Abstract
Dysbindin-1 regulates D2-receptor trafficking and is implicated in schizophrenia and related cognitive abnormalities, but whether this molecular effect mediates the clinical manifestations of the disorder is unknown. We explored in dysbindin-1-deficient mice (dys–/–) (1) schizophrenia-related behaviors, (2) molecular and electrophysiological changes in medial prefrontal cortex (mPFC) and (3) the dependence of these on D2-receptor stimulation. Dysbindin-1 disruption altered dopamine-related behaviors and impaired working memory under challenging/stressful conditions. Dys–/– pyramidal neurons in mPFC layers II/III were hyperexcitable at baseline but hypoexcitable following D2 stimulation. Dys–/– were also respectively more and less sensitive to D2 agonist- and antagonist-induced behavioral effects. Dys–/– had reduced expression of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and CaMKKβ in mPFC. Chronic D2 agonist treatment reproduced these changes in protein expression, and some of the dys–/– behavioral effects. These results elucidate dysbindin's modulation of D2-related behavior, cortical activity and mPFC CaMK components, implicating cellular and molecular mechanisms of the association of dysbindin with psychosis.
Keywords: dopamine, genes, mice, prefrontal cortex, schizophrenia, working memory
Introduction
Dysbindin-1, hereafter referred to as dysbindin, is encoded by the dystrobrevin-binding protein 1 gene (DTNBP1), is located in synaptic sites throughout human and mouse brain1 and has been implicated in the regulation of exocytosis and vesicle biogenesis in endocrine cells and neurons.2 Genetic variations in DTNBP1 impact human cognitive abilities.3,4 Moreover, several studies have associated DTNBP1 genetic variations with risk for schizophrenia,5–7 and reduced dysbindin gene and protein expression have been reported in the hippocampus and prefrontal cortex (PFC) of schizophrenic patients.8–11 These changes might be linked to risk associated variation in the gene.12 Decreased dysbindin protein levels in schizophrenia have been consistently reported, though contrasting evidence exists for changes in mRNA expression.8–11 Reduced dysbindin levels have also been linked to increased D2-receptor abundance on the neuronal surface in mice and in cell culture,13,14 suggesting a potential pathophysiological link to psychosis, which has long been thought to involve D2 mechanisms.15
Dopamine (DA) signaling is critical for modulating higher order cognitive functions, impacting many domains of human behavior, thought and emotion.16 Dysbindin mRNA and protein are present in the substantia nigra pars compacta,1 where DA neurons are abundant. Knockdown of dysbindin in PC12 cells increases DA release, and dysbindin overexpression tends to have the opposite effect, whereas neither manipulation impacts total tissue DA levels.17 Sandy (sdy) mice, which bear a spontaneous genetic deletion in the Dtnbp1 gene that results in the complete loss of dysbindin protein,18 display a higher homovanillic acid/DA ratio compared with normal wild-type mice in cortico-limbic brain regions.19 Downregulation of dysbindin in human Sh-SY5Y neuroblastoma cells, rat primary cortical neurons and in dysbindin mutant mice, all cause increased cell surface DA D2-receptor abundance, through enhanced recycling and insertion of D2 receptors into the neuronal membrane. Consequently, downregulation of dysbindin strengthens D2 signaling, whereas D1 receptors are unchanged.13,14 Thus, dysbindin may regulate DA signaling pre- and post-synaptically by modulating DA release and turnover and D2 receptors cell surface cycling. In addition to its role in DA signaling, dysbindin has been implicated in regulating both pre- and post-synaptic aspects of glutamatergic signaling and synaptic stability,20–22 which represent further potential pathogenic components of psychosis.
To elucidate the potential impact of these molecular effects of dysbindin on cognitive function, emotional arousal and the neurobiological basis of altered behavioral phenotypes, we have performed a series of experiments in dysbindin mutant mice to test the behavioral, pharmacological, electrophysiological and molecular implications of reduced dysbindin expression in the context of altered DA signaling. The dysbindin-1 null mutant mouse is an animal model of reduced dysbindin protein expression reported in schizophrenia.1,8–11,23 In addition to cognitive and behavioral tests thought to model schizophrenia-associated behaviors, we also examined the impact of dysbindin genetic modifications on neuronal excitability in the superficial layers of mPFC and the cortical expression of components of the Ca2+/calmodulin-dependent protein kinase (CaMK) superfamily. We investigated the expression of CaMK components because of their roles in learning and memory processes24 and in DA trafficking in the brain.25–28 Moreover, we also investigated the behavioral and CaMK molecular changes following pharmacologic manipulations of DA/D2 pathways and compared them with the effects of reduced dysbindin expression. Our results support the view that dysbindin may add to risk for psychosis by disrupting DA/D2-related mechanisms regulating cortical function and neuronal excitability.
Materials and methods
Subjects
All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and followed the NIH Guidelines ‘Using Animals in Intramural Research.’ Sdy is an autosomal recessive coat mutation that occurred spontaneously in the inbred DBA/2J strain in 1983 at The Jackson Laboratory. The mutation present in our mice was transferred to the C57BL/6J (B6) genetic background by 11 generations of backcrossing into B6 at The Jackson Laboratory and National Institute of Mental Health. The purpose of this backcrossing procedure was to remove the effects on behavior and DA function of the native sdy mouse background strain (DBA/2J), which has been shown to have abnormal behavioral phenotypes referable to the DA system.29 In particular, previous reports have shown that on the DBA/2J genetic background, sdy mice exhibit strong locomotor activity and coordination deficits, as demonstrated by poor performance in the rotarod test and even death during a forced swimming test.30 Also, DBA/2J mice are impaired in θ burst long-term potentiation, in aspects of learning and memory, have higher dopaminergic activity in the forebrain, and are homozygous for four other mutations (cadherin, glycoprotein, tyrosinase-related protein 1 and hemolytic complement) compared with B6 mice.29,31,32 Therefore, the role of the dysbindin mutation is potentially confounded when studied on the DBA/2J genetic background.1,23 In these experiments, we used male littermates that were dysbindin-1 null mutant (dys–/–), heterozygous (dys+/–), and wild-type (dys+/+) bred by a heterozygous (dys+/– × dys+/–) mating strategy. Genotypes were identified by PCR analysis of tail DNA (for details see Supplementary Information and Supplementary Figure 10).
Behavioral tasks, immunoblotting and electrophysiology
Experiments were performed as previously described.14,27,33 In the discrete paired-trial variable-delay T-maze task, mice were presented with a sequence of randomly chosen forced runs, each followed by a choice run so that they were required to integrate information held online (the forced run) with the learned rule (non-match to sample). In the spatial T-maze task of reference memory, we used the same T-maze apparatus but mice were only required to acquire a simple spatial rule: the same arm of the maze was baited on every trial. For detailed information on behavioral testing, immunoblotting and electrophysiological methods, see Supplementary Information.
Acute treatment with a dopaminergic D2-receptor agonist (quinpirole) and antagonist (eticlopride)
Mice were injected intraperitoneally, in a volume of 10 ml kg–1 of body weight. Within each genotype, mice were assigned to receive an injection of vehicle (0.9% saline) or eticlopride (Sigma-Aldrich, St Louis, MO, USA; either 0.1 or 0.5 mg kg–1) and vehicle or quinpirole (LY-171,555; Sigma-Aldrich; either 0.5 or 1 mg kg–1), according to a full Latin-square design, wherein each mouse was randomly treated with all of the agonist/antagonist doses used and twice with vehicle. At least 1 week elapsed between exposures to the different drug doses. Mice were injected immediately before the test.
Chronic treatment with the dopaminergic D2-receptor agonist quinpirole
To determine the effect of the chronic quinpirole treatment on mPFC molecular measures, naive wild-type (dys+/+) mice were repeatedly treated for 15 consecutive days with vehicle or one of two doses of quinpirole, 0.5 mg kg–1 (Quin. 0.5) or 1 mg kg–1 (Quin. 1). The brains of these mice were removed 75 min after the final injection, and the mPFC was dissected and stored at –80 °C. The dissected cortical area refers to medial PFC, which comprise anterior cingulate, prelimbic and infralimbic cortices. Note that the rodent prelimbic cortex is considered the nearest homolog of the dorsolateral PFC in humans.34 To evaluate the effect of chronic quinpirole treatment on behavioral measures, naive B6 wild-type (dys+/+) mice were treated daily with vehicle or 0.5 mg kg–1 (Quin. 0.5). The behavioral tests were performed exactly as reported for the dysbindin mutant mice and started after 12 days of treatment. Mice were injected 60 min before the start of the behavioral tests.
Statistical analysis
Results are expressed as mean±standard error of the mean (s.e.m.) throughout. Two-tailed Fisher's exact analyses and one- or two-way analysis of variance were used. For details see Supplementary Information. Post hoc analyses for individual group comparisons used Newman–Keuls analyses. The accepted value for significance was P≤0.05.
Results
Faster acquisition but worse working memory performance in dysbindin mutant mice
Dys–/– and +/– mice have undetectable and reduced dysbindin protein levels, respectively.1,18,23 Reduced dysbindin levels did not affect the general health and sensorimotor abilities (including physical and reflex functions) of genetically modified mice (Supplementary Table 1).1 Working memory deficits have been described as core cognitive features of schizophrenia and are related to PFC DA function.35 Human subjects carrying dysbindin-risk haplotypes for schizophrenia manifest cognitive dysfunction3 and deficits in spatial working memory performance.36 We therefore tested dys–/– ,+/– and +/+ littermates in an mPFC-dependent spatial working memory T-maze task.27,33
Dys–/– , +/– and +/+ mice readily learned to run quickly through the maze to retrieve the reward (F1,29 = 119.46, P < 0.0001; Figure 1a), with no geno-type differences (F2,29 = 1.41, P = 0.26). However, dys–/– acquired the task in fewer days than +/+ littermates (F2,29 = 4.50, P < 0.05; Figure 1b). Thus, disruption of the dysbindin gene resulted in faster acquisition of this working memory task.
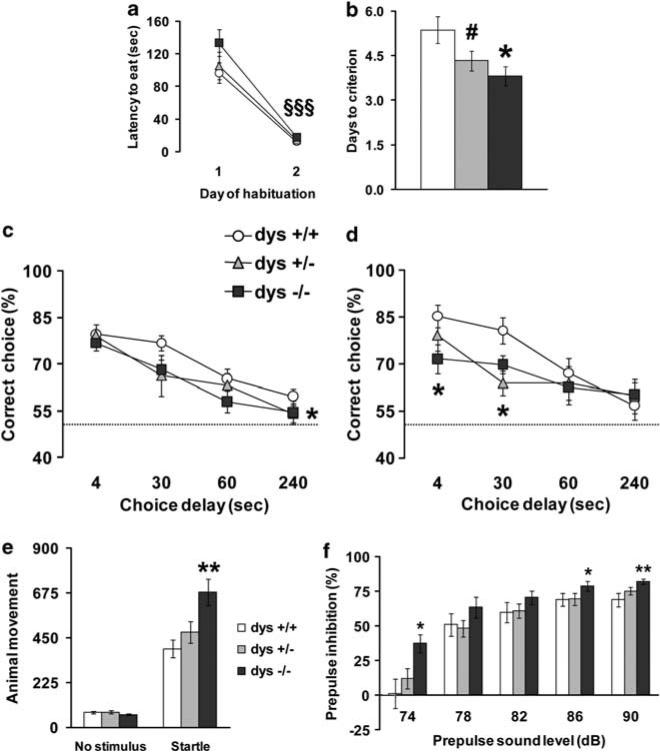
Figure 1.
Dysbindin mutant mice show faster acquisition to criteria but impaired working memory performance in a prefrontal cortex-dependent T-maze task. (a) Latency to retrieve the hidden food pellet and (b) days needed to reach criterion, by dys+/+, +/– and –/– littermates during the discrete paired-trial T-maze task; *P < 0.05 and #P = 0.07 vs dys+/+, §§§P < 0.0005 vs day 1. (c, d) Percentage of correct choices displayed by the same dys+/+, +/– and –/– mice during the discrete paired-trial variable-delay T-maze test with different intra-trial delays randomly presented (4, 30, 60 and 240 s) and an inter-trial delay of 20 s under normal conditions (c) and during the mild stress of placement in a new home cage 15 min before testing (d). The dotted line corresponds to chance levels (50%) of correct choices. *P < 0.05 vs +/+ mice. Values represent mean±s.e.m. in all the figures. A similar number of the dys–/– (N=11), +/– (N=10) and +/+ (N=11) mice tested reached the criteria (100, 90 and 100%, respectively; P = 0.6). Acoustic startle and prepulse inhibition (PPI) are increased in dysbindin mutant mice. (e) Animal movements displayed by dys+/+, +/– and –/– littermates during no stimulus trials or following the presentation of a 120-dB stimulus (Startle). (f) Percent PPI of the acoustic startle response displayed by the same mice after the presentation of 74, 78, 82, 86 and 90 dB prepulse sound stimuli. Ns: +/+ = 15, +/–= 12, –/–= 16. *P < 0.05 and **P < 0.005 vs +/+. For all genotypes, PPI progressively increased with higher prepulse intensities (F4,160 = 81.00, P < 0.0001).
Mice were then tested in the discrete paired-trial T-maze paradigm under more demanding conditions consisting of four different intra-trial delays distributed over 16 trials a day, instead of 10 (increasing handling), with inter-trial delays reduced to 20 s instead of 20 min (increasing proactive interference).27 All groups displayed the same delay-dependent performance, progressively increasing the number of mistakes with longer delays (F3,112 = 20.12, P < 0.0001; Figure 1c) with dys–/– and +/– mice showing overall worse performance compared with +/+ performance mice considering all delays used (main effect of genotype: F2,112 = 2.97, P = 0.05; post hoc: P = 0.05 and P = 0.09, respectively). Thus, although genetic modification resulting in reduced dysbindin levels produced cognitive advantages in the acquisition of this mPFC-dependent working memory task, dysbindin reduction tended to impair working memory performance under more challenging conditions.
Mild stress exacerbates working memory deficits in dysbindin mutant mice
Mild uncontrollable stressors impair PFC working memory function and increase PFC DA levels.37,38 Therefore, we further tested these same mice under mildly stressful conditions consisting of housing each mouse in an empty clean new cage instead of their home cage during the test. When the new cage stress was introduced, the percentage of correct choices at the different intra-trial delays revealed a strong genotype effect with the short delays of 4 and 30 s (F2,56 = 5.26, P = 0.01) and a main effect of the choice delays (F3,112 = 10.78, P < 0.0001). All groups displayed delay-dependent performance deterioration, but both dys–/– and +/+ mice displayed impaired performan cecompared with +/+ littermates with the 4- and 30-s intra-trial interval (P < 0.05; Figure 1d). This profile suggests that under more stressful conditions, the detrimental effects of the Dtnbp1 mutation on working memory performance are exacerbated. Finally, the cognitive deficits seen in dysbindin mutant mice appear to be relatively specific to the working memory task as demonstrated by normal performance in a spatial T-maze paradigm for reference memory and in olfactory discrimination in the habituation/dishabituation test (see Supplementary Information and Supplementary Figures 1 and 2).
Acoustic startle and prepulse inhibition are increased in dysbindin mutant mice
The acoustic startle response is enhanced in human patients suffering from anxiety and stress-related disorders39 and is correlated with stress reactivity in mice.27 Moreover, abnormal prepulse inhibition (PPI) of an acoustic startle stimulus is found in patients with schizophrenia.40 Thus, we explored the startle response and PPI in dysbindin mutant mice. As compared with dys+/+ and +/– littermates, dys–/– mice showed higher acoustic startle reactivity to the 120-dB stimulus (genotype × acoustic sound interaction effect: F2,40 = 6.97, P = 0.005; post hoc on interaction: P < 0.005), whereas the levels of basal activity in the apparatus when no stimulus was presented did not differ (post hoc on interaction: P = 0.84; Figure 1e). Analysis of PPI of a 120-dB acoustic startle stimulus showed a main genotype effect (F2,40 = 6.60, P < 0.005; Figure 1f), with –/– mice displaying more PPI than +/+ (P < 0.005) and +/– (P < 0.05) mice. A trend for a genotype × prepulse intensity interaction was detected (F8,160 = 1.80, P = 0.08), with –/– mice displaying more PPI than+/+ mice with 74, 86 and 90 prepulse sound levels (P < 0.05; Figure 1f). Thus, these results indicate that genetic modifications resulting in reduced dysbindin levels increase reactivity to stressful events, though the pattern of effects is not consistent with PPI deficits seen in patients with schizophrenia.
Higher baseline locomotor activity but normal amphetamine-induced hyperlocomotion in dysbindin mutant mice
Hyperactivity and increased amphetamine sensitivity are considered as a rodent correlate of schizophrenia-like positive symptoms.41 To test whether dysbindin genetic modification may affect general measures of locomotor activity and locomotor activity following amphetamine treatment, we tested dys–/– ,+/– and +/+ littermates in an open field arena. Dys–/– mice were more active than +/– and +/+ mice in the novel environment. Moreover, dys–/– mice showed increased number of rearing events during the first exposure to the open field arena compared with +/+ mice (Supplementary Information; Supplementary Figure 3).
On the following day, dys–/– mice were again more active than +/+ . However, amphetamine injection (0.75 mg kg–1,+intraperitoneally) did not have any dysbindin-dependent genotype effects in locomotor activity, rearing and stereotypic behaviors (Supplementary Information; Supplementary Figures 3 and 4). These results indicate that dysbindin genetic disruption produces a hyperactive phenotype, consistent with previous findings,31 but does not affect the responses to amphetamine.
DA/D2-receptor modulation of acoustic startle and PPI in wild-type and dysbindin mutant mice
We explored if and how acute injection of a DA/D2 antagonist (eticlopride) and an agonist (quinpirole) might differently affect acoustic startle and PPI responses in the dysbindin mutant mice.
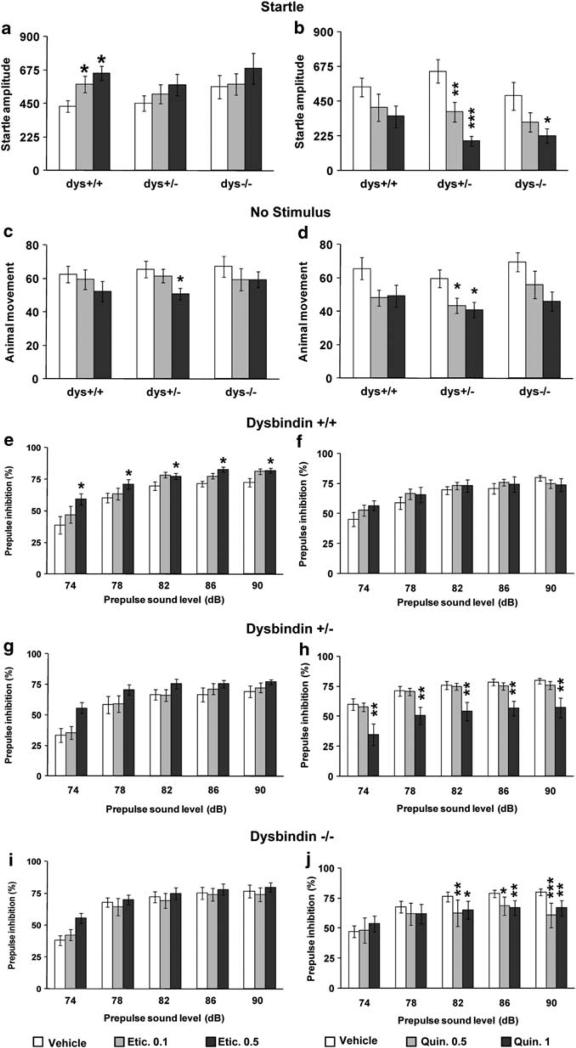
The analysis of the acoustic startle reactivity to the 120-dB stimulus following vehicle or eticlopride injection revealed a treatment-dose effect (F2,141 = 3.86, P < 0.05). Post hoc analysis revealed that in dys+/+ mice eticlopride increased startle reactivity (P < 0.05; Figure 2a). No treatment-dependent differences were present for +/– (P = 0.40; Figure 2a) or –/– littermates (P = 0.54; Figure 2a). For basal activity, a treatment-dose effect was present (F2,141 = 3.34, P < 0.05). Dys+/– showed an effect of the higher eticlopride dose to decrease the basal activity (P < 0.05; Figure 2c), whereas no treatmentdependent differences were present for +/+ (P = 0.44; Figure 2c) or –/– (P = 0.56; Figure 2c).
Figure 2.
Dopamine (DA) D2-receptor modulation of acoustic startle and prepulse inhibition (PPI) in wild-type and dysbindin mutant mice. Animal movement displayed by dys+/+, +/– and –/– littermates following the presentation of (a, b) a 120-dB stimulus (Startle) or (c, d) of no stimulus. (a, c) After acute injection with vehicle (Vehicle) or one of two doses of the D2 antagonist eticlopride, that is 0.1mgkg–1 (Etic. 0.1) or 0.5mgkg–1 (Etic. 0.5); (b, d) after acute injection with vehicle (Vehicle) or one of two doses of the D2 agonist quinpirole, that is 0.5mgkg–1 (Quin. 0.5) or 1 mg/kg (Quin. 1). (e–j) Percent PPI of the acoustic startle response displayed by (e, f) dys+/+, (g, h) dys+/– and (i, j) dys–/– littermates after the presentation of 74, 78, 82, 86 and 90 dB prepulse sound stimuli and after acute injection with (e, g, i) vehicle (Vehicle) or one of the two doses of eticlopride or (f, h, j) with vehicle (Vehicle) or one of the two doses of quinpirole. Etic. Ns: +/+ = 14, +/– = 21, –/– = 15; Quin. Ns: +/+ = 10, +/– = 16, –/–= 10. *P < 0.05, **P < 0.005 and ***P < 0.0005 vs vehicle-treated mice.
Analysis of PPI of a 120-dB acoustic startle stimulus showed a treatment-dose effect in dys+/+ (F2,39 = 5.40, P < 0.01; Figure 2e), but not in +/– (F2,60 = 2.29, P = 0.11; Figure 2g) or in –/– (F2,42 = 0.75, P =0.48; Figure 2i). Dys+/+ displayed increased PPI when treated with eticlopride as compared with vehicle (P < 0.01; Figure 2e). Thus, acute treatment with a D2 antagonist modulated both startle and PPI in mice. Moreover, dysbindin mutant mice were more resistant to the D2 antagonist effects, in line with the evidence of increased D2 receptors following dysbindin genetic reductions.
Acoustic startle reactivity to the 120-dB stimulus following vehicle or quinpirole injection revealed a treatment-dose effect (F2,99 = 14.61, P < 0.0001). Dys+/– and –/– mice both showed an effect of quinpirole to decrease the startle reactivity (P < 0.05; Figure 2b), whereas no treatment-dependent differences were present for dys+/+ littermates (P = 0.20; Figure 2b). For the basal activity, a treatment-dose effect was present (F2,99 = 6.28, P < 0.005; Figure 2d). Dys+/– mice showed an effect of quinpirole to decrease the basal activity (P < 0.05; Figure 2d), whereas no treatment-dependent differences were present for dys+/+ (P = 0.24; Figure 2d) or dys–/– mice (P = 0.14; Figure 2d).
Analysis of PPI of a 120-dB acoustic startle stimulus showed a main treatment-dose effect in dys+/– (F2,45 = 6.64, P < 0.005; Figure 2h), and a treatment × prepulse sound level interaction effect in dys–/– (F8,108 = 4.26, P < 0.0005; Figure 2j), but no treatment effects in +/+ (F2,27 = 0.32, P =0.73; Figure 2f). In particular, the higher dose of quinpirole decreased PPI in dys+/– mice, whereas both doses of quinpirole decreased PPI in dys–/– mice, compared with the vehicle treatment (P < 0.01; Figures 2h and j). Thus, these results show that acute treatment with a D2 agonist modulates both startle and PPI and does so in a manner opposite to a D2 antagonist. Moreover, dysbindin mutant mice were more sensitive to the D2 agonist effects, consistent with the increased cell surface expression of D2 receptors shown in our earlier studies on these mice.13,14 In these experiments, we did not replicate the increased startle reactivity in the dysbindin mutant mice. This was related to the injection manipulations of these last experiments (Supplementary Information; Supplementary Figure 5) and was probably dependent on the different sensitivity to stressful events between dys+/+ and –/– mice.
Enhanced excitability of pyramidal neurons in mPFC layer II/III of dysbindin mutant mice
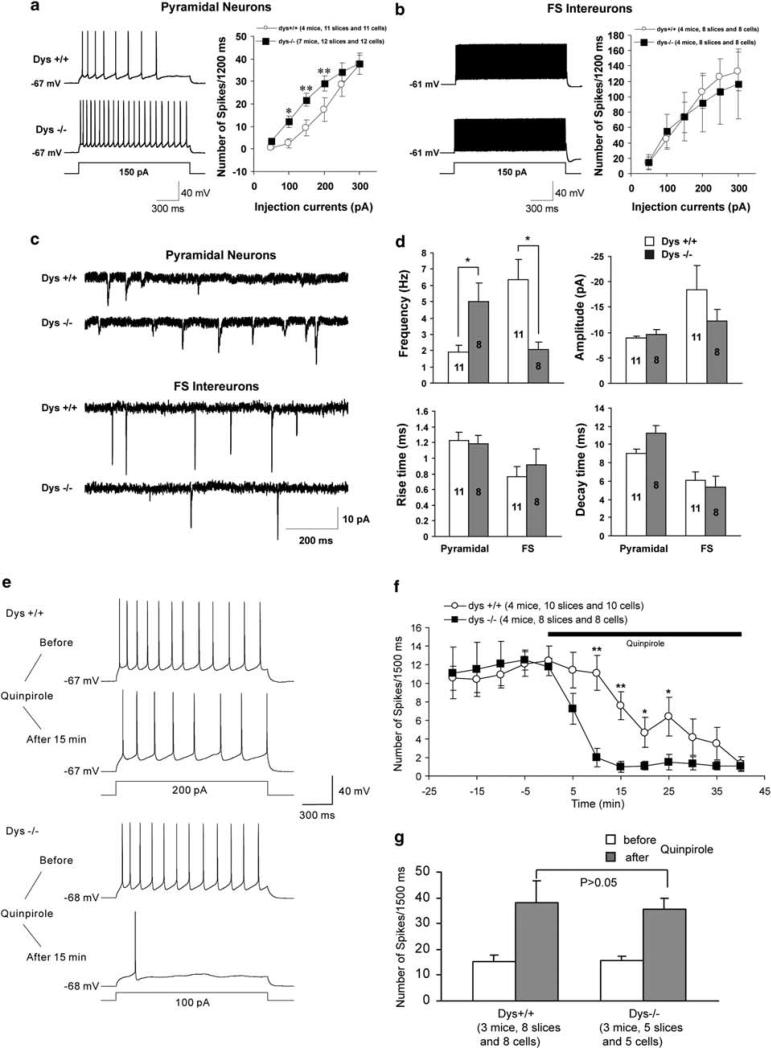
Dtnbp1 gene disruption produced working memory advantages under basal conditions but disadvantages under more challenging situations in an mPFC-dependent T-maze task. The PFC is prominently implicated in schizophrenia pathophysiology, particularly in terms of prefrontal networks involving other cortical regions. Layer II/III pyramidal neurons are prominently involved in intracortical pathways, 42,43 and thus have a central role in mediating working memory processes.44 Thus, we explored the physiological role of dysbindin in layer II/III mPFC neurons with whole-cell current clamp recordings. Application of a depolarizing step (1.5 s) induced more spikes in dys–/– pyramidal neurons compared with dys+/+ pyramidal neurons (P < 0.05; Figure 3a). In contrast, there was no difference between dys+/+ and –/– fast-spiking (FS) interneurons in the spike frequency in a range of depolarizing currents (Figure 3b).
Figure 3.
Enhanced excitability of dysbindin null mutant mice pyramidal neurons in medial prefrontal cortex (mPFC) layer II/III. (a, b) Repetitive firings were evoked by various depolarizing steps, and action potential numbers were plotted against the depolarizing currents injected into the pyramidal cells and fast-spiking (FS) interneurons in mPFC. Representative traces are shown on the left and quantifications are shown on the right. (a) Firing frequency from dys–/– layer II/III pyramidal neurons was much higher than that from +/+ at the same injection currents. (b) The FS interneurons were identified by their shape, location and FS characters. No difference in firing frequency was observed between dys–/– and +/+ layer II/III FS interneurons. (c) Sample traces of spontaneous excitatory post-synaptic currents (sEPSCs) recorded from the layer II/III pyramidal neurons (upper panel) and FS interneurons (lower panel) from dys+/+ and –/– mice. (d) Quantification of sEPSCs recorded from the layer II/III pyramidal neurons and FS interneurons from dys+/+ and –/– mice. The frequency of sEPSCs from dys–/– pyramidal neurons was remarkably higher than that from +/+ pyramidal neurons. In contrast, the frequency of sEPSCs from dys–/– FS interneurons was decreased compared with +/+. No differences were observed in the amplitude and rise time as well as decay time of sEPSCs between dys–/– and +/+ mice. *P < 0.05, **P < 0.01. Effect of quinpirole on excitability of pyramidal neurons and FS interneurons in layer II/III. (e) Sample traces showing the changes in firing rates recorded in layer II/III pyramidal neurons in the mPFC upon application of quinpirole. Current clamp recordings were performed by holding the membrane potentials at their resting potentials. A depolarizing pulse (1.5 s) was applied to evoke typically 7–16 spikes in the baseline every 5 min. Note the decrease in firing frequency after treatment with quinpirole in both dys+/+ and –/– neurons. (f) Time course of the quinpirole effect. *P < 0.05, **P < 0.01. (g) The effect of quinpirole on the excitability of FS interneurons. A depolarizing pulse (1.5 s) was applied to evoke about 13–19 spikes in the baseline every 5 min. Note the increase in firing frequency after treatment with quinpirole in both dys+/+ and –/– neurons. No differences were observed in firing frequency after exposure to quinpirole between dys+/+ and –/– FS interneurons.
The change in pyramidal cell excitability prompted us to examine excitatory inputs to layer II/III pyramidal cells in mPFC slices. In the presence of the GABAA antagonist bicuculline (10 μm), the frequency of spontaneous excitatory post-synaptic currents recorded at the holding potential of –70mV was significantly increased in dys–/– pyramidal cells compared with dys+/+ pyramidal cells (Figures 3c and d). In contrast, no changes were found in the amplitude, rise time and decay time of spontaneous excitatory post-synaptic currents between dys+/+ and –/– pyramidal neurons. Interestingly, the frequency of spontaneous excitatory post-synaptic currents was remarkably decreased in dys–/– FS interneurons compared with dys+/+ FS cells (Figures 3c and d). Taken together, these results suggest that dysbindin modulates layer II/III pyramidal neuron excitability and regulates excitatory inputs to layer II/III pyramidal and FS neurons.
Pyramidal neurons in mPFC layer II/III of dysbindin mutant mice are more sensitive to quinpirole excitability effects
To examine the effect of dysbindin mutation on D2 responses of layer II/III pyramidal neurons, we applied the D2-receptor agonist quinpirole (10 μm) to mPFC slices and measured changes in neuronal excitability, as reflected by the number of action potentials induced by a fixed step depolarization. Consistent with previous reports,45 the application of quinpirole resulted in a decrease in firing frequency in pyramidal neurons derived from +/+ mPFC slices (Figure 3e). In dys–/– pyramidal neurons, quinpirole elicited a much greater effect on firing frequency (Figure 3e). Time course experiments indicated that the quinpirole-induced decrease in neuronal excitability occurred faster and stronger in dys–/– than that in +/+ mice (Figure 3f). Thus, in layer II/III, dys–/– pyramidal neurons have higher basal excitability and respond more to D2-receptor activation compared with pyramidal neurons of dys+/+, perhaps consistent with their increased D2-receptor density at the cell surface. We further examined the effect of quinpirole on excitability of GABAergic interneurons in layer II/III of the mPFC, and found increased excitability of FS GABAergic neurons after treatment of quinpirole in both dys+/+ and –/– mice. Interestingly, there were no significant differences in the excitability of FS cells in response to quinpirole between dys+/+ and –/– mice (Figure 3g).
Dysbindin modulates the Ca2+/calmodulin kinase superfamily in PFC
DA modulation of excitatory synaptic transmission in layer II/III pyramidal neurons involve CaMKII-dependent mechanisms.25 CaMK components also are implicated in working memory,24 and their protein levels in mPFC correlate with the acquisition and performance of mice with a different DA altering genetic mutation (catechol-O-methyltransferase, COMT) in the same mPFC-dependent T-maze task used for the dysbindin mutant mice.27
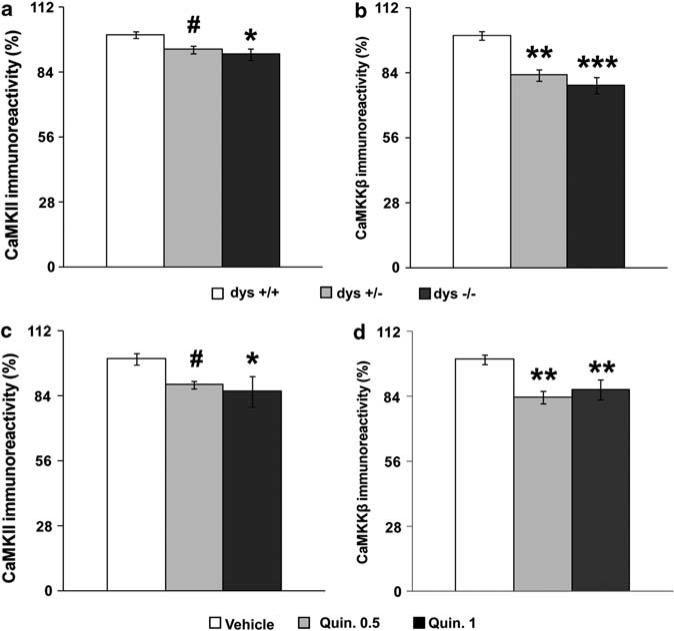
We therefore investigated the impact of disrupted dysbindin gene expression on mPFC levels of the different components of the CaMK family. mPFC CaMKII protein immunoreactivity was decreased in dys–/– mice compared with +/+ littermates (F2,50 = 3.21, P < 0.05; Figure 4a). Both dys–/– and +/– mice displayed lower mPFC CaMKKβ protein levels of both the brain-specific heavier band (F2,19 = 12.32, P < 0.0005; Figure 4b), and the lighter non-specific ubiquitous band (F2,19 = 8.48, P < 0.005; Supplementary Figures 6 and 7). No dysbindin-dependent changes were found for CaMKIV and CaMKKα (Supplementary Figure 7).
Figure 4.
Dysbindin disruption and chronic dopamine (DA)/D2 stimulation modulate medial prefrontal cortex (mPFC) Ca2+/calmodulin-dependent protein kinase (CaMK) components similarly. (a) CaMKII and (b) CaMKKβ protein levels in the mPFC of dys+/+, +/– and –/– littermates by immunoblot analysis. CaMKII Ns: +/+ = 10, +/– = 24, –/– = 19; CaMKKβ Ns: +/+ = 5, +/– = 10, –/– = 7. *P < 0.05, **P < 0.005, ***P < 0.0005 and #P = 0.06 vs +/+. Sample western blots are shown in Supplementary Figure 6. There was no significant difference between dys–/–, +/– and +/+ mice on mPFC CaMKIV (F2,19 = 0.55, P = 0.59; Supplementary Figure 7) and CaMKKα (F2,19 = 0.54, P = 0.59; Supplementary Figure 7) protein immunoreactivity. (c) CaMKII and (d) CaMKKβ protein levels in the mPFC of B6 mice after 15 daily repeated treatment with vehicle (Vehicle) or one of two doses of the D2 agonist quinpirole, that is 0.5mgkg–1 (Quin. 0.5) or 1mg kg–1 (Quin. 1). CaMKII Ns: vehicle = 7, Quin. 0.5 = 5, Quin. 1 = 5; CaMKKβ Ns: vehicle = 9, Quin. 0.5 = 4, Quin. 1 = 5. Results are expressed as percentage of the +/+ or vehicle group for each experiment. *P < 0.05, **P < 0.005 and #P = 0.05 vs vehicle-treated mice. Sample western blots are shown in Supplementary Figure 6. Chronic D2 stimulation did not affect mPFC CaMKIV (F2,15 = 1.74, P = 0.21; Supplementary Figure 8), CaMKI (F2,15 = 0.06, P = 0.94; Supplementary Figure 8) and CaMKKα (F2,15 = 0.53, P = 0.60; Supplementary Figure 8) protein immunoreactivity.
Chronic DA D2-receptor stimulation also modulates expression of Ca2+/calmodulin kinase components in PFC
To test whether increased stimulation of D2-receptor pathways would produce similar changes seen in the CaMK components in the mPFC of dysbindin mutant mice, we chronically treated normal wild-type (dys+/+) mice with the DA D2-preferring agonist quinpirole. Analysis of mPFC CaMKII protein levels revealed a drug treatment effect (F2,14 = 4.16, P < 0.05). In particular, both groups of dys+/+ mice treated with two different doses of quinpirole (0.5 and 1mgkg–1) displayed decreased mPFC CaMKII protein immunoreactivity as compared with vehicle-treated littermates (P≤0.05; Figure 4c). Both Quinpirole 0.5- and Quinpirole 1-treated mice showed decreased mPFC CaMKKβ protein levels (F2,15 = 10.16, P < 0.005; Figure 4d). No quinpirole-dependent changes were found for CaMKIV and CaMKKα (Supplementary Figure 8). These results dramatically parallel the changes in CaMK components derived from reduced dysbindin gene expression, suggesting that decreased dysbindin gene expression reduced mPFC CaMKII and CamKKβ levels through chronic stimulation of D2-receptors pathways.
Chronic DA D2-receptor stimulation impairs working memory performance
Chronic pharmacological stimulation of D2 receptors produced similar mPFC changes in CaMK components to those found in dysbindin mutant mice. Thus, to investigate whether some behaviors of the dysbindin mutant mice might also arise following chronic stimulation of DA D2-receptors pathways, we treated another group of naive B6 wild-type mice with vehicle or quinpirole (0.5mgkg–1). After 12 days of daily injections, we tested these mice in the same behavioral tasks used for the dysbindin mice.
No drug treatment effect was seen on the number of days needed to acquire the working memory T-maze task (F1,13 = 0.53, P =0.48; Figure 5a). During the habituation phase, vehicle- and quinpirole-treated mice readily learned to run quickly through the maze to retrieve the food reward (F1,13 = 121.69, P < 0.0001; Figure 5b). Thus, quinpirole treatment did not affect the acquisition of this working memory task, a departure from the results in the dysbindin-altered mice.
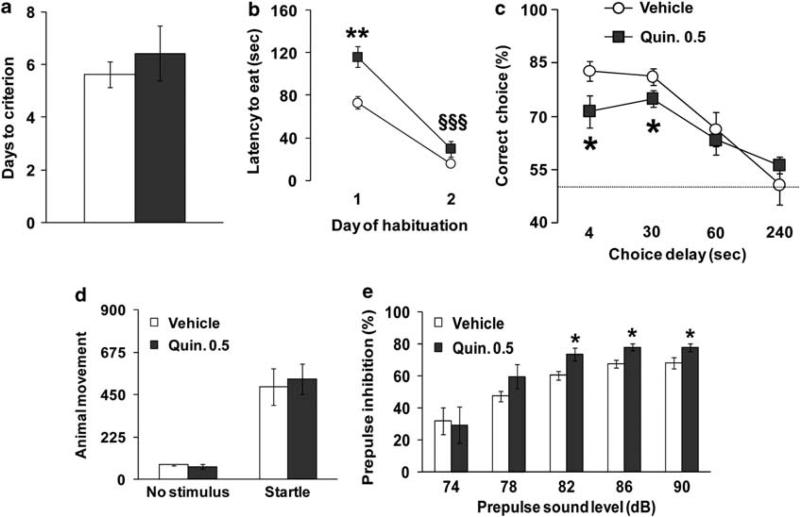
Figure 5.
Chronic dopamine (DA) D2-receptor stimulation impairs working memory performance. (a) Days needed to reach criterion and (b) latency to retrieve the hidden food pellet displayed by B6 littermates after at least 15 daily repeated treatment with vehicle (Vehicle) or 0.5mgkg–1 (Quin. 0.5) of quinpirole during the discrete paired-trial T-maze task. Ns: vehicle = 8, Quin. 0.5 = 7; **P < 0.001 vs vehicle-treated mice, §§§P < 0.0005 vs day 1. All mice used reached the criterion. The habituation performance of the quinpirole-treated mice was different from their vehicle-treated littermates on day 1 (P < 0.001), but not on day 2 (P = 0.16). It is worth noting that a similar pattern was present for the dys–/– mice (Figure 1b). (c) Percentage of correct choices displayed by the same vehicle- and quinpirole-treated B6 littermates during the discrete pairedtrial variable-delay T-maze test with different intra-trial delays randomly presented (4, 30, 60 and 240 s) and an inter-trial delay of 20 s. Both groups displayed the same delay-dependent performance, progressively increasing the number of mistakes with longer delays (F3,52 = 18.01, P < 0.0001). The dotted line corresponds to chance levels (50%) of correct choices. *P < 0.05 vs vehicle-treated mice. Prepulse inhibition (PPI) is increased in chronically quinpirole-treated mice. (d) Animal movement displayed by chronically vehicle- and quinpirole-treated littermates during no stimulus trials or following the presentation of a 120-dB stimulus (Startle). (e) Percent PPI of the acoustic startle response displayed by the same mice after the presentation of 74, 78, 82, 86 and 90 dB prepulse sound stimuli. Ns: vehicle = 8, Quin. 0.5 = 7. *P < 0.05 vs vehicle-treated mice. In both groups, PPI progressively increased with higher prepulse intensities (F4,48 = 25.41, P < 0.0001).
Mice were then tested under more demanding conditions, as previously described. Analysis of the percentage of correct choices at 4 and 30 s intra-trial delays revealed a genotype effect (F1,26 = 8.18, P < 0.01), with quinpirole-treated mice showing worse performance compared with vehicle-treated littermates (P < 0.01; Figure 5c). Thus, chronic D2 agonist administration resulted in some behavioral changes similar to those found in our dys–/– mice, but not all. However, under more challenging conditions, chronic quinpirole treatment impairs working memory performance analogous to that found in dysbindin mutant mice.
PPI is increased in chronically quinpirole-treated mice
We found an effect of dysbindin genetic modification in both acoustic startle responses and PPI. Thus, we explored the startle response and PPI phenomenon in chronic vehicle- and quinpirole-treated B6 mice. Both vehicle- and quinpirole-treated mice showed the same acoustic startle reactivity to the 120-dB stimulus, and same levels of basal activity (F1,12 = 0.06, P =0.81; Figure 5d). Quinpirole-treated mice displayed increased PPI as compared with vehicle-treated littermates with the prepulse sound levels of 82, 86 and 90 dB (P < 0.05; Figure 5e). Thus, chronic hyperstimulation of DA/D2 pathways result in increased PPI responses in mice, as similarly found in dys–/– mice (Figure 1f).
Hypoactive behavior but normal locomotor response to amphetamine in quinpirole-treated mice
Dysbindin genetic modification increased locomotor activity and rearing behavior, thus we tested vehicle- and quinpirole-treated B6 mice in the same open field arenas under the same conditions. During the first exposure to the apparatus, quinpirole-treated mice were less active than vehicle-treated littermates (Supplementary Figure 9). Moreover, quinpirole-treated mice showed fewer rearing events as compared with vehicle-treated mice (Supplementary Figure 9).
On the following day, during the first 10 min spent in the open field, quinpirole-treated mice were again less active than vehicle-treated littermates (Supplementary Figure 9). After these 10 min of baseline, amphetamine treatment (0.75mgkg–1) produced a consistent quinpirole-independent increase in locomotor activity (Supplementary Figure 9). Quinpirole-treated mice showed a decreased number of rearing events during the first 10 min (Supplementary Figure 9). Immediately after the baseline portion of the test, amphetamine treatment produced an increase in rearing behavior in a quinpirole-treatment-independent way (Supplementary Figure 9). Neither chronic quinpirole nor dysbindin mutation altered the effects of amphetamine, but chronic quinpirole produced a hypoactive phenotype in baseline conditions. In contrast, genetic disruption of dysbindin produced a hyperactive phenotype at baseline.
Discussion
Using a dysbindin null mutant mouse line derived from the sdy mouse, but transferred to a B6 inbred background, we have studied the impact of reduced dysbindin expression on behaviors, pharmacological responses, protein expression and layer II/III neural activity relevant to DA D2/pathways. Genetic mutation of the dysbindin gene affects (1) working memory and stress reactivity, (2) intracortical pyramidal cell excitability and (3) mPFC DA and CaMK systems. We also have demonstrated that dysbindin genetic disruption (1) modulates sensitivity to D2 agents and (2) produces some behavioral and molecular effects similar to those produced by direct pharmacological manipulation of the DA D2/pathways. See Supplementary Table 2 for a summary of the experiments performed. These results offer a potential mechanism for the association of DTNBP-1 and psychosis in humans.
Dysbindin, D2 and working memory
The dysbindin-deficient mice exhibited an improvement in acquiring, but impairment in performing a spatial working memory task. Dysbindin disruption results in an accumulation of DA D2, but not D1 receptors, on the plasma membranes of cortical neurons.13,14 Some studies had linked D2 receptors to working memory circuitry in monkeys and humans.46,47 Moreover, acute injection of a D2-receptor agonist into medial PFC in rats impaired whereas the antagonist sulpiride improved cognitive performance in a spatial U-maze task.48 Here, we show similarly that chronic pharmacologic stimulation of DA/D2 pathways impairs working memory performance but not the acquisition of the T-maze task. Thus, these various results suggest that working memory deficits in dysbindin-1 mutant mice are due at least in part to their increased DA D2 signaling. The behavioral discrepancies between dysbindin mutant mice and chronic D2-treated mice might be related to dysbindin modulation of other neurotransmitters systems, such as glutamatergic signaling and NR2A trafficking.2,20–22 Alternatively, reductions in dysbindin may have different effects pre- and post-synaptically. There are multiple isoforms of dysbindin with differential pre- and/or post-synaptic localization.1 Only a predominantly post-synaptic isoform, dysbindin-1C, is reduced in the dorsolateral PFC of schizophrenia cases.11
Interestingly, working memory deficits were not present during the acquisition of the working memory task when long inter-trial intervals were used. Small deficits were seen when proactive interference, different delays and greater handling were used. Additionally, these working memory deficits were exacerbated by more stressful conditions. The ability to acquire this discrete paired-trial working memory T-maze task in mice depends on the mPFC.33 Previous results suggested that, under baseline conditions, dysbindin mutant mice have reduced DA levels in cortico-limbic brain regions.19 Moreover, recent microdialysis data further indicate that dysbindin mutant mice have decreased DA PFC levels as shown by a decreased releasable pool of DA but not serotonin in the PFC of these mice.49 This may represent a compensatory effect in response to the increased density of D2 receptors on the surface of cortical neurons in dysbindin knockout mice,14 or it may be an effect of Dtnbp1 on pre-synaptic DA release.49 Thus, under less challenging conditions, the reduced DA levels in the dysbindin mutant mice may understimulate upregulated D2 receptors and thus not impair working memory acquisition. Indeed, reduced synaptic DA, in the context of increased D2-receptor abundance, may translate into optimal D2 DA signaling. Conversely, under more challenging and stressful situations, that in rodents increase PFC DA release,38 the more abundant D2 PFC receptors in the dysbindin mutant mice might then become relatively overstimulated, ultimately impairing working memory performance. Further support for this formulation comes from our electrophysiological data and our data showing that dysbindin mutant mice are more sensitive to the effects of quinpirole in electrophysiological, startle reactivity and PPI experiments.
It is noteworthy that we previously found in this same mouse line a profile of effects on neuronal excitability in mPFC layer V that is different from our current findings in layers II/III. In layer V, which projects subcortically, we observed changes in interneuron but not pyramidal neuron excitability, both in baseline conditions and after D2 activation.14 In the current study on layer II/III, the opposite pattern of effects was found. These alternative effects may reflect differences in the laminar distribution of D2 and D1 receptors in the mouse,50 an indirect effect of PFC microcircuit abnormalities in other layers or in intrinsic homeostatic mechanisms related to cortical excitability. Neurons in the cortex have the ability to maintain synaptic or intrinsic excitability to maintain stable total firing rates.51 Thus, homeostatic plasticity from chronic increases of D2 receptors in the dys–/– mice neuron surface may be engaged to enhance the baseline excitability of pyramidal neurons in layer II/III and reduce the baseline excitability of interneurons in layer V. Alternatively, the differences in excitability between layer II/III and V might be related to the role played by dysbindin in the more general homeostatic modulation of neurotransmission.21 In particular, this may have a more important role in regulation of excitability in layer II/III as the source and termination of cortico-cortical connections, and thus in the formation and maintenance of cognitive networks, compared with layer V pyramidal cells, which project cortico-fugally. In addition, it has been reported that the inhibitory effect of DA on PFC pyramidