.86** | –0.02 | 0.74** | ||||||
| Na+ | –0.77** | 0.88** | 0.78** | 0.85** | 0.13 | –0.77** | –0.91*** | ||||||
| Cl– | –0.87** | 0.89** | 0.80** | 0.91*** | 0.29 | –0.74** | –0.89** | 0.94*** | |||||
| K+ | 0.55* | –0.70** | –0.57* | –0.67** | –0.07 | 0.62* | 0.72** | –0.78** | –0.76** | ||||
| K+:Na+ | 0.71** | –0.87** | –0.72** | –0.80** | –0.06 | 0.75** | 0.90*** | –0.94*** | –0.91*** | 0.93*** | |||
| Leaf OP | –0.75** | 0.80** | 0.63* | 0.79** | 0.37 | –0.54* | –0.74** | 0.80** | 0.77** | –0.73** | –0.80** | ||
| Pot ECe 15.3 | ST | 0.76** | –0.83** | –0.66* | –0.74** | –0.06 | 0.66* | 0.90*** | –0.88** | –0.85** | 0.58* | 0.82** | –0.75** |
| Na+ | –0.37 | 0.46 | 0.69** | 0.52* | –0.05 | –0.69** | –0.43 | 0.55* | 0.43 | –0.66* | –0.60 | 0.54 | |
| Cl– | –0.35 | 0.29 | 0.36 | 0.37 | 0.20 | –0.32 | –0.22 | 0.46 | 0.47 | –0.71** | –0.56 | 0.55 | |
| K+ | 0.18 | –0.31 | –0.16 | –0.06 | –0.37 | 0.07 | –0.12 | 0.02 | –0.17 | 0.01 | 0.00 | –0.06 | |
| K+:Na+ | 0.46 | –0.57 | –0.72 | –0.52* | –0.20 | 0.65* | 0.32 | –0.50 | –0.51 | 0.63* | 0.56 | –0.56 | |
| Leaf OP | –0.63* | 0.83** | 0.59* | 0.69** | 0.21 | 0.23 | 0.08 | –0.14 | –0.05 | 0.10 | –0.79** | 0.77** | |
| Hydroponic | ST | –0.12 | 0.11 | –0.08 | –0.01 | 0.39 | 0.23 | 0.08 | –0.14 | –0.05 | 0.10 | 0.10 | 0.35 |
| Na+ | 0.30 | –0.25 | –0.23 | –0.19 | –0.29 | 0.09 | 0.08 | –0.03 | 0.00 | –0.22 | –0.08 | –0.41 | |
| Cl– | –0.01 | 0.08 | 0.07 | 0.00 | –0.35 | –0.22 | –0.06 | 0.28 | 0.14 | –0.26 | –0.25 | 0.08 | |
| K+ | 0.10 | 0.27 | –0.02 | 0.10 | –0.18 | –0.05 | –0.20 | 0.25 | 0.21 | –0.25 | –0.25 | –0.02 | |
| K+:Na+ | –0.38 | 0.53* | 0.33 | 0.36 | 0.20 | –0.24 | –0.32 | 0.25 | 0.22 | 0.02 | –0.15 | 0.47 | |
| Leaf OP | –0.10 | 0.20 | 0.11 | 0.03 | 0.32 | –0.07 | 0.09 | –0.09 | 0.08 | –0.23 | –0.09 | 0.11 | |
*, **, *** Significant at 0.05, 0.01, and 0.001 levels, respectively (n=13).
Table 5.
The Spearmans’s rank correlations between grain yield of field-grown plants and salt tolerance of plants grown in the hydroponic and pot (n=13)
| Field Grain yield | Pot ECe 7.2 Salt tolerance | Pot ECe 15.3 Salt tolerance | ||
| POT ECe 7.2 | Salt tolerance | 0.79** | ||
| POT ECe 15.3 | Salt tolerance | 0.82** | 0.81** | |
| Hydroponic | Salt tolerance | –0.22 | 0.11 | –0.12 |
Discussion
A critical aspect of improving the salt tolerance of crop plants is identifying the intraspecific differences in growth under salt stress. Selecting the most effective procedure to do this, based on specific physiological factors and which predicts the differences in salt tolerance in the field, is often overlooked, yet arguably this is the crucial step in developing robust screening methods for salt tolerance. Further, the relative importance of different mechanisms can vary between closely related species (Rush and Epstein, 1981) and varieties (Yeo and Flowers, 1983) and also with the severity of salinity stress (Tavakkoli et al., 2010a ). The study reported here addressed three important questions for the use of physiological mechanisms as selection criteria for improving salt tolerance. (i) Is there genetic variation in the expression of the mechanism? (ii) Is the targeted mechanism important in affecting the whole plant tolerance to salinity? (iii) Is the screening method used for selecting a tolerant variety with a specific mechanism of salt tolerance under controlled conditions able to predict grain yield in the field?
