Abstract
Roots are highly plastic and can acclimate to heterogeneous and stressful conditions. However, there is little knowledge of the effect of moisture gradients on the mechanisms controlling root growth orientation and branching, and how this mechanism may help plants to avoid drought responses. The aim of this study was to isolate mutants of Arabidopsis thaliana with altered hydrotropic responses. Here, altered hydrotropic response 1 (ahr1), a semi-dominant allele segregating as a single gene mutation, was characterized. ahr1 directed the growth of its primary root towards the source of higher water availability and developed an extensive root system over time. This phenotype was intensified in the presence of abscisic acid and was not observed if ahr1 seedlings were grown in a water stress medium without a water potential gradient. In normal growth conditions, primary root growth and root branching of ahr1 were indistinguishable from those of the wild type (wt). The altered hydrotropic growth of ahr1 roots was confirmed when the water-rich source was placed at an angle of 45° from the gravity vector. In this system, roots of ahr1 seedlings grew downward and did not display hydrotropism; however, in the presence of cytokinins, they exhibited hydrotropism like those of the wt, indicating that cytokinins play a critical role in root hydrotropism. The ahr1 mutant represents a valuable genetic resource for the study of the effects of cytokinins in the differential growth of hydrotropism and control of lateral root formation during the hydrotropic response.
Keywords: Abscisic acid, Arabidopsis thaliana, cytokinins, gravitropism, hydrotropism, root architecture, root cap, water potential gradients
Introduction
The degree to which plants depend on environmental cues to orchestrate growth and development is unmatched in the animal kingdom. One of the most important traits that have evolved in plants is the ability to sense environmental cues and transduce them as a basis for governing their growth orientation. The directional growth of plant organs relative to the direction of environmental stimuli is a tropism. Gravitropism and phototropism are the most studied tropisms, and many genes that regulate them have been identified (Morita, 2010). The lack of sufficient water is one of the major constrains on world agriculture, and interest in hydrotropism has fluctuated over the years (Cassab, 2008; Miyazawa et al., 2009). Hydrotropism implies the perception of water gradients and, in response, the alteration of growth patterns of plants. This phenomenon has a crucial role in establishing the structure of the root system, and thus has implications on the ability of plants to survive under limiting water conditions. Hydrotropism is a long documented, but not well understood plant behaviour (Eapen et al., 2005; Cassab, 2008; Takahashi et al., 2009). Thus far, not much is known about the mechanisms that regulate root growth responses to water gradients. Moisture gradients, like other stimuli, are perceived by the root cap. Root cap cells are thus unique for their capacity to sense different types of stimuli, some of which are fixed in direction and intensity (gravity) while others vary in time, space, direction, and intensity (obstacles, moisture gradients, and nutrients) (Hawes et al., 2003). Hydrotropism has been recently characterized in the model plant Arabidopsis. The root hydrotropic response in Arabidopsis, compared with other plants such as pea and cucumber (Takahashi and Suge, 1991), is readily observed even in the presence of gravity (Takahashi et al., 2002; Eapen et al., 2003, 2005). A genetic screen based on the ability of Arabidopsis seedlings to develop hydrotropic root curvature allowed isolation of ahydrotropic mutants such as no hydrotropic response 1 (nhr1) (Eapen et al., 2003). Later, Kobayashi et al. (2007) also isolated a series of hydrotropic mutants termed ‘mizu-kussei’ (miz). The miz1 mutant did not display hydrotropism, and exhibited regular gravitropism, reduced phototropism, and a modified wavy root growth response (Kobayashi et al., 2007). This implies that both MIZ1 and NHR1 are not sole players in hydrotropism and supports the view that root cap cells have assessment mechanisms that merge diverse stimuli to generate an ultimate integrated response (Trewavas, 2003). Among the six miz mutants isolated, only two mutated genes, MIZ1 and MIZ2, have been identified (Kobayashi et al., 2007; Miyazawa et al., 2009). MIZ1 encodes a protein containing a domain of an unknown function (DUF617) that is highly conserved among terrestrial plants such as rice and moss. Since MIZ1 is responsible for the root hydrotropic response, the MIZ domain may have an important role in the adaptation of terrestrial plants. Another mutant, miz2, is affected in GNOM, a guanine-nucleotide exchange factor for ADP-ribosylation-type G proteins, that lacks a hydrotropic but not a gravitropic response, implying distinct roles for vesicular trafficking in these tropisms (Miyazawa et al., 2009). It has been recently stated that MIZ1 participates in lateral root development by maintaining auxin levels and that its function requires GNOM activity (Moriwaki et al., 2011). One of the visible changes associated with the early phases of root hydrotropism in Arabidopsis and radish is that starch in root cap columella cells is readily degraded upon hydrostimulation (Takahashi et al., 2003). These authors concluded that the reduction in starch content in columella cells came with a declined responsiveness to gravity, a response that allows roots to exhibit hydrotropism. Because the recently isolated hydrotropic mutant miz1 maintains the ability to degrade starch in columella cells upon hydrostimulation (Kobayashi et al., 2007), in contrast to the nhr1 mutant (Ponce et al., 2008a ), it can be suggested that NHR1 and starch degradation reside at early steps, whereas MIZ1 resides further downstream in the hydrotropic pathway. Moreover, the root hydrotropic response is observed in starch excess mutants of Arabidopsis (mex1 and sex1; Ponce et al., 2008a ), indicating that starch depletion is not fundamental for hydrotropism.
Crop plants usually confront heterogeneous water distribution in the field and develop their root system accordingly (Canadell et al., 1996; Dordolot et al., 2007). The goal of this study was to develop a novel screening system with a water potential gradient for selecting Arabidopsis mutants that showed altered root hydrotropism. In this system, the mutant seedlings were challenged to grow from a low water potential region towards a high water potential region, a behaviour not shown by the wild type (wt). Here, a newly identified mutant of Arabidopsis (named altered hydrotropic response; ahr1) is described whose growth is strongly directed to the region with higher water availability when the water-rich source is placed downward relative to the root tip. When the position of the water-rich source was placed obliquely, the mutant was non-hydrotropic, unless cytokinins were added to the medium. Furthermore, the morphology of the root cap of ahr1 mutants was conserved in the water deficit conditions of the screening system, while the root cap cells of the wt were severely damaged. Lastly, the phenotype of the ahr1 mutant shown in the screening system was enhanced in the presence of abscisic acid (ABA), indicating that cytokinins and ABA play a crucial role in hydrotropism.
Materials and methods
Plant materials, growth media, and mutant screen
Wt Arabidopsis thaliana seeds of the ecotypes Col 0, WS, and Ler, and ahk1 were provided by the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Wt Col 0 was used to conduct mutagenesis with 0.3% (v/v) ethyl methanesulphonate (EMS; Sigma, St Louis, MO, USA). Seeds were surface sterilized and kept at 4 °C for 24 h in 0.1% (w/v) agar. miz1 (Kobayashi et al., 2007) and miz2 (Miyazawa et al., 2009) were donated by Dr Hideyuki Takahashi (Tohoku University, Sendai, Japan). The screening system with a water potential gradient consisted of two different media that were horizontally split. The top half was a water stress medium (WSM), which consists of half-strength Murashige and Skoog (MS) salts supplemented with 0.5% (w/v) sucrose, 2.5% (v/v) glycerol, and 0.5% (w/v) alginic acid. The lower half consisted of a normal medium (NM), which contained half-strength MS salts supplemented with 0.5% (w/v) sucrose. The pH of the medium was adjusted to 5.6 before autoclaving. Alginic acid was added to improve WSM solidification. Both media were solidified with 0.9% Bacto agar. The WSM was poured into one half (upper part) of a square plate with an acrylic slab (9.0×0.5 cm) in the middle of the plate that divided it into two equal parts. After solidification of the WSM, the piece of acrylic was removed and the NM was poured into the other half (lower part) of the plate. Before sowing seeds, plates were stored at 4 °C for 24 h. In this way the water potential gradient was established before seeds were sown. Seeds were placed in the upper half of the plate at 1.1 cm above the interphase of both media. Plates were sealed with Parafilm, set in a vertical position at 23 °C, and subjected to 16 h light/8 h dark cycles in a growth chamber. Under these conditions, seedlings from wt plants remained at the WSM and never contacted the NM. For the mutant screen, seedlings whose roots reached the NM after 12 d were transferred to soil. Seeds from these plants were harvested and tested in the re-screen. Selected mutants were then backcrossed three times to wt plants. Progeny derived from the third backcross were used in all assays.
Water potential analysis of the screening system
The water potential of the screening system was measured at time zero and every 24 h for 8 d at various distances from the WSM–NM junction of the square Petri plate by the dew point psychometric combined method with the PSYPRO water potential data logger (Wescor Inc., Logan UT, USA). Each measurement was repeated three times for each time point during the time-course study. The data are presented as the mean ±SD. The osmolarity of the screening system containing glycerol, sorbitol, or NaCl was measured with an Osmomat 030 cryoscopy osmometer (Gonotec, Berlin, Germany).
Measurements of root growth and root curvature
After growing for the indicated time (usually 3–15 d) on the screening system or NM, seedlings were photographed with a Nikon D-1 digital camera (Nikon, Co., Chiyoda-ku, Tokyo, Japan). Photographs were taken at different times and images were imported into Adobe Photoshop CS 8.0 (Adobe Systems Inc., San Jose, CA, USA). The images were analysed using National Institute of Health (NIH) Image J software version 1.34 (http://rsb.info.nih.gov/ij/). The length of primary roots, the number and length of visible lateral roots, and the root tip curvature were measured using this software. Data were transferred to a Microsoft Excel spreadsheet, and the average length of primary roots and lateral roots, root tip curvature, the SD, and Student’s t-test (two-tail t-test with two-sample unequal variance) were calculated and plotted. Data derived from root growth analysis of ahr1 and the wt treated with different osmotic effectors, salt, and hormones were analysed by two-way analysis of variance (ANOVA) with Bonferroni post-tests using the Prism 5 program for Mac OS X (GraphPad Software, Inc.).
Microscopy
To observe amyloplasts in columella cells of root caps, seedlings were treated as described by Takahashi et al. (2003). Root tips were observed under a light microscope (Nikon Eclipse E600, Nikon Co.) and photographed with a Nikon D1 digital camera. For confocal microscopy analysis, 10-day-old wt and ahr1 seedlings were stained with propidium iodide (10 μg ml−1) for 10 min and then were observed with a Zeiss LSM 510 confocal microscope using a 63× C Apochromat W Korr (1.2 numerical aperture and 0.25 water diffraction) water immersion objective (Zeiss, Jena, Germany).
Hormone treatments
ABA and kinetin were purchased from Sigma, prepared as 10 mM stock solutions, and diluted in drops of methanol and 1 N NaOH, respectively. Filter-sterilized ABA was included in lukewarm 0.8% (w/v) agar, and added locally to seeds or root tips with the help of a Pasteur pipette at the concentrations defined in the text. Filter-sterilized kinetin was directly added to lukewarm MS culture media.
Mapping the ahr1 mutation
The mapping population was generated by manually crossing ahr1 mutants selected from the test system to the Ler and WS ecotypes. The resulting F1 plants were allowed to self-pollinate to generate F2 populations for mapping. Tissue from F2 plants was collected for DNA isolation. DNA pools were bulked and used to assign a rough position on the genetic map by identifying linked genetic markers [a set of simple sequence length polymorphism (SSLP) markers spaced at regular intervals throughout the genome]. For fine-scale mapping, DNA was prepared from individual F2 plants of both Ler and WS mapping populations (892 plants, 1784 chromosomes), and recombination frequencies were obtained with flanking SSLP markers from the region.
Results
Isolation of mutants with altered hydrotropic responses
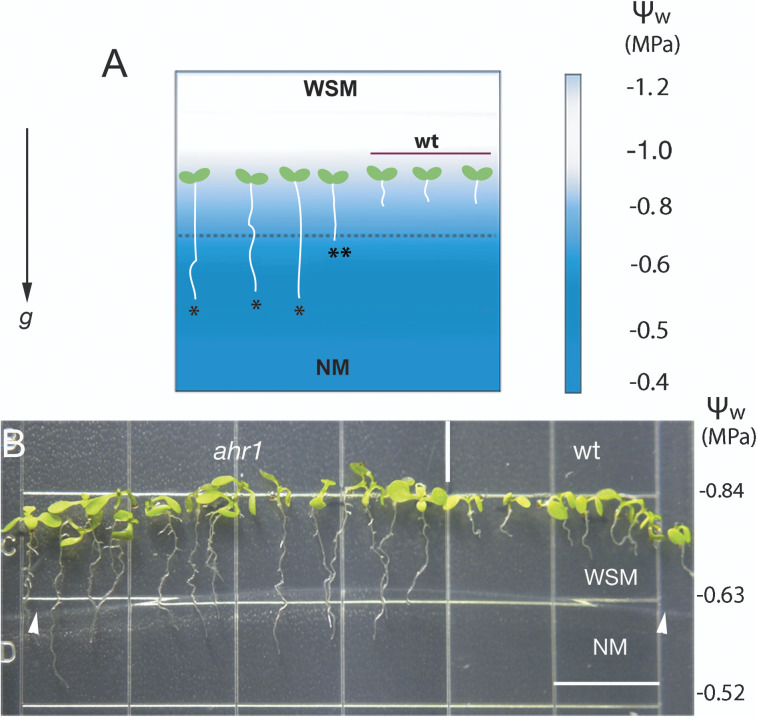
A testing system was designed for the screening of an EMS-mutagenized population of Arabidopsis seedlings with altered hydrotropic response (Fig. 1A). Mutagenized seeds were sown in the upper half of a square plate containing a WSM, and those seedlings whose primary roots grew to reach the lower part of the plate, containing the NM (with higher water availability), were considered as having an altered root hydrotropic response (Fig. 1A). Hereafter, this system will be referred to as the horizontal WSM→NM screening system. In this system, primary roots of growing wt seedlings never reached the water-rich source, stopping their growth in the WSM after 4–6d. Wt roots showed an average root length of 0.4±0.04 cm (five replicates ±SD, n=350) (Fig. 1B). Changes in water potential of the screening system over time were determined as described by Eapen et al. (2003) (Fig. 1). A total of 10 500 EMS-mutagenized seeds were screened in this system, which resulted in the isolation of five putative mutants that showed considerable root growth after 10 d (Figs 1B, 2A). However, only two of them, named ahr1 and ahr2, maintained the altered hydrotropic response phenotype after backcrossing four times with wt plants. Here, the characterization of ahr1 is reported. The segregation ratio of F2 seedlings after the fourth backcross of ahr1 with the wt (83 wt:206 intermediate root:96 altered hydrotropic response) indicated that the inheritance pattern occurred from segregation of a single semi-dominant gene with a 1:2:1 segregation (χ2=0.323 < χ2 0.05(1)=3.841). Intermediate root phenotype referred to seedlings whose primary roots stopped their growth once they reached the interphase between the WSM and NM (Fig. 1A).
Fig. 1.
The horizontal WSM→NM system for selecting mutants with altered hydrotropic response. (A) Diagram illustrating the screening system. The experimental set-up consists of a square Petri dish containing two types of medium, one supplemented with 2.5% (v/v) glycerol and 0.5% (w/v) alginic acid (WSM) placed in the upper half, and a normal medium (NM) in the lower half. The numbers on the right denote the data regarding the water potential (Ψw) throughout the screening system at time zero. The water potential of the system in the lower half of the plate gradually decreased once glycerol diffused from above over time, and became more negative in positions closer to the NM. The diagram illustrates the phenotypes obtained after screening for altered hydrotropic mutants (single asterisk), intermediate root phenotype (double asterisk), and the wt response. (B) Phenotype of 10-day-old ahr1 mutant and wt seedlings in the screening system. Roots of ahr1 plants continued to grow after crossing the boundary between WSM and NM and thus sustained downward growth towards the zone with higher water availability (higher water potential). Wt roots arrested their growth after 4 d. The numbers on the right designate the data of the water potential (Ψw) in three different sections of the screening system after 10 d. Arrowheads delimit the border between the WSM and NM. The imags are representative of 10 different experiments (n=360). Scale bar=13 mm.
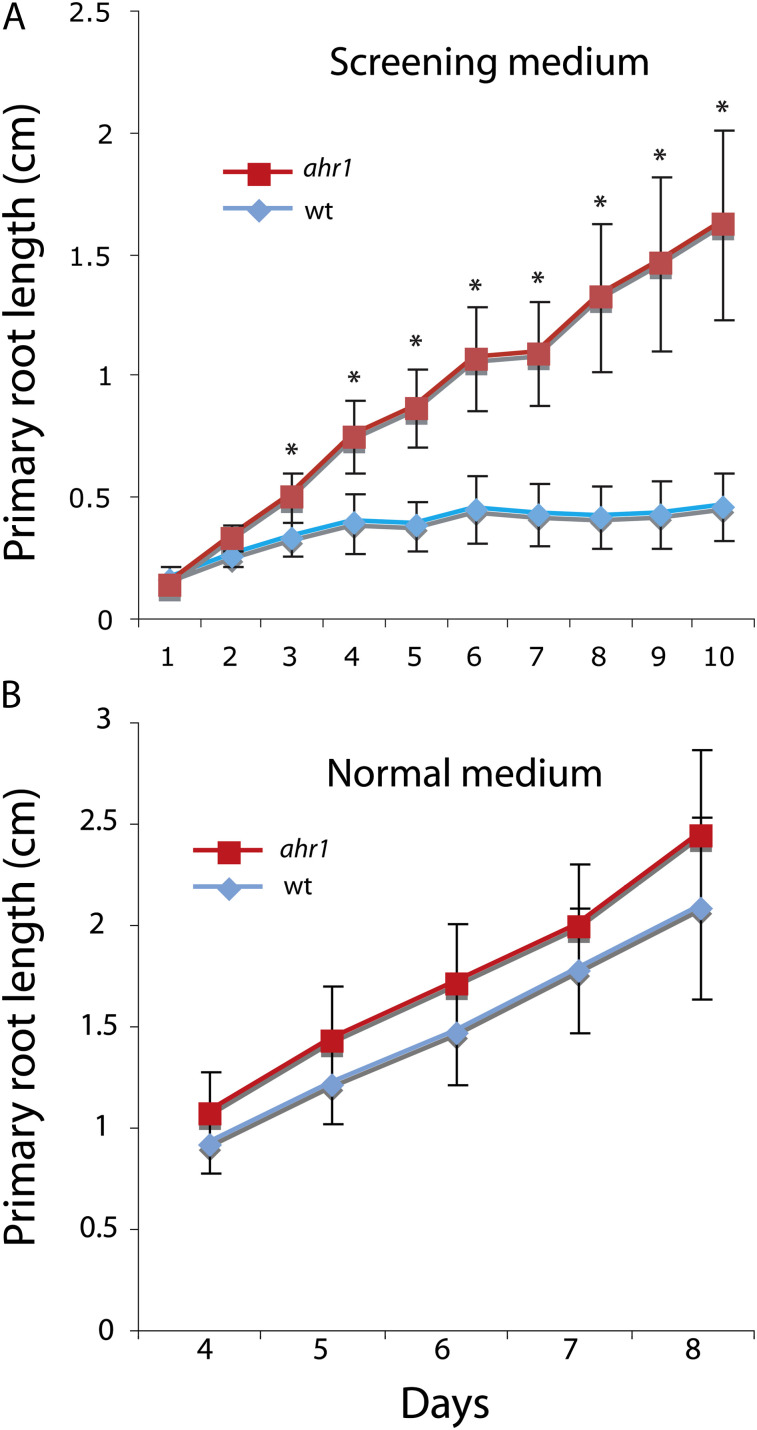
Fig. 2.
Dynamics of root growth of wt and ahr1 seedlings in the horizontal WSM→NM screening system and in NM. (A) Seeds were sown in the horizontal WSM→NM screening system and measurements were taken every 24 h for 10 d. (B) Seeds of the wt and ahr1 were sown in NM plates and, once seedlings had the same root size at 4 d, measurements were taken every 24 h for the next 4 d. Data are the average ±SD of three independent experiments (n=105). NM, normal medium. Root growth of ahr1 in the screening system is significantly different from that of the wt (Student’s t-test, p < 0.001), and is not significant in the NM (Student’s t-test, p=0.4). Asterisks denote significant differences.
Mapping of the ahr1 mutation
The chromosomal position of the AHR1 gene was determined by SSLP linkage analysis (Lukowitz et al., 2000). The ahr1 mutation was induced in the Col ecotype and crossed to Ler to generate a mapping F2 population derived from a Col/Ler F1 plant with the genotype ahr1/AHR1. The site of the ahr1 mutation was mapped to the region between two SSLP markers, nga168 and nga1126, since these markers showed a clear bias toward the Col-specific band in the mutant pool. This indicated that the mutation maps in the lower arm of chromosome II. The recombination frequency was 5.1% for the SSLP marker nga168 and 3.13% for nga361. Additionally, the recombination frequency with the molecular single nucleotide polymorphism markers (collection of Cereon genomics) was 0.056% for CERB4A and 0.056% for CERB3 (0.1121% across the interval).
Root growth responses of ahr1
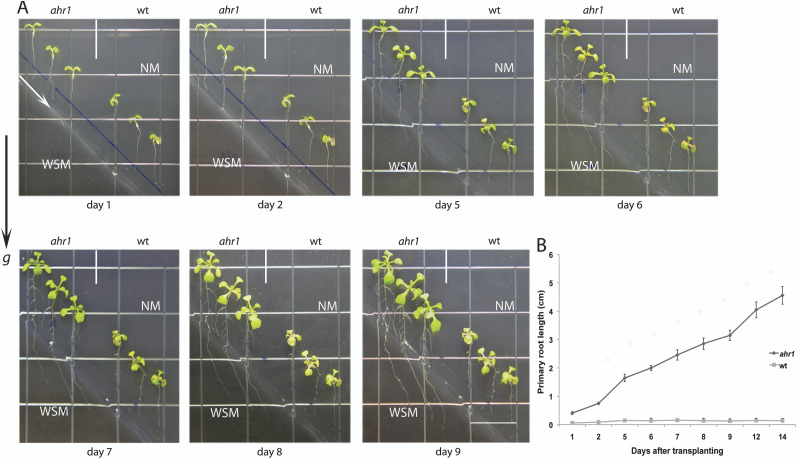
Root growth of ahr1 mutants in NM after 8 d or 14 d was identical to that of the wt (Fig. 2B, and data not shown), indicating that the increased root growth of ahr1 in the horizontal WSM→NM screening system reflected only a short-term growth adjustment to water potential gradients in search for higher water availability. However, in this screening system (Fig. 1A), ahr1 seedlings dramatically differed from those of the wt (Fig. 2A). First, in the early post-germinative phase, ahr1 roots were longer than those of the wt. For instance, root lengths of 10-day-old ahr1 mutants were on average 1.5±0.35 cm, whereas those of the wt were 0.4±0.14 cm (an ∼4-fold inhibition, n=60, three replicates ±SD, Student’s t-test, p ≤ 0.001) (Fig. 2A). Secondly, ahr1 seedlings survived in this screening system (Fig. 1A) after 26 d of germination and set seed after they were transplanted to soil, whereas those of the wt eventually dried out (data not shown). Thirdly, ahr1 developed a highly branched root system in the screening system (see Fig. 8A–D). On the other hand, ahr1 and wt seedlings accumulated glycerol in similar amounts when grown in this screening system (data not shown) and hence the increased growth of ahr1 was not due to a differential accumulation of glycerol.
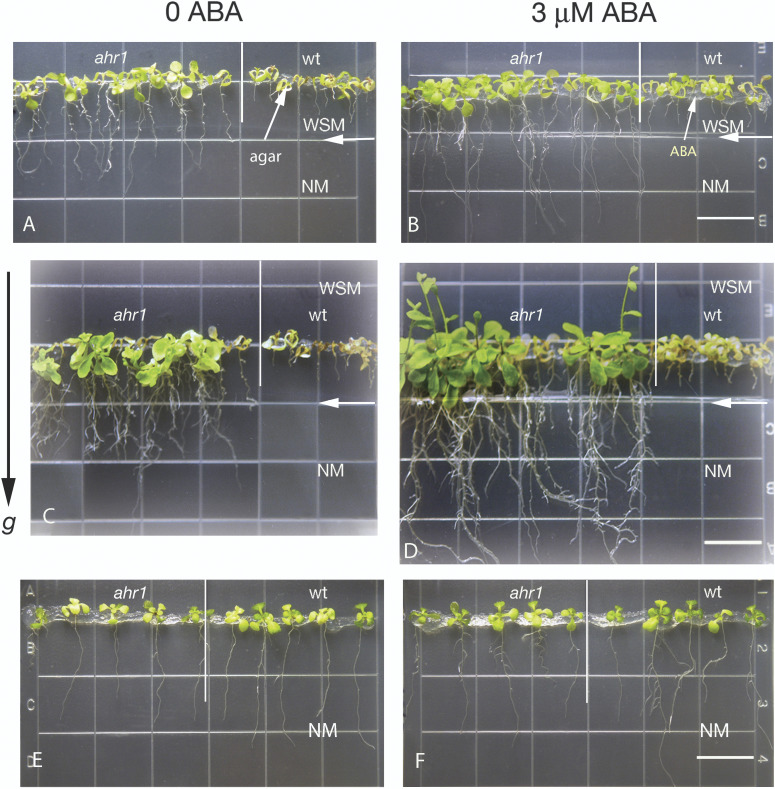
Fig. 8.
Contrasting effects of ABA on root architecture of ahr1 depending upon the presence or absence of osmotic potential gradients. The root system architecture of ahr1 was increased by adding to seeds a thin layer of agar containing 3 μM ABA (oblique arrow) when germinated in the WSM portion of the horizontal WSM→NM screening system (B, D). (A, C) Control plates with 0 ABA, in which seeds were covered only with agar (oblique arrow). Horizontal arrows indicate the border between the WSM and the NM. (E, F) ABA decreased the root system size of ahr1 in normal conditions (NM). Control seeds treated with (E) a thin layer of agar (0 ABA), and (F) with 3 μM ABA. Photographs were taken after 10 d (A, B, E, F) or 20 d (C, D), and are representative of three independent experiments (n=125). Scale bar=13 mm. (This figure is available in colour at JXB online.)
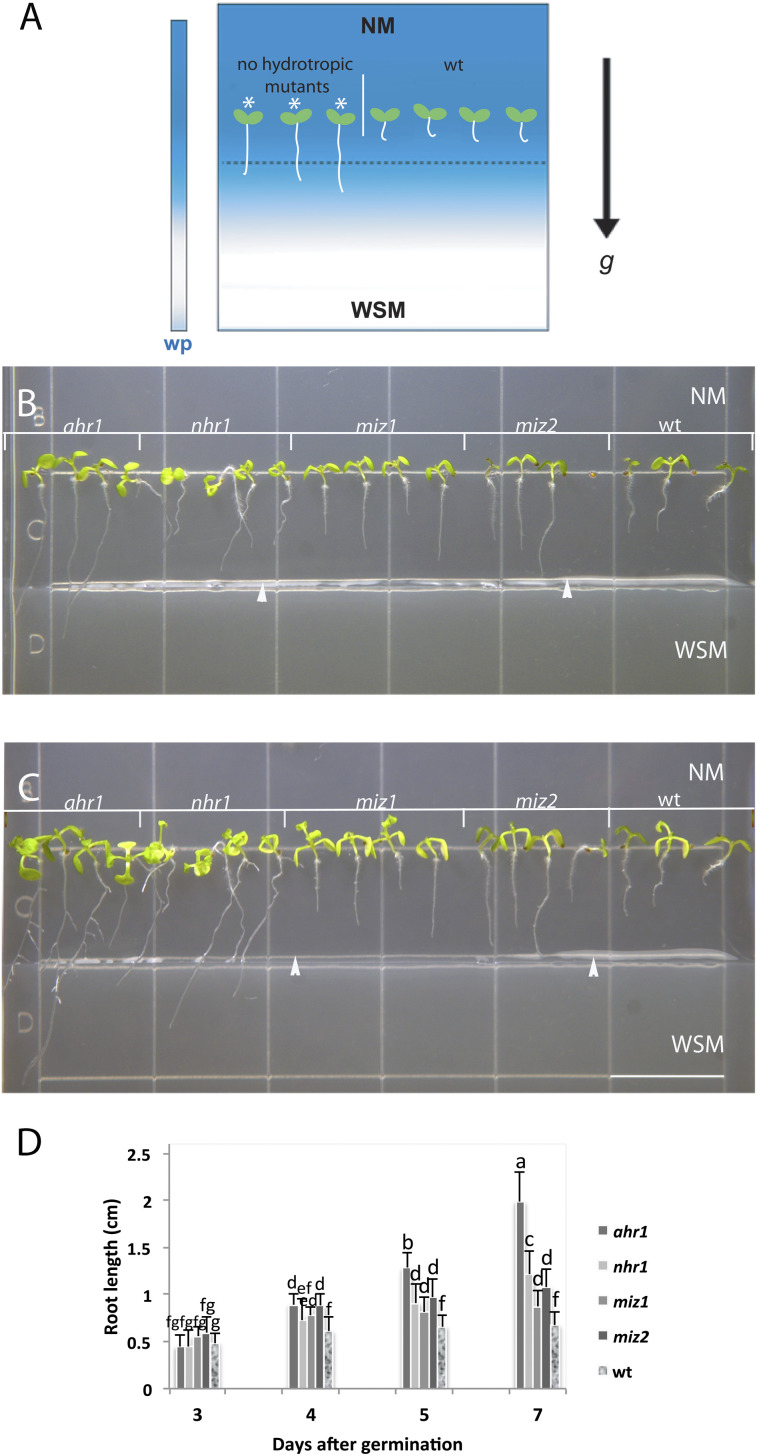
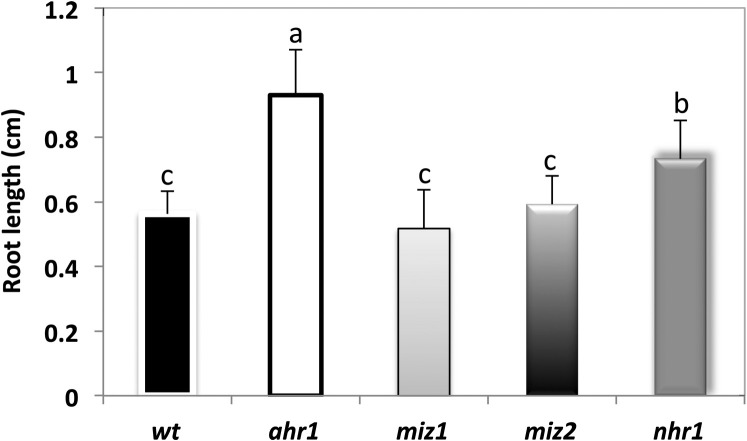
To characterize further the response of the primary root of the ahr1 mutant to water potential gradients, growth was analysed in the test system for hydrotropism previously reported for the isolation of nhr mutants (Eapen et al., 2003). In this system, the medium is inverted; that is, the NM is positioned in the upper part and the WSM is placed in the lower section of the plate (Fig. 3). Hereafter this system is referred to as the horizontal NM→WSM system. Wt root seedlings, which displayed hydrotropism, remained in the NM and never reached the WSM (Fig. 3), while the ahr1 mutant displayed a no hydrotropic response, reaching the WSM in a similar fashion to the nhr1 mutant (Eapen et al., 2003). Another class of known hydrotropic mutants, the ahydrotropic miz1 and miz2 mutants (Kobayashi et al., 2007; Miyazawa et al., 2009), were also tested in the horizontal NM→WSM system. Both miz1 and miz2 displayed a no hydrotropic response equivalent to that shown by ahr1 and nhr1, indicating that the horizontal NM→WSM screening system could have also been suitable to select all these no hydrotropic mutants (Fig. 3) However, ahr1 roots were the longest compared with nhr1, miz1, and miz2, indicating that this mutant displayed a different no hydrotropic response phenotype and was resistant to the water potential conditions of the horizontal NM→WSM system. In contrast to ahr1 mutants, miz1 and miz2 showed a similar response to the wt in the horizontal WSM→NM screening system designed for the isolation of the ahr mutants (Fig. 1A). However, nhr1 roots were significantly longer than those of the wt, miz1, and miz2 but significantly shorter than ahr1 roots, although this no hydrotropic mutant could not reach the NM below (Fig. 4). Hence, the phenotypes of nhr1, miz1, and miz2 mutants were not comparable with that of ahr1 because ahr1 root growth was not restrained in the lower water potential conditions of the WSM.
Fig. 3.
Root growth of ahr1, miz1, miz2, and nhr1 seedling mutants in the horizontal NM→WSM test system for hydrotropism. (A) Diagram illustrating the horizontal NM→WSM screening system for testing hydrotropism as described in Eapen et al. (2003). (A) The experimental set-up contains two types of medium in a square Petri dish. The upper medium is the control medium (NM), and the medium at the bottom is supplemented with 2.5% (v/v) glycerol and 0.5% (w/v) alginic acid (WSM). The water potential of the system in the upper half of the plate gradually decreased once glycerol diffused from below over time and became more negative in positions closer to the NM. The diagram illustrates the phenotypes obtained with the screening for no hydrotropic mutants (single asterisk) and the wt response. (B) Five-day-old seedlings, (C) 7-day-old seedlings. Roots of nhr1, miz1, and miz2 grow to the boundary between the NM and WSM (arrow), showing a lack of hydrotropic response. Wt roots arrested their growth after 5 d and developed a hydrotropic curvature. Arrowheads point to the border between the NM and WSM. (D) Root growth measured in hydrotropic mutants showed that these were significantly longer after 5 d of germination compared with the wt. ahr1 roots were the longest compared with nhr1, miz1, and miz2. Each data point is the average of 38 individuals from three independent experiments. Error bars represent the SD. Bars with different letters differ significantly after two-way ANOVA with Bonferroni post-tests, p < 0.001. Scale bar in (B)=13 mm.
Fig. 4.
Root growth of the ahr1, nhr1, miz1, and miz2 mutants in the horizontal WSM→NM screening system for the selection of ahr mutants. Comparison among the nhr1, miz1, miz2, and wt genotypes showed that their roots were significantly shorter after 11 d of germination than those of ahr1. Each data point is the average of 22 individuals from three independent experiments. Error bars represent the SD. Bars with different letters differ significantly after two-way ANOVA with Bonferroni post-tests, p < 0.001 in a versus b and c; and p < 0.05 in b versus c.
Because glycerol was used as an osmolyte in the WSM region of both horizontal screening systems (NM→WSM and WSM→NM), ahr1 and wt seedlings were grown at different glycerol concentrations to evaluate the sensitivity of their primary roots to glycerol in the absence of a water potential gradient. Root growth of ahr1 and wt seedlings decreased in NM plates containing 50, 163, or 217 mM glycerol [equivalent to 0.5, 1.5, and 2.0% (w/v), respectively] (Supplementary Fig. S1 available at JXB online). Both wt and ahr1 seedlings diminished their root length at all glycerol concentrations tested. The root length of wt seedlings was reduced >60% at 50 mM and 163 mM glycerol, and by >75% at 217 mM glycerol, whereas that of ahr1 was reduced by 40% at 50 mM and 163 mM glycerol and by >60% at 217 mM glycerol. The response to 2.5% (w/v) glycerol was not tested since in the screening system this concentration decreased after gradient formation. This indicated that the ahr1 mutation confers a slightly increased resistance to glycerol, providing a more efficient osmotic adjustment capacity in the presence of a water potential gradient.
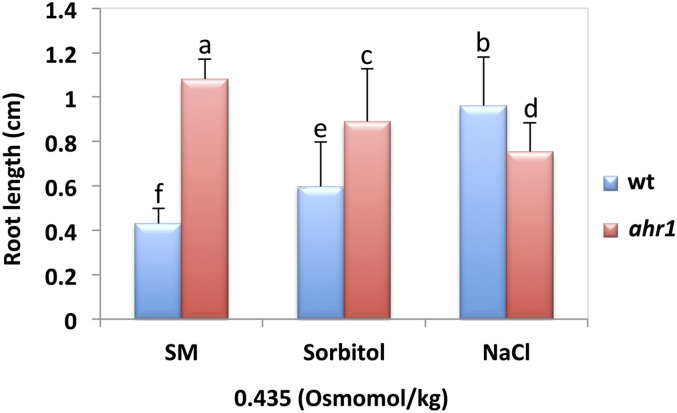
To discern whether the ahr1 phenotype is specific to glycerol or rather a general response to changes in water potential gradients, glycerol was substituted with sorbitol or with NaCl in the horizontal WSM→NM screening system. Sodium chloride was used for testing ionic sensitivity (Fig. 5). Root growth of ahr1 after 11 d was significantly higher compared with that of the wt when the screening system contained 2.5% (w/v) glycerol and/or 6.5% (w/v) sorbitol (with comparable osmolality, 0.435 Osmomol/kg), but significantly decreased in the presence of 200 mM NaCl (0.444 Osmomol/kg) (Fig. 5). Hence, ahr1 roots did not respond to the gradient of glycerol as a distinct substance and showed an enhanced capacity to grow in water potential gradients generated with two different osmolytes (Fig. 5).
Fig. 5.
ahr1 mutant seedling roots display long-term growth resistance in the presence of a water potential gradient generated by sorbitol. Seeds of the wt and ahr1 were sown in a horizontal WSM→NM system with a water potential gradient where the WSM region contained glycerol, NaCl, or sorbitol. Measurements were scored after 11 d of germination. Similar osmolarity (Osmomol/kg) was used in the preparation of the medium with glycerol, sorbitol, or NaCl. Data represent the mean root length of three different experiments (n=24 for each treatment and plant). Error bars represent the SD. Bars with different letters differ significantly after two-way ANOVA with Bonferroni post-tests, p < 0.01.
In addition, growth of ahr1 seedlings was tested on a steeper gradient of water potential in order to establish the threshold of the response. For this, ahr1 and wt seeds were plated diagonally in the upper section of the horizontal WSM→NM screening system. ahr1 roots kept growing when confronted with water potential gradients from –0.8 MPa to –1.1 MPa (Supplementary Fig. S2A at JXB online). On the other hand, ahr1 seedlings maintained their proximal meristem in all roots tested in this gradient, indicating that ahr1 root cells have a better osmotic adjustment capacity, in clear contrast to those of the wt (Supplementary Fig. S2B).
The horizontal WSM→NM screening system described in Fig. 1 was designed for selecting plants with an altered hydrotropic response operating along the gravity vector. Thus far, ahr1 roots directed their downward growth towards the higher water potential zone, but would they be able to direct their growth to the WSM from the NM distributed diagonally? To answer this question, a diagonal NM→WSM test system was utilized. In this, the NM was positioned in the upper triangular half of a square Petri plate, and the WSM was placed in the lower triangular half. Five-day-old seedlings that germinated in NM with straight roots of uniform size (1.5–2.0 cm in length) were positioned vertically 0.5 cm above the boundary between both substrates (Fig. 6A). Roots of ahr1 showed hydrotropic bending and diagonal root growth by presenting an average of 29° towards the NM on the right side of the plate (viewed from the front). The mean angles for ahr1 and wt roots were 28.6±2.2 ° and 10.1±2.9°, respectively (n = 20 per experiment, three replicates ±SD, Student’s t-test, p <0.001) (Fig. 6A, B). Moreover, wt seedlings did not show significant elongation of the primary root and developed few lateral roots. Roots of ahr1 also developed more lateral roots than the wt under these conditions (35±0.8 and 10.3±0.04 in ahr1 and the wt, respectively, n = 20 per experiment, three replicates ±SD, Student’s t-test, p < 0.001). A water potential gradient also developed over time in this experimental set-up (data not shown). To evaluate if the response of ahr1 in this oblique experimental system was indeed hydrotropic, the growth pattern of ahr1 was examined in a control set that consists of two NMs placed diagonally in the upper and lower triangles of a square Petri plate. It was found that the gravitational set-point angle of the root was very similar in ahr1 and the wt (5±0.2 ° and 4.5±0.4 ° in ahr1 and the wt, respectively, n = 20 per experiment, three replicates ±SD, Student’s t-test, p > 0.2). These analyses demonstrated that the effect of the ahr1 mutation on the conditional growth of the primary root, in response to an osmotic gradient, correlated with hydrotropism since ahr1 roots showed significant growth toward water-rich substrates, which was to some extent released from gravitropic behaviour.
Fig. 6.
Experimental set-up for analysing the direction of root growth in an oblique NM→WSM water potential gradient (positive to negative). NM was placed diagonally in the upper section of the square plate, and WSM was positioned in the lower section. (A) Five-day-old seedlings with straight roots of uniform size (1.5–2.0 cm) were placed vertically with their tips 0.5 cm above the boundary between both substrates. Plates were maintained in the vertical position for the duration of the experiment. Photographs were taken at different times after the transfer. The arrow in the first image delimits the border between NM and WSM. NM, normal medium; WSM, water stress medium. (B) Root growth of ahr1 and the wt in the oblique NM→WSM experimental system. The root length of ahr1 seedlings was considerably different from that of the wt (Student’s t-test, p < 0.001). Each point represents the mean ±SD. The images are representative of three independent experiments (n=60). Scale bar=13 mm.
ahr1 root tip morphology
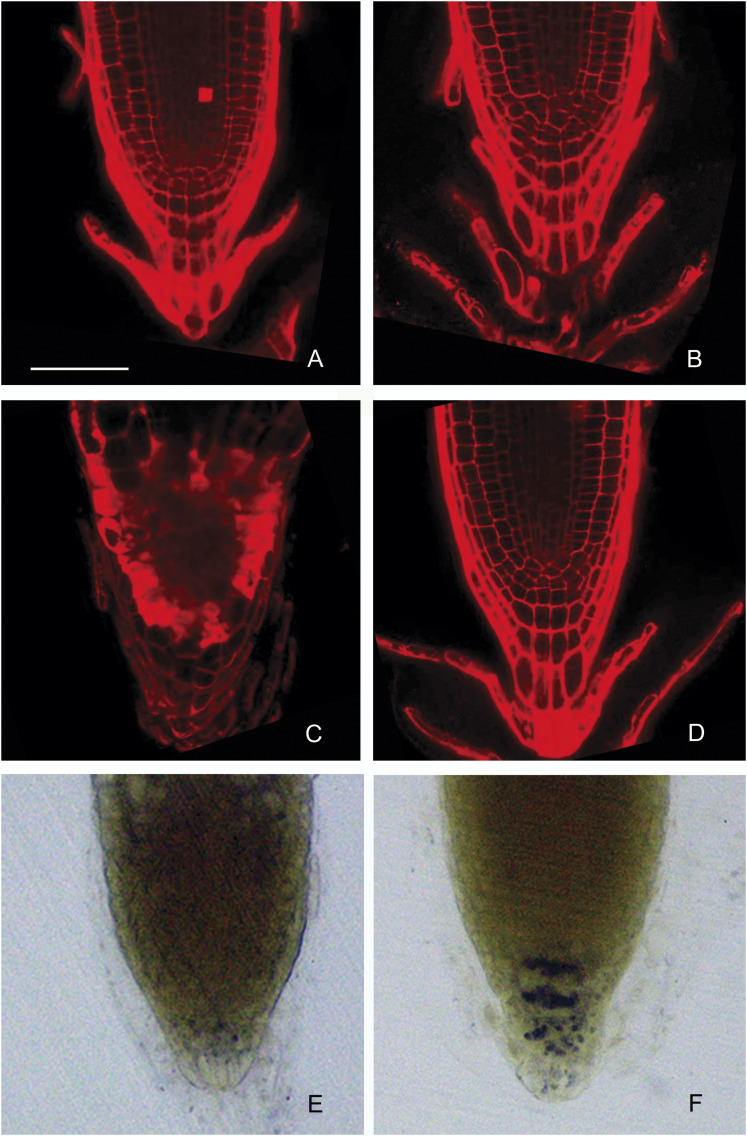
Since moisture gradients are perceived in the root cap (Jaffe et al., 1985), the root tip morphology of ahr1 seedlings was analysed. Comparison of wt and ahr1 root tips revealed that the water deficit conditions of the horizontal WSM→NM screening system damaged almost all the cells in root tips of the wt, since these readily accumulated propidium iodide (Fig. 7C). Most notably, the root meristematic cells of wt seedlings were the most affected (Fig. 7C). Root cap cells of the wt were also altered in a horizontal WSM→NM screening system since they were usually larger in size compared with those grown in the NM (Fig. 7C). In contrast, ahr1 root tip morphology in the screening system was normal and comparable with that of the wt grown in NM (Fig. 7A, D), indicating that cells in the root cap and in the root proper contended quite well with the low water potential of the horizontal WSM→NM screening system, maintaining not only cell turgor but ultimately cell growth. Previous studies have shown that water stress conditions produced a significant reduction in starch-filled amyloplasts of columella cells in the wt but not in the nhr1 mutant (Takahashi et al., 2003; Ponce et al., 2008a , b ). To investigate whether the water deficit conditions of the horizontal WSM→NM screening system induced a decrease in starch-filled amyloplasts of ahr1 root cap columella cells, amyloplasts were visualized by staining starch with I2–KI solution in 10-day-old ahr1 seedlings. ahr1 columella cells maintained several starch-filled amyloplasts, in clear contrast to those of the wt (Fig. 7E, F), indicating that these cells are well acclimated to the water potential gradient of the horizontal WSM→NM screening system. Furthermore, the amyloplasts of ahr1 root tips also stained strongly in lugol solution compared with those of the wt when grown in a steeper water potential gradient generated with glycerol (Supplementary Fig. S2B at JXB online).
Fig. 7.
Morphology of wt and ahr1 root tips grown in the horizontal WSM→NM screening system. Wt (A) and ahr1 (B) seedlings after 10 d in NM. The wt (C) and ahr1 (D) after 10 d in the screening system. Seedlings were stained with propidium iodide for microscopic examination with a confocal microscope. Starch in amyloplasts was maintained in columella cells of ahr1 roots grown in the screening system (F), while it severely declined in 99% of wt roots (E). Wt (E) and ahr1 (F) seedlings after 10 d in the screening system. Amyloplasts were visualized with I2–KI solution. Images are representative of three independent experiments (n=45). Scale bar=60 μm.
ABA contributes to the altered hydrotropic response phenotype of ahr1
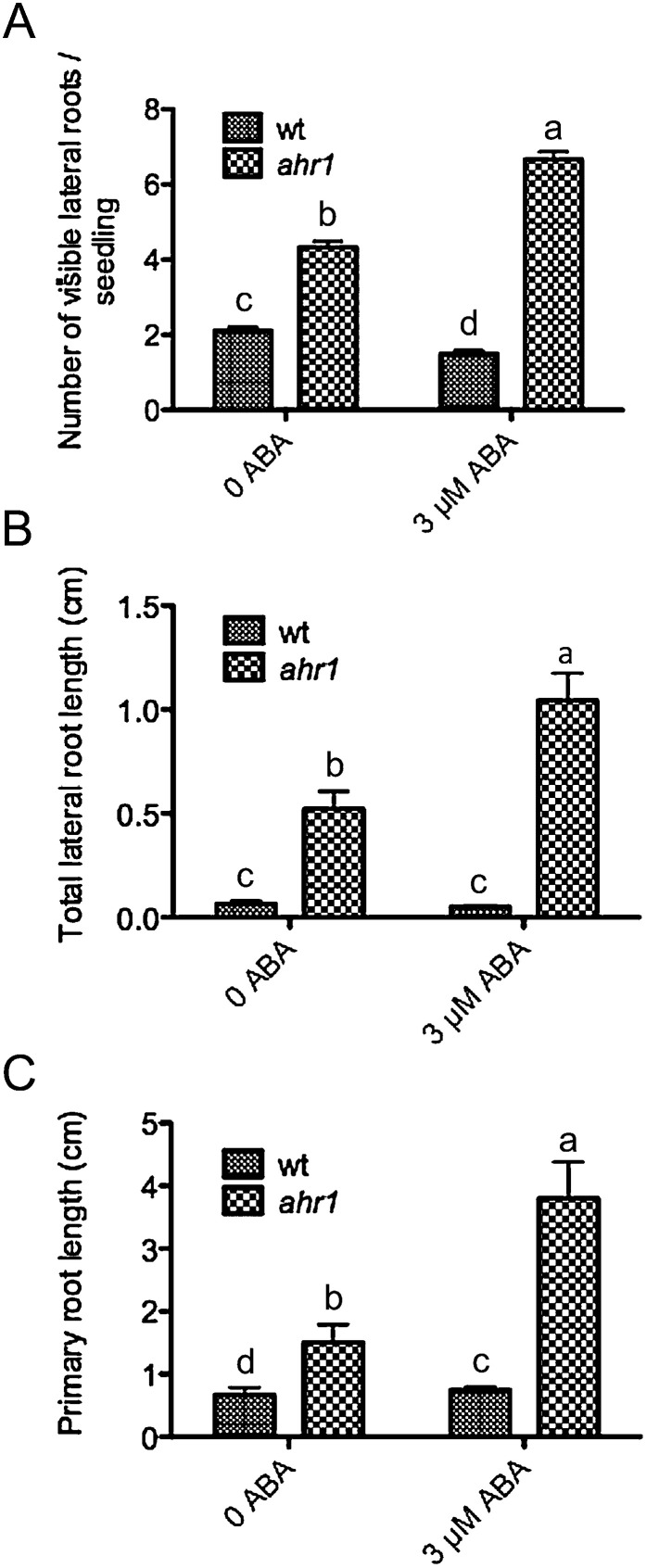
Earlier studies indicated that ABA and water stress are critical regulators of root tropic responses in Arabidopsis since nhr1 roots germinated from seeds treated with ABA in a horizontal NM→WSM system for testing hydrotropism (as in Fig. 3) showed an enhanced non-hydrotropic response growth whereas those of the wt showed an orthogravitropic rather than a hydrotropic response (Ponce et al., 2008a , b ). Hence, as a first step toward analysing whether ABA also influenced the phenotype of ahr1 in the screening system, ahr1 seeds were locally treated with ABA. ABA substantially enhanced the development of a deep and highly branched root system in ahr1 seedlings in the horizontal WSM→NM system (Figs 8A–D, 9A–C). In contrast, ABA slightly reduced the number of lateral roots in the wt, although their length was no affected (Fig. 9A, B). In addition, ABA slightly increased the length of the primary root of the wt (Fig. 9C).
Fig. 9.
The root architecture of 10-day-old ahr1 mutants was altered when treated locally with 3 μM ABA in the horizontal WSM→NM screening system. (A) Number of visible lateral roots. (B) Total lateral root length. (C) Primary root growth. Each value represents the mean ±SD, and were acquired from three different experiments (n=75). Bars with different letters differ significantly after two-way ANOVA with Bonferroni post-tests, p < 0.01 in (A), p < 0.001 in (C), and p < 0.001 in (C).
Cytokinin influences the hydrotropic response of ahr1
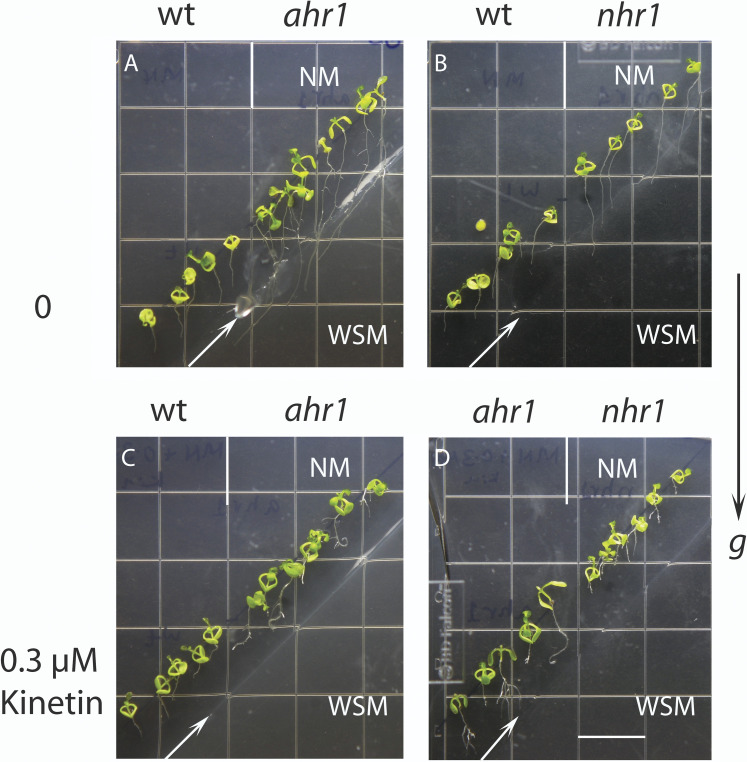
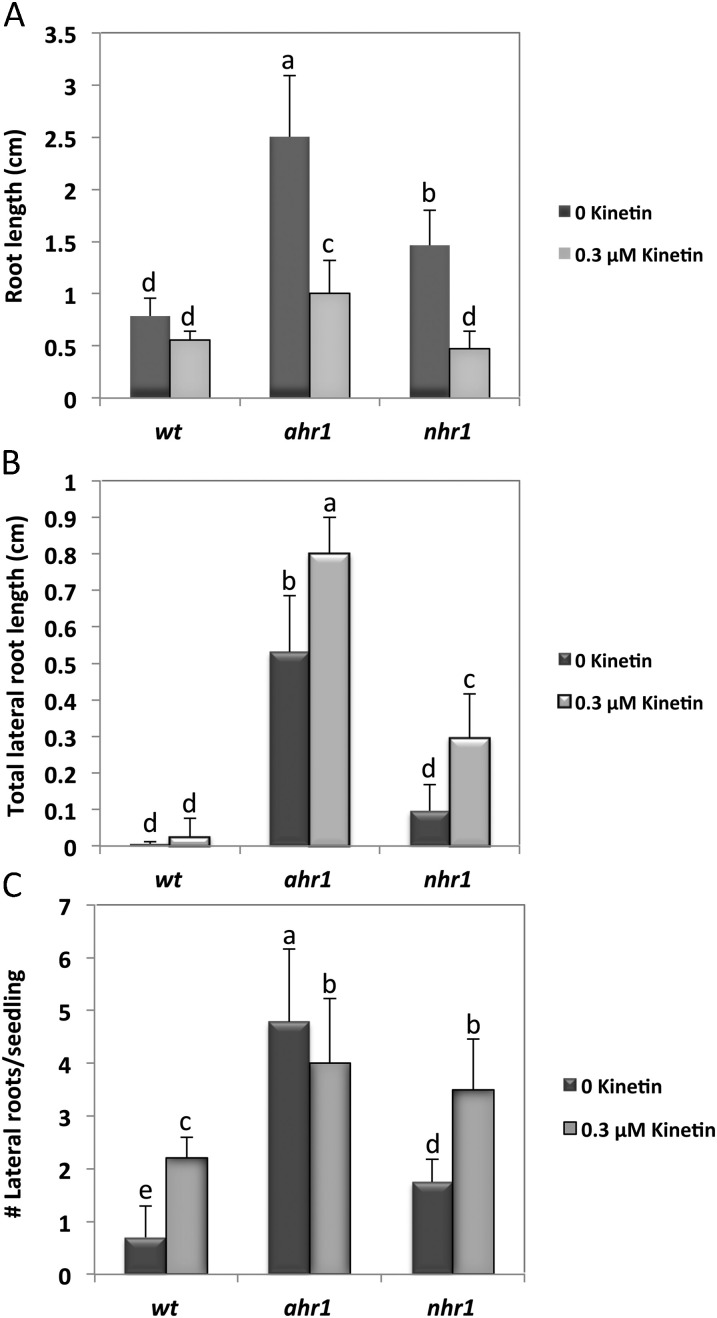
Since one of the aims of this study was to discern whether plant growth regulators influence root hydrotropism, the role of cytokinins was also analysed. Cytokinins play a negative regulatory role in root growth (Werner et al., 2003, 2010). The main site of cytokinin synthesis occurs in columella cells of root caps (Aloni et al., 2005). Cytokinins are also involved in the regulation of the early rapid phase of root gravitropic curvature initiation (Aloni et al., 2004). Exogenous cytokinin applied to vertically positioned roots induced root bending towards the application site, confirming the inhibitory effect of cytokinin in root gravitropism (Aloni et al., 2004). Consequently, the hydrotropic response of ahr1 and wt roots was tested in the diagonal NM→WSM screening system with a water potential gradient positioned obliquely in the presence of kinetin. However, in this case, seeds were germinated directly on the plates and were not transplanted as in the experiment depicted in Fig. 6. Surprisingly, the no hydrotropic response phenotype of ahr1 seedlings (Fig. 10A) was modified or reduced in the presence of kinetin, since roots were shorter and showed a stronger or higher hydrotropic curvature than in the absence of kinetin (Fig. 10C, D). Roots of ahr1 roots treated with kinetin deviated from the vertical by 53±4.1°, which was significantly different from those left untreated (34±4.7°, p < 0.001). Roots of wt seedlings were unresponsive to kinetin and developed a hydrotropic curvature similar to that of untreated roots (Figs 10A, C, 11A); however, they showed a significantly higher number of lateral roots when treated with kinetin (Fig. 11C). Arabidopsis histidine kinase ATHK1 can function as an osmosensor in yeast and has been implicated in some plant stress responses (Urao et al., 1999) since ahk1 mutants showed significant sensitivity to drought (Wohlbach et al., 2008). The ahk1 mutants were more affected than those of the wt in the water potential conditions of the diagonal NM→WSM screening system (data not shown). The total root length of the three genotypes tested decreased in the presence of kinetin (Fig. 11A). The number of lateral roots was severely affected in the ahr1 and nhr1 genotypes tested in oblique plates plus kinetin compared with those of the wt (Fig. 11B, C). However, the total lateral root length per seedling was affected in wt and mutants seedlings.
Fig. 10.
Cytokinin eliminates the altered hydrotropism of ahr1 and the no hydrotropic response phenotype of nhr1 in a diagonal NM→WSM test system for hydrotropism. In this system, the NM (top) and WSM (bottom) were obliquely split in a square Petri dish. Seeds of wt, ahr1, and nhr1 genotypes were sown in the NM portion of the oblique system in the absence (A, B) or presence of 0.3 μM kinetin (C, D). (A) ahr1 roots showed an altered hydrotropic response with longer roots that developed a hydrotropic curvature in the WSM towards the NM. (A, B) Wt roots developed a hydrotropic curvature in the NM and were shorter than those of ahr1 and nhr1. (B) nhr1 roots displayed a no hydrotropic response. (C) ahr1 roots supplied with kinetin were shorter and developed a hydrotropic curvature in the NM. (C, D) Root growth of the wt was reduced by kinetin. (D) nhr1 roots were shorter than those left untreated. Images were taken 7 d after germination. Arrows delimit the border between NM and WSM. NM, normal medium; WSM, water stress medium. Scale bar=13 mm.
Fig. 11.
Kinetin at 0.3 μM modified the root architecture of 11-day-old hydrotropic mutants ahr1 and nhr1 in the diagonal NM→WSM test system. Roots of ahr1 and nhr1 seedlings were significantly shorter in the presence of kinetin than those left untreated. The total lateral root length of ahr1 seedlings was significantly increased by kinetin. Wt and nhr1 roots increased their number of lateral roots in the presence of kinetin. (A) Root growth. (B) Total lateral root length. (C) Number of lateral roots per seedling. Each value represents the mean ±SD, and were acquired from three different experiments (n=25). Bars with different letters differ significantly after two-way ANOVA with Bonferroni post-tests, p < 0.01 in (A, C), and p <0.001 in (B).
Kinetin at a concentration of 0.3 μM slightly diminished root growth of 7-day-old ahr1, nhr1, and wt seedlings in the horizontal NM→WSM screening system for testing no hydrotropic response mutants such as nhr1 (Eapen et al., 2003) (Supplementary Fig. S4A, B, D at JXB online). In this testing system, the downward root growth (orthogravitropism) of ahr1 was unaffected in the presence of kinetin; however, orthogravitropic growth of nhr1 and the wt was significantly limited since nhr1 roots deviated ∼27° and 22° from the vertical, respectively (Supplementary Fig. S4B, C, E). This indicated that the hydrotropic curvature displayed by ahr1 roots in the presence of kinetin was not caused by an inhibition of the orthogravitropic response (Fig. 10; Supplemental Fig. S10).
In normal conditions (NM plates), 0.3 μM kinetin decreased root growth similarly in all genotypes tested, although miz1 showed reduced sensitivity (Supplementary Fig. S5A–E at JXB online). However, at 3 μM and 10 μM kinetin, the root growth of all mutants and that of wt seedlings was strongly inhibited. The slight resistant phenotype to exogenous cytokinins of miz1 was recently reported by Moriwaki et al. (2011). Interestingly, kinetin significantly repressed the orthogravitropic growth in all five genotypes tested, although ahr1, wt, and nhr1 roots were the most affected since these moved ∼75 ° away from the vertical compared with miz1 and miz2 roots, which deviated to a lesser extent (∼30 °) (Supplementary Fig. S6B, C). The root gravitropic response of ahr1 and the wt in NM was not affected after two reorientation stimuli (Supplemental Fig. S7). Thus, kinetin primarily influenced root orthogravitropic responses in normal conditions (Supplementary Fig. S6B, C) and root hydrotropic responses in the presence of water potential gradients in ahr1, nhr1 (Fig. 10C, D), miz1, and miz2 mutants (Supplementary Fig. S8C, D). The cytokinin receptor histidine kinase mutant ahk4/cre1/wol1 has been shown to develop longer roots in normal conditions (Riefler et al., 2005) and displayed a salt-tolerant phenotype (Tran et al., 2007). Interestingly, ahk4/cre1/wol1 mutant roots displayed a faster hydrotropism than those of the wt and in clear contrast to the hydrotropic mutants ahr1, nhr1, miz1, and miz2 (Supplementary Figs S9, S8; Figs 3, 10). Overall, these results suggested that cytokinins play a critical role in root hydrotropism.
Discussion
Plant roots in search of water optimize their root architecture accordingly (Lynch, 1995; Malamy, 2005). More specifically, the spatial positions of the water supply and the root hydrotropic response have a profound effect on root architecture (Tsutsumi et al., 2003; Eapen et al., 2005). Therefore, studies that assess how root hydrotropism controls the regulation of root architectural traits are beneficial to determine the precise role of these traits in improving plant performance under given environmental conditions. In this work, the identification of a gene in Arabidopsis that affects the root hydrotropic response by allowing the primary root to elongate and increased the size of the root system in response to a gradient in water potential over time is described (Figs 1, 8). Interestingly, the ahr1 phenotype is comparable with that of the wt under normal conditions in primary root growth, root tip morphology, and number and total length of lateral roots (Figs 2B, 7A, B, 9E, F), but showed a distinctive rapid osmotic adjustment in order to respond hydrotropically in the different systems tested, which included an osmotic potential gradient. Additionally, in the absence of a water potential gradient, the growth of ahr1 and wt roots was equally sensitive at different glycerol concentrations in the medium. A similar behaviour was seen in the presence of other effectors (sorbitol or mannitol; data not shown), further indicating that the ahr1 phenotype is basically manifested in the presence of a water potential gradient in the medium, a condition that usually takes place in the soil (Nilsen et al., 1983).
The oblique test system for studying hydrotropism allowed the detection of the altered hydrotropic root growth of the ahr1 mutant towards the substrate with higher water potential. In this system, ahr1 roots grew towards the low water potential medium (WSM) following the gravitropic vector. However, as soon as they entered the WSM, they turned the direction of their growth at a 30 ° angle towards the substrate with higher water potential (NM). In the absence of a water potential gradient, the angle of deviation from the gravitational vector was ∼5 °, indicating that ahr1 differential root growth is the consequence of an altered hydrotropic response since wt roots stopped their growth in the oblique test system. ahr1 roots deviated from the vertical and grew toward regions with higher water potential (Fig. 6). The altered hydrotropic response revealed by ahr1 in this system seems to be related to two different processes: (i) the ability to overcome the arrest of primary root growth seen in the wt by maintaining the integrity of its root cap (Fig. 1); and (ii) the ability to maintain the capacity of its root cap to respond to water potential gradients (Fig. 7). The structure of the root cap in hydrostimulated ahr1 seedlings was preserved, in contrast to the wt, and there were no features among ahr1 root caps to distinguish them from seedlings growing in a normal or screening system (Fig. 7B, D). Consequently, the preservation of root cap structure and functions is crucial for growing under these conditions. A previous study indicated that root caps contain essential components of the signalling system that determines root architecture (Tsugeki and Fedoroff, 1999). Genetic root cap ablation altered root architecture both by inhibiting root meristematic activity and by stimulating lateral root initiation (Tsugeki and Fedoroff, 1999). The histological analyses also revealed that the preservation of root cap columella cells with amyloplasts in ahr1 mutants is probably correlated with the capacity for continuing root growth in different osmotic potential gradients. The analyses presented in this work demonstrated the plasticity of ahr1 roots in response to gradually changing environments. The development of the root system in plants growing in dry soil is generally less inhibited than shoot growth, and may even be promoted, as has been shown in maize (Sharp et al., 2004). Maintenance of root growth during water deficits is a clear advantage to sustain an adequate water supply and is under genetic control (O’Toole and Bland, 1987). To determine the threshold level of the water potential perceived by ahr1 roots, ahr1 seeds were planted diagonally in the WSM section of the horizontal WSM→NM screening system (Supplementary Fig. S2A at JXB online). All ahr1 roots grew, but only those of seeds that were planted 1.1 cm above the boundary between the two media reached the lower half of the screening system, where the water potential is higher (NM, –0.6 MPa). Interestingly, all roots showed preservation of the apical region as well as the morphology or structure of the root cap with amyloplasts in columella cells (Supplementary Fig. S2B). Future research on the mechanism sustaining cell elongation under water potential gradients must focus on the apical region of ahr1 roots, its vacuole and amyloplast regeneration mechanism, which allow ahr1 roots to grow in the low water potential gradients.
Previous work had shown that there is substantial osmotic adjustment, mostly by proline deposition in the tips of maize primary roots growing at low water potential (Sharp et al., 1990). It still remains to be deciphered how ahr1 roots accomplish their osmotic adjustment. Thus far, it has been shown that ABA is necessary for the maintenance of maize primary root elongation during water stress (Sharp and LeNoble, 2002; Sharp et al., 2004). Local addition of ABA to seeds enhanced the root proliferation phenotype of the ahr1 mutant in the screening system, and negatively affected that of the wt (Figs 8A–D, 9A–C). In contrast, ABA showed no influence on the root system size of ahr1 grown in NM (Fig. 8E, F; Supplementary Fig. S3 at JXB online). Hence, these results illustrate that maintenance of root elongation and increase in root proliferation of ahr1 by ABA under water deficit conditions is based on the environmental cues which modify growth response to ABA. ABA plays an inductive role in drought rhizogenesis, which is characterized by the formation of short, tuberized, hairless roots (Vartanian et al., 1994). This is an adaptive strategy that occurs in Brassicaceae and related families when subjected to a progressive water stress (Vartanian et al., 1994). These roots are capable of enduring a prolonged drought period and give rise to a new functional root system upon rehydration. In contrast, ABA-deficient (aba1) and ABA-insensitive (abi1) mutants showed a dramatic reduction in the number of lateral roots produced per mg of root biomass after progressive drought stress (Vartanian et al., 1994). Interestingly, in the absence of drought stress, ABA also plays a direct role in lateral root regulation since wt seedlings treated with ABA showed a significant reduction in the number of visible lateral roots (De Smet et al., 2003, 2006). Here ahr1 roots sustained the capacity for proliferation under water potential gradients in the presence of ABA, indicating that ABA inhibition of meristem activation after lateral root emergence under non-optimal conditions is released in ahr1 mutants.
Another interesting finding is the connection between hydrotropism and cytokinins. The altered hydrotropic response phenotype of the ahr1 mutant in the screening system seems to be related to cytokinin regulation of root elongation under higher osmotic conditions (Tran et al., 2007; Wohlbach et al., 2008; Huang et al., 2009; Werner et al., 2010). It is hypothesized that when wt roots are hydrostimulated, cytokinins apparently inhibit root elongation and enable root hydrotropic curvature. Hence, the ahr1 mutant might have modified this regulatory mechanism since, in the presence of exogenous cytokinins, it showed an exaggerated hydrotropic root growth response (Fig. 10; Supplementary Fig. S10 at JXB online). Cytokinins also altered root architecture in ahr1 seedlings in the obliquely positioned NM→WSM test system since ahr1 roots not only displayed hydrotropic curvature but were also significantly shorter, and had fewer and longer lateral roots compared with those which were left untreated (Fig. 11). Similarly, orthogravitropic growth of ahr1, miz1, and miz2 roots was sensitive to cytokinins when the higher water potential condition was positioned at 45 ° to their root tips. In contrast, the root architecture of ahr1 seedlings treated with kinetin in the horizontal NM→WSM screening system also developed shorter roots; however, these did not develop a hydrotropic curvature (Supplementary Fig. S4B). These results indicate that the orthogravitropic growth of ahr1 roots was insensitive to cytokinins or to the presence of low water potential conditions which are more unique to the horizontal NM→WSM screening system. Thus, orthogravitropism in ahr1 or lack of hydrotropism in miz1 and miz2 could only be abated if the water potential gradient was positioned at 45° and not at 0° solely in the presence of cytokinins. Why did ahr1 primary roots not grow or showed a hydrotropic response in the horizontal NM→WSM screening system in the presence of kinetin? The root growth response of ahr1 might be related to the direction of the water potential gradient since the water potential gradient developed in the two systems was similar (data not shown). Conceivably, the orthogravitropic response of ahr1 roots is stronger when the direction towards the higher water potential gradient follows the gravity vector, but can be overcome when the direction of the gradient is positioned obliquely.
ahr1 mutant seedlings showed major alterations in ABA and cytokinin responses, indicating that ABA and cytokinin signalling play a role in the adaptation of root architecture to changes in water deficit conditions. Recently, it has been shown that MIZ1 also regulates the root architecture under stressed conditions (Moriwaki et al., 2011). miz1 mutants did not show a defect in lateral root development under normal and water stress conditions. However, overexpression of MIZ1 resulted in a reduced number of lateral roots and greater inhibition of root growth in the presence of cytokinins; hence, MIZ1 negatively regulated cytokinin sensitivity of root development (Moriwaki et al., 2011). Previous results and those of the present study indicated that root hydrotropism and root system development are closely linked via cytokinin signalling. In contrast to ahr1, the cytokinin receptor mutant cre1/ahk4/wol1, which also displayed drought tolerance (Tran et al., 2007), showed an enhanced root hydrotropic response and short roots in the horizontal NM→WSM test medium for hydrotropism even compared with those of the wt (Supplementary Fig. S9 at JXB online). This indicated that cytokinin perception is required for root growth under water deficit conditions.
Overall, the present data suggest that a mutation in AHR1 alleviates root growth arrest in the presence of a gradient in the water potential, thereby allowing an altered hydrotropic response and increased branching relative to the wt. It remains to be deciphered whether the ahr1 mutation has direct effects on the conditional regulation (upon a water gradient) of general root growth and branching compared with water sensing. Basically, the identification of all other genes involved in water sensing and growth arrest by high osmoticum is needed in order to determine how AHR1 relates to these pathways. The ability of plants to produce an optimal root system is a critical adaptive response for survival, particularly in seasonal water deficit conditions. Therefore, the present results demonstrate that there is considerable potential to increase the fitness of crop plants by altering pathways that regulate the response of roots to water potential gradients. Ongoing work will determine the molecular identity of AHR1 and elucidate the mechanisms by which it maintains root cap morphology under different osmotic conditions, leading to their long-term growth adjustment to osmotic potential gradients.
Supplementary data
Supplementary data are available at JXB online online:
Figure S1. The effect of glycerol on root growth of 5-day-old ahr1 and wt seedlings.
Figure S2. Roots of ahr1 grow in the presence of a gradient of water potential in the substrate.
Figure S3. Effect of ABA on root growth of ahr1 and wt seedlings in normal conditions.
Figure S4. Cytokinins did not affect root downward growth of ahr1 seedlings in the horizontal NM→WSM test system for hydrotropism.
Figure S5. Tolerance of ahr1, nhr1, miz1, miz2, and the wt to exogenous cytokinins.
Figure S6. The effect of kinetin in the orthogravitropic root growth of the hydrotropic mutants and the wt.
Figure S7. Root gravitropic response of ahr1 and wt seedlings in the presence of cytokinins.
Figure S8. Cytokinin affects the impaired hydrotropism of miz1 and miz2 mutants in a diagonal NM→WSM test system for hydrotropism.
Figure S9. Behaviour of cre1/wol1/ahk4, nhr1, and wt roots in the horizontal NM→WSM test system for hydrotropism.
Figure S10. Fourteen-day-old ahr1and nhr1 hydrotropic mutants in the diagonal NM→WSM test system with exogenous cytokinins.
Figure S11. ahr1 root seedlings showed an altered hydrotropic response in the presence of moisture gradients in a closed chamber.
Acknowledgments
The authors are grateful to Dr Barbara Pickard and Dr Clifford LaMotte for valuable discussions and suggestions, and to Dr Jorge Nieto-Sotelo for critically reviewing the manuscript. We also thank Hideyuki Takahashi and members of his lab for sharing their experimental set-up for hydrotropism with a moisture gradient and for valuable scientific input for this research. Shirley Ainsworth is gratefully acknowledged for helpful library support, Roberto Rodríguez for valuable computer assistance, Yulemi Escamilla for the genetic analysis of ahr1 and some of the analyses of miz1 and miz2 mutants, Luis Romero-Carachuri for bulk mapping analysis, and Adrián Rodríguez-Acosta for water potential determinations of the screening systems. This work was supported by the Consejo Nacional de Ciencia y Tecnología [46022Q and 81533], by the Dirección General de Asuntos del Personal Académico [DEGAPA-UNAM IN220807 and IN226810], and by a grant from the University of California Institute for Mexico and the United States [UC-Mexus CN-05-166].
References
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ulrich CI. Root synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. Journal of Experimental Botany. 2005;56:1535–1544. doi: 10.1093/jxb/eri148. [DOI] [PubMed] [Google Scholar]
- Aloni R, Langhans M, Aloni E, Ulrich CI. Role of cytokinin in the regulation of root gravitropism. Planta. 2004;220:177–182. doi: 10.1007/s00425-004-1381-8. [DOI] [PubMed] [Google Scholar]
- Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. Maximum rooting depth of vegetation type at the global scale. Oecologia. 1996;108:5833–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- Cassab GI. Other tropisms and relationship to gravitropism. In: Gilroy S, Masson PH, editors. Plant tropisms. London: Blackwell Publishing; 2008. pp. 123–139. [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis . The Plant Journal. 2003;33:543–555. doi: 10.1046/j.1365-313x.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- De Smet I, Zhang H, Inzé D, Beeckman T. A novel role of abscisic acid emerges from underground. Trends in Plant Science. 2006;11:435–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Dordolot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X. Root systems architecture: opportunities and constrains for genetic improvement of crops. Trends in Plant Science. 2007;12:474–481. doi: 10.1016/j.tplants.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Eapen D, Barroso ML, Campos ME, Ponce G, Corkidi G, Dubrovsky JG, Cassab GI. A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis . Plant Physiology. 2003;131:536–546. doi: 10.1104/pp.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen D, Barroso ML, Ponce G, Campos ME, Cassab GI. Hydrotropism: root growth responses to water. Trends in Plant Science. 2005;10:44–50. doi: 10.1016/j.tplants.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Bengough G, Cassab GI, Ponce G. Root caps and rhizosphere. Journal of Plant Growth Regulation. 2003;21:352–367. [Google Scholar]
- Huang J-G, Yang M, Liu P, Yang G-D, Wu C-A, Zheng C-C. GhDREB1 enhances abiotic stress tolerance, delays GA-mediated development and represses cytokinin signaling in transgenic Arabidopsis. Plant, Cell and Environment. 2009;32:1132–1145. doi: 10.1111/j.1365-3040.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Takahashi H, Biro RL. A pea mutant for the study of hydrotropism in roots. Science. 1985;230:445–447. doi: 10.1126/science.230.4724.445. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Takahashi A, Kakimoto Y, Miyazawa Y, Fuji N, Higashitani A, Takahashi H. A gene essential for hydrotropism in roots. Proceedings of the National Academy of Sciences, USA. 2007;104:4724–4729. doi: 10.1073/pnas.0609929104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Gilmor CS, Scheible WR. Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiology. 2000;123:795–805. doi: 10.1104/pp.123.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- Miyazawa Y, Takahashi A, Kobayashi A, Kaneyasu T, Fujii N, Takahashi H. The GNOM-mediated vesicular trafficking plays an essential role in hydrotropism of Arabidopsis roots. Plant Physiology. 2009;149:835–840. doi: 10.1104/pp.108.131003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita MT. Directional gravity sensing in gravitropism. Annual Review of Plant Biology. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- Moriwaki T, Miyasawa Y, Kobayashi A, Uchida M, Watanabe C, Fujii N, Takahashi H. Hormonal regulation of lateral roots development in Arabidopsis modulated by MIZ1 and requirement of GNOM activity. Plant Physiology. 2011;157:1209–1220. doi: 10.1104/pp.111.186270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen ET, Sharifi MR, Rundel PW, Jarrel WM, Virginia RA. Diurnal and seasonal water relations of the desert phreatophyte Prosopis glandulosa (honey mesquite) in the Sonoran desert of California. Ecology. 1983;64:1381–1393. [Google Scholar]
- O’Toole JC, Bland WL. Genotypic variation in crop plant root systems. Advances in Agronomy. 1987;41:91–145. [Google Scholar]
- Ponce G, Razgado F, Cassab GI. Roles of amyloplasts and water deficit in root tropisms. Plant, Cell and Environment. 2008a;31:205–217. doi: 10.1111/j.1365-3040.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- Ponce G, Razgado F, Cassab GI. How amyloplasts, water deficit and root tropism interact? Plant Signaling and Behavior. 2008b;3:460–462. doi: 10.4161/psb.3.7.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell. 2005;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Hsiao TC, Silk WK. Growth of the maize primary root at low water potentials. II. The role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiology. 1990;93:1337–1346. doi: 10.1104/pp.93.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME. ABA, ethylene, and the control of shoot and root growth under water stress. Journal of Experimental Botany. 2002;53:33–37. [PubMed] [Google Scholar]
- Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT. Root growth maintenance during water deficits: physiology to functional genomics. Journal of Experimental Botany. 2004;55:2343–2351. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Miyazawa Y, Fujii N. Hormonal interactions during root tropic growth: hydrotropism versus gravitropism. Plant Molecular Biology. 2009;69:489–502. doi: 10.1007/s11103-008-9438-x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Suge H. Root hydrotropism of an agravitropic mutant, ageotropum . Physiologia Plantarum. 1991;82:24–31. [Google Scholar]
- Takahashi N, Goto N, Okada K, Takahashi H. Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana . Planta. 2002;216:203–211. doi: 10.1007/s00425-002-0840-3. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Yamazaki Y, Kobayashi A, Higashitani A, Takahashi H. Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiology. 2003;132:805–810. doi: 10.1104/pp.018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L-S, Urao T, Qin T, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis . Proceedings of the National Academy of Sciences, USA. 2007;104:20623–20628. doi: 10.1073/pnas.0706547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. Aspects of plant intelligence. Annals of Botany. 2003;92:1–20. doi: 10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugeki R, Fedoroff NV. Genetic ablation of root cap cells in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 1999;96:12941–12946. doi: 10.1073/pnas.96.22.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi D, Kosugi K, Mizuyama T. Effect of hydrotropism on root system development in soybean (Glycine max): growth experiments and a model simulation. Journal of Plant Growth Regulation. 2003;21:441–458. [Google Scholar]
- Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, Shinozaki K. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. The Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian N, Marcotte L, Giraudat J. Drought rhizogenesis in Arabidopsis thaliana . Plant Physiology. 1994;104:761–767. doi: 10.1104/pp.104.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schülling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. The Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbach DJ, Quirino BF, Sussman MR. Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. The Plant Cell. 2008;20:1101–1117. doi: 10.1105/tpc.107.055871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.