Screening in hydroponics failed to predict differences in salt tolerance and ion uptake in soil, whether under controlled conditions or in the field. The hydroponic method used—exposing seedlings to short periods of salinity stress—is one commonly used to assess salt tolerance and to examine the mechanisms of salt tolerance among genotypes. Despite the emphasis that has been placed on Na+ and/or Cl– exclusion as a selection criterion for salt tolerance (Munns et al., 2006), no relationship was observed between the level of exclusion and salt tolerance in the genotypes used in hydroponic study based on early growth (Fig. 3). The different rankings in salt tolerance and in the relationships between ion concentration and salt tolerance between soil and hydroponics suggest there are fundamental differences in the nature of the two systems that influences the responses to salinity between the plants grown. This has important implications for the development of salt-tolerant germplasm and for elucidating the relative importance of the mechanisms of salt tolerance in the field.
Genetic differences in Na+ and Cl– exclusion among barley genotypes were not associated with salt tolerance in hydroponics (Fig. 3). A similar result has been found for wheat (Genc et al., 2007), which brings into question the rationale for using hydroponic screening, at least at the salt concentrations commonly used in much of the current work (100–150 mM NaCl). By contrast, in the pot and field experiments, genetic differences in Na+ and Cl– exclusion and their association with plant growth were expressed (Figs 5, 6). However, just as important was the observation that the rankings in Na+ and Cl– exclusion in hydroponics were unrelated to the rankings found in soil and in the field. Genetic differences in Na+ exclusion have been previously demonstrated in hydroponic studies (Schachtman et al., 1991; Houshmand et al., 2005; Munns et al., 2006; Genc et al., 2007), but the different results for selected barley genotypes suggest that the level of discrimination is much lower in hydroponics than in soil (Rivandi et al., 2010, Tavakkoli et al., 2010a ), although the cause of this difference between the two systems is not yet understood. The concentrations of Na+ and Cl– in leaves of the hydroponically-grown plants were much greater than those in soil- and field-grown plants. Drew and Lauchli (1985) showed an oxygen-dependent exclusion of Na+ ion from shoots by roots of maize. Under fully aerobic conditions, roots partially excluded Na+ from the shoots over a wide range of NaCl concentration 0.2–200 mM). With root anoxia, the exclusion mechanism broke down so that much greater amounts of Na+ reached the shoots, with the simultaneous inhibition of K+ transport. While the supported hydroponic system used in this study and by many other researchers (Munns et al., 2002; Genc et al., 2007) was filled and drained with 25 l of nutrient solution every 30 min to provide aeration, the quantity of oxygen at the root surface may not be sufficient for an efficient Na+ exclusion. Moreover, for soil-grown plants, the salt concentration in the soil solution may not only change due to mass flow exceeding uptake, but also as a result of decreasing water content in the vicinity of the roots due to high transpirational demand and low unsaturated hydraulic conductivity. This does not occur in solution culture, where the matric potential is zero as is resistance to water movement (Vetterlein et al., 2004).
Maintenance of high K+ concentrations in salt-tolerant genotypes was observed only among plants grown in soil at ECe 7.2 dS m-1 in Experiment 2, which may be one of the mechanisms underlying their higher salt tolerance (Table 4). However, for plants grown in hydroponics, at a soil ECe ∼15.3 dS m−1 and in the field there was no significant relationship between salt tolerance and/or grain yield and the shoot concentrations of K+. The ratio of K+:Na+ has been associated with salt tolerance (Gorham et al., 1990; Dvořak et al., 1994; Chen et al., 2007), however, in the current study, the significant positive correlation between grain yield and K+:Na+ appears to be due to the genotypic variation in shoot Na+ concentration rather than maintenance of high shoot K+ concentration: there was a negative correlation between salt tolerance and Na+ and no significant relationship with K+.
In soil-grown plants measuring the biomass production at 70 d after sowing (Harvest 3) showed the strongest relationship with salt tolerance, and has revealed substantial variation among genotypes at both levels of salinity stress which was also predictive of grain yield in the field (see Supplementary Table S3 at JXB online). However, there was no significant correlation between the salt tolerance of 15 genotypes after 70 d (Harvest 3) and their salt tolerance at earlier growth stages. This finding confirmed the unsuitability of using an early assessment of salinity tolerance at the seedling stage (Krishnamurthy et al., 2007). Tolerance to salinity is necessary at the whole plant level through the complete life cycle in grain-producing species. The determination of salt tolerance in saline conditions presents simple and useful parameters, the differences in the levels of salt tolerance at the seedling stage did not reflect enhanced salinity tolerance at the adult plant level. Similarly, most investigators have been unable to demonstrate a relationship between tolerance under laboratory high-salt conditions and later growth stages across a range of species, particularly bread wheat (Kingsbury et al., 1984; Ashraf and McNeilly, 1988), durum wheat (Almansouri et al., 2001), and barley (Norlyn and Epstein, 1982). Nevertheless while using relative growth at the early stage seems to be a convenient test for screening large numbers of genotypes in a rapid manner, it must first be demonstrated that it is correlated to tolerance during vegetative growth, flowering, and maturity if it is to be of value (Maas, 1986; Ashraf and Harris, 2004). The heritability for salt tolerance, which ranged from 0.36 to 0.66, show that genetic differences explain a major part of the phenotypic differences. There may be scope to improve the screening efficiency for shoot biomass ratio further and thereby the operational heritability values by sampling larger numbers of plants at one time.
Salinized crops produce osmotically active organic substances, which often accumulate in the cytoplasm to balance the vacuole solute potential. Soluble sugars, proline, and betaines are some of the compatible organic solutes found in glycophyte plants (Hasegawa et al., 2000; Ashraf and Harris, 2004). In our study, salt stress caused an increase in ions and organic solutes in all genotypes, but the more salt-tolerant varieties had a significantly higher concentration of soluble sugars glucose, fructose, and sucrose., glycine betaine, and proline when grown in the soil or in the field (Table 3). Cram 1976., showed that of the various organic osmotica, sugars contribute up to 50% of the total osmotic potential in glycophytes subject to saline conditions. Ion accumulation in plants can also play a major role in osmotic adjustment to high salinities. It would seem, however, from the relationships between ion accumulation and measured leaf osmotic potential observed here for genotypes, that the simple accumulation of Na+ and Cl– alone cannot account for the osmotic behaviour of these varieties (Table 3). While the ability to restrict Na+ and Cl– accumulation could prevent the development of an osmotic imbalance in some genotypes, the concentration of Na+ and Cl– accumulated when considered together with the reduction in shoot K+ would seem to necessitate the synthesis of additional osmotically active solute in order to prevent an osmotic imbalance with respect to the external salt in soil solution (Table 3). However, the contribution of organic solutes to osmotic potential of hydroponically grown plants was not significant. The leaf osmotic potential of plants grown under hydroponic systems was significantly lower than those grown in soil which reflects the large difference between the two cultures in terms of the rate of Na+ and Cl– uptake by plants (Table 3). Much previous research has involved exposing plants suddenly to similar high concentrations of NaCl (>100 mM) that cause osmotic shock rather than osmotic stress, which induces major trauma that rarely if ever occurs in nature. Although an attempt was made to overcome the trauma of osmotic shock by increasing the concentration of salt gradually in several small steps over a few days rather than in one large step (see the Materials and methods), even this cautious approach may have been too sudden to identify useful genetic variation in salt tolerance. The important point is that it can take weeks for such variation to become evident in soil and especially under field conditions, and soil-grown plants will have more time to adapt to the salt concentration than plants in hydroponic systems (Passioura, 2010). This is of particular importance for an adaptation mechanism such as osmotic adjustment, which requires the uptake of ions and the formation of compatible solutes which are absent in hydroponics (Vetterlein et al., 2004; Tavakkoli et al., 2010a ).
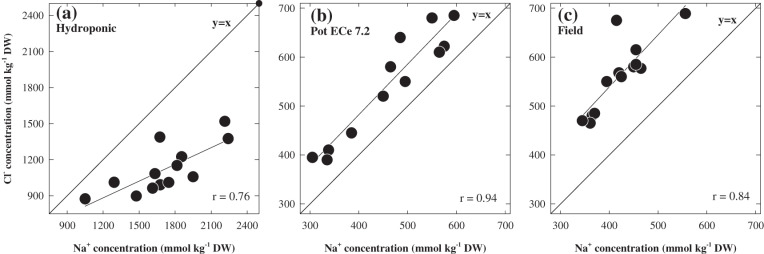
An important finding of this study was that the correlations with Na+ and Cl– concentrations and salt tolerance in the pots (Figs 5, 6c, e) was strongest when the plant Na+ and Cl– concentrations were close to those found in field study (Fig. 8a, b) and this was related to the differences in the relationship between Na+, Cl–, and salt tolerance at the three different harvests. This also occurred at different harvests, depending on the level of soil salinity at which plants were grown. Chloride toxicity has not been considered to be a major factor in salt tolerance in cereal crops. However, to a large extent, the different responses to elevated Na+ between hydroponics and soil were also seen with Cl–. In both pot screening and field study, the concentrations of Cl– were higher than those of Na+, but in hydroponics the Na+ concentration was generally higher than Cl– (Fig. 9).
Fig. 9.
The relationship between Na+ and Cl- concentration of 13 genotypes of barley grown in (a) hydroponic (49 days after germination) , (b) pot (70 days after sowing) and (c) field (ZGS 65). Values are averages (n = 3 or 4).
Within any given field, large fluctuations in salinity, drought, and extremes of temperature can occur. As a consequence, a large degree of heterogeneity between the stress levels that impact different plants in the same field can be present. This heterogeneity, in turn, can affect plant performance and yield. In addition to heterogeneity in saline conditions in differing parts of a given field, the simultaneous occurrence of different abiotic stresses should also be addressed. Abiotic stresses such as salinity and drought, salinity and heat, and distinct combinations of drought and temperature, or high light intensity are common to many agricultural areas and could affect plant productivity. It was recently shown that the response of plants to a combination of salinity and heat stress is unique and cannot be directly extrapolated from the response of plants to salinity or heat stress applied individually (Keles and Oncel, 2002; Koussevitzky et al., 2008). Because different abiotic stresses are most likely to occur simultaneously under field conditions, a greater attempt must be made to mimic these conditions in laboratory studies. The timing of the salinity stress event with respect to the developmental stage of the plant should also be addressed. Although plants can differ in their sensitivity to various abiotic stresses during different developmental stages including germination, vegetative growth, reproductive cycle, and senescence, from a strictly agronomic point of view there appears to be only one main consideration (Mittler and Blumwald, 2010): how would this interaction between stress and development affect overall yield? Most crops are highly sensitive to abiotic stresses during flowering, with devastating effects on yield (Sanchez et al., 2002; Humphreys et al., 2006; Barnabas et al., 2008). Another key difference between laboratory studies and field conditions is the intensity and duration of the stress. In the field, salinity conditions are generated gradually during a period of several weeks and months and plants do not experience a sudden stress. Thus, artificial soil mixtures containing a high content of peat moss, vermiculite, sand or high organic matter and solution culture methods should be avoided because they cannot reproduce natural soil drying conditions (Mittler and Blumwald, 2010; Tavakkoli et al., 2010a ). Conditions of water deficiency similar to those occurring in the field can be mimicked in the laboratory by growing plants under limited daily amounts of water rather than by withholding water altogether (see the Materials and methods).
Conclusions
This study demonstrated that solution culture may not allow differences in salt tolerance between genotypes to be discerned and the diverse genotypic variation found in hydroponics did not correlate with pot and field experiments. The findings suggested that assessing salinity tolerance at the seedling stage may not predict salinity tolerance at the later stages. The exclusion of Na+ and Cl– significantly contributed to salt tolerance and grain yield production in pot and field studies. This work has also established a screening procedure (Experiment 2) that correlated with a field evaluation of grain yield of the barley varieties at a moderately saline site. This study also shows that several processes are involved in salt tolerance and that the correlation of these traits with salt tolerance can differ with the severity of the salt stress. Specific-ion exclusion was correlated with salinity tolerance under mild salinity stress but at high salinity stress osmotic potential rather than ion exclusion was more strongly correlated with salinity tolerance. The present study also suggests that salt exclusion coupled with a synthesis of organic solutes are important components of salt tolerance in the tolerant genotypes and further field tests of these plants under stress conditions will help to verify their potential utility in crop-improvement programmes.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Grains Research and Development Corporation to ET and by the University of Adelaide. We thank Mr S Coventry (The University of Adelaide) for kindly supplying the seed for this study and excellent HPLC guidance, Mr P Hooper (manager of the Hart Field Site) for his support with the field study, and Dr G Lyons, for useful comments on the manuscript.
References
- Almansouri M, Kinet JM, Lutts S. Effect of salt and osmotic stresses on germination in durum wheat Triticum durum Desf. Plant and Soil. 2001;231:547–550. [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Science. 2004;166:3–16. [Google Scholar]
- Ashraf M, McNeilly T. Variability in salt tolerance of nine spring wheat cultivars. J Agron Crop Science. 1988;160:14–21. [Google Scholar]
- Aslam M, Huffaker RC, Rains WD. Early effects of salinity on nitrate assimilation in barley seedlings. Plant Physiology. 1984;76:321–325. doi: 10.1104/pp.76.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabas B, Jager K, Feher A. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell and Environment. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Britto DT, Ebrahimi-Ardebili S, Hamam AM, Coskun D, Kronzucker HJ. 42K analysis of sodium-induced potassium efflux in barley: mechanism and relevance to salt tolerance. New Phytologist. 2010;186:373–384. doi: 10.1111/j.1469-8137.2009.03169.x. [DOI] [PubMed] [Google Scholar]
- Browning LS, Bauder JW, Phelps SD. Effect of irrigation water salinity and sodicity and water table position on water table chemistry beneath Atriplex lentiformis and Hordeum marinum . Arid Land Research and Management. 2006;20:101–115. [Google Scholar]
- Chen Z, Pottosin I, Cuin TA, et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology. 2007;145:1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Shabala S, Mendham N, Newman I, Zhang G, Zhou M. Combining ability of salinity tolerance on the basis of NaCl-induced K+ flux from roots of barley. Crop Science. 2008;48:1382–1388. [Google Scholar]
- Cram WJ. Negative feedback regulation of transport in cells. The maintenance of turgor, volume and nutrient supply. In: Luttge U, Pitman MG, editors. Encyclopaedia of plant physiology. Berlin: Springer-Verlag; 1976. pp. 284–316. [Google Scholar]
- Cramer GR, Epstein E, Läuchli A. Effects of sodium, potassium and calcium on salt-stressed barley. I. Growth analysis. Physiologia Plantarum. 1990;80:83–88. [Google Scholar]
- Dang YP, Dalal RC, Mayer DG, et al. High subsoil chloride concentrations reduce soil water extraction and crop yield on Vertisols in north-eastern Australia. Australian Journal of Agricultural Research. 2008;59:321–330. [Google Scholar]
- Drew MC, Lauchli A. Oxygen-dependent exclusion of sodium ions from shoots by roots of Zea mays (cv. Pioneer 3906) in relation to salinity damage. Plant Physiology. 1985;79:171–176. doi: 10.1104/pp.79.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořak J, Noaman MM, Goyal S, Gorham J. Enhancement of the salt tolerance of Triticum turgidum L. by the Kna1 locus transferred from the Triticum aestivum L. chromosome 4D by homoeologous recombination. Theoretical and Applied Genetics. 1994;87:872–877. doi: 10.1007/BF00221141. [DOI] [PubMed] [Google Scholar]
- El-hendawy SE, Hu Y, Schmidhalter U. Growth, ion content, gas exchange, and water relations of wheat genotypes differing in salt tolerances. Australian Journal of Agricultural Research. 2005;56:123–134. [Google Scholar]
- Ellis RP, Forster BP, Gordon DC, et al. Phenotype/genotype associations for yield and salt tolerance in a barley mapping population segregating for two dwarfing genes. Journal of Experimental Botany. 2002;53:1163–1176. doi: 10.1093/jexbot/53.371.1163. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Hajibagheri MA. Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant and Soil. 2001;231:1–9. [Google Scholar]
- Garthwaite AJ, von Bothmer R, Colmer TD. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl– into the shoots. Journal of Experimental Botany. 2005;56:2365–2378. doi: 10.1093/jxb/eri229. [DOI] [PubMed] [Google Scholar]
- Genc Y, McDonald GK, Tester M. Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant, Cell and Environment. 2007;30:1486–1498. doi: 10.1111/j.1365-3040.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- Genc Y, Tester M, Mcdonald GK. Calcium requirement of wheat in saline and non-saline conditions. Plant and Soil. 2010;327:331–345. [Google Scholar]
- Gorham J, Jones WRG, Bristol A. Partial characterization of the trait for enhanced K+–Na+ discrimination in the D genome of wheat. Planta. 1990;180:590–597. doi: 10.1007/BF02411458. [DOI] [PubMed] [Google Scholar]
- Gorham J, Papa R, Aloy-Lleonart M. Varietal differences in sodium uptake in barley cultivars exposed to soil salinity or salt spray. Journal of Experimental Botany. 1994;45:895–901. [Google Scholar]
- Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young IM. Root phenomics of crops: opportunities and challenges. Functional Plant Biology. 2009;36:922–929. doi: 10.1071/FP09150. [DOI] [PubMed] [Google Scholar]
- Hall J, Maschmedt D, Billing B. The soils of Southern South Australia. Department of Water, Land and Biodiversity Conservation, Government of South Australia; 2009. [Google Scholar]
- Halperin SJ, Kochian LV, Lynch JP. Salinity stress inhibits calcium loading into the xylem of excised barley Hordeum vulgare. roots. New Phytologist. 1997;135:419–427. [Google Scholar]
- Harris B, Sadras V, Tester M. A water-centred framework to assess the effects of salinity on the growth and yield of wheat and barley. Plant and Soil. 2010;336:1–13. [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Houshmand S, Arzani A, Maibody SAM, Feizi M. Evaluation of salt-tolerant genotypes of durum wheat derived from in vitro and field experiments. Field Crops Research. 2005;91:345–354. [Google Scholar]
- Huang S, Spielmeyer W, Lagudah ES, Munns R. Comparative mapping of HKT genes in wheat, barley, and rice, key determinants of Na+ transport, and salt tolerance. Journal of Experimental Botany. 2008;59:927–937. doi: 10.1093/jxb/ern033. [DOI] [PubMed] [Google Scholar]
- Humphreys MRS, Cairns AJ, Turner LB, Humphreys J. A changing climate for grassland research. New Phytologist. 2006;169:9–26. doi: 10.1111/j.1469-8137.2005.01549.x. [DOI] [PubMed] [Google Scholar]
- Isbell RF. The Australian soil classification. CSIRO, Melbourne: 1996. [Google Scholar]
- James RA, Caemmerer SV, Condon AGT, Zwart AB, Munns R. Genetic variation in tolerance to the osmotic stress component of salinity stress in durum wheat. Functional Plant Biology. 2008;35:111–123. doi: 10.1071/FP07234. [DOI] [PubMed] [Google Scholar]
- James RA, Munns R, Von Caemmerer S, Trejo C, Miller C, Condon AG. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl– in salt-affected barley and durum wheat. Plant, Cell and Environment. 2006;29:2185–2197. doi: 10.1111/j.1365-3040.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Roche D, Monaco TA, Durham S. Gas exchange, chlorophyll fluorescence parameters, and carbon isotope discrimination of 14 barley genetic lines in response to salinity. Field Crops Research. 2006;96:269–278. [Google Scholar]
- Keles Y, Oncel I. Response of antioxidative defense system to temperature and water stress combinations in wheat seedlings. Plant Science. 2002;163:783–790. [Google Scholar]
- Kingsbury R, Epstein E. Salt sensitivity in wheat. A case for specific ion toxicity. Plant Physiology. 1986;80:651–654. doi: 10.1104/pp.80.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury R, Epstein E, Pearcy RW. Physiological responses to salinity in selected lines of wheat. Plant Physiology. 1984;74:417–423. doi: 10.1104/pp.74.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Interactions among Ca2+, Na+, and K+ in salinity toxicity: quantitative resolution of multiple toxic and ameliorative effects. Journal of Experimental Botany. 1999;50:1495–1505. [Google Scholar]
- Klute A. Water retention: laboratory methods. In: Klute A, editor. Methods of soil analysis, Part 1. Agronomy Monograph 9. Madison, WI: ASA and SSSA; 1986. pp. 635–662. [Google Scholar]
- Kopittke PM, Blamey FPC, Kinraide TB, Wang P, Reichman SM, Menzies NW. Separating multiple, short-term, deleterious effects of saline solutions on the growth of cowpea seedlings. New Phytologist. 2011;189:1110–1121. doi: 10.1111/j.1469-8137.2010.03551.x. [DOI] [PubMed] [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. Journal of Biological Chemistry. 2008;283:34197–34203. doi: 10.1074/jbc.M806337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy L, Serraj R, Hash C, Dakheel A, Reddy B. Screening sorghum genotypes for salinity tolerant biomass production. Euphytica. 2007;156:15–24. [Google Scholar]
- Lynch J, Lauchli A. Salt stress disturbs the calcium nutrition of barley (Hordeum vulgare L) New Phytologist. 1985;99:345–354. [Google Scholar]
- Maas EV. Salt tolerance in plants. Applied Agricultural Research. 1986;1:12–25. [Google Scholar]
- Marigo G, Peltier JP. Analysis of the diurnal change in osmotic potential in leaves of Fraxinus excelsior L. Journal of Experimental Botany. 1996;47:763–769. [Google Scholar]
- McDonald GK. Effects of soil properties on variation in growth, grain yield and nutrient concentration of wheat and barley. Australian Journal of Experimental Agriculture. 2006;46:93–105. [Google Scholar]
- Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Annual Review of Plant Biology. 2010;61:443–462. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- Muehlbauer GJ, Feuillet C, Maccaferri M, Sanguineti MC, Giuliani S, Tuberosa R. In: Genetics and genomics of the Triticeae. New York: Springer; 2009. Genomics of tolerance to abiotic stress in the Triticeae; pp. 481–558. [Google Scholar]
- Munns R, Hare RA, James RA, Rebetzke GJ. Genetic variation for improving the salt tolerance of durum wheat. Ausralian Journal of Agricultural Research. 2000;51:69–74. [Google Scholar]
- Munns R, Husain S, Rivelli AR, James R, Condon AG, Lindsay M, Lagudah ES, Schachtman DP, Hare RA. Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant and Soil. 2002;247:93–105. [Google Scholar]
- Munns R, James RA. Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant and Soil. 2003;253:201–218. [Google Scholar]
- Munns R, James RA, Lauchli A. Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany. 2006;57:1025–1043. doi: 10.1093/jxb/erj100. [DOI] [PubMed] [Google Scholar]
- Munns R, James RA, Sirault XRR, Furbank RT, Jones HG. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. Journal of Experimental Botany. 2010;61:3499–3507. doi: 10.1093/jxb/erq199. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nair KPP, Khulbe NC. Differential response of wheat and barley genotypes to substrate-induced salinity under North Indian conditions. Experimental Agriculture. 1990;26:221–225. [Google Scholar]
- Norlyn JD, Epstein E. Barley production: irrigation with seawater on coastal soil. In: San Pietro A, editor. Biosaline research: a look to the future. New York: Plenum Press; 1982. pp. 525–529. [Google Scholar]
- Nuttall JG, Hobson KB, Materne M, Moody DB, Munns R, Armstrong RD. Use of genetic tolerance in grain crops to overcome subsoil constraints in alkaline cropping soils. Australian Journal of Soil Research. 2010;48:188–199. [Google Scholar]
- Othman Y, Al-Karaki G, Al-Tawaha AR, Al-Horani A. Variation in germination and ion uptake in barley genotypes under salinity conditions. World Journal of Agricultural Sciences. 2006;2:11–15. [Google Scholar]
- Passioura JB. Scaling up: the essence of effective agricultural research. Functional Plant Biology. 2010;37:585–591. [Google Scholar]
- Poustini K, Siosemardeh A. Ion distribution in wheat cultivars in response to salinity stress. Field crops Research. 2004;85:125–133. [Google Scholar]
- R Development Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Rajendran K, Tester M, Roy SJ. Quantifying the three main components of salinity tolerance in cereals. Plant, Cell and Environment. 2009;32:237–249. doi: 10.1111/j.1365-3040.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- Ramagopal S. Salinity stress induced tissue-specific proteins in barley seedlings. Plant Physiology. 1987;84:324–331. doi: 10.1104/pp.84.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson HM. Gas exchange, growth in wheat and barley grown in salt. Australian Journal of Plant Physiology. 1986;13:475–489. [Google Scholar]
- Rengasamy P. Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Australian Journal of Experimental Agriculture. 2002;42:351–361. [Google Scholar]
- Richards RA. Should selection for yield in saline regions be made on saline or non-saline soils? Euphytica. 1983;32:431–438. [Google Scholar]
- Richards RA, Dennett CW, Qualset CO, Epstein E, Norlyn JD, Winslow MD. Variation in yield of grain and biomass in wheat, barley, and triticale in a salt-affected field. Field Crops Research. 1987;15:277–287. [Google Scholar]
- Rivandi J, Miyazaki J, Hrmova M, Pallotta M, Tester M, Collins NC. A SOS 3 homologue maps to HvNax4, a barley locus controlling an environmentally sensitive Na+ exclusion trait. Journal of Experimental Botany. 2010 doi: 10.1093/jxb/erq346. doi: 10.1093/jxb/erq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell D. Soil science: methods and applications. Wiley, New York: 1994. [Google Scholar]
- Royo A, Aragüés R. Salinity–yield response functions of barley genotypes assessed with a triple line source sprinkler system. Plant and Soil. 1999;209:9–20. [Google Scholar]
- Rush DW, Epstein E. Comparative studies on the sodium, pottasium, and chloride relations of a wild halophytic and a domestic salt-sensitive tomato species. Plant Physiology. 1981;68:1308–1313. doi: 10.1104/pp.68.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AC, Subudhi PK, Rosenow DT, Nguyen HT. Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench) Plant Molecular Biology. 2002;48:713–726. doi: 10.1023/a:1014894130270. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Munns R, Whitecross MI. Variation in sodium exclusion and salt tolerance in Triticum tauschii . Crop Science. 1991;31:992–997. [Google Scholar]
- Shavrukov Y, Gupta N, Miyazaki J, Baho M, Chalmers K, Tester M, Langridge P, Collins N. HvNax3—a locus controlling shoot sodium exclusion derived from wild barley (Hordeum vulgare ssp. spontaneum) Functional and Integrative Genomics. 2010;10:277–291. doi: 10.1007/s10142-009-0153-8. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh M, Zhou MX, Chen ZH, Mendham NJ. Physiological and cytological response of salt-tolerant and non-tolerant barley to salinity during germination and early growth. Australian Journal of Experimental Agriculture. 2006;46:555–562. [Google Scholar]
- Tavakkoli E. PhD thesis, University of Adelaide; 2011. Limitations to yield in saline–sodic soils: quantification of the osmotic and ionic regulations that affect the growth of crops under salinity stress. [Google Scholar]
- Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, Mcdonald GK. Additive effects of Na+ and Cl– ions on barley growth under salinity stress. Journal of Experimental Botany. 2011;62: 2189–2203. doi: 10.1093/jxb/erq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkoli E, Rengasamy P, Mcdonald GK. The response of barley to salinity stress differs between hydroponics and soil systems. Functional Plant Biology. 2010a;37:621–633. [Google Scholar]
- Tavakkoli E, Rengasamy P, Mcdonald GK. High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. Journal of Experimental Botany. 2010b;61:4449–4459. doi: 10.1093/jxb/erq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetterlein D, Kuhn K, Schubert S, Jahn R. Consequences of sodium exclusion on the osmotic potential in the rhizosphere: comparing of two maize cultivars differing in Na+ uptake. Plant Nutrition and Soil Science. 2004;167:337–344. [Google Scholar]
- Yeo AR, Flowers TJ. Varietal differences in the toxicity of sodium ions in rice leaves. Physiologia Plantarum. 1983;59:189–195. [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Research. 1974;14:415–421. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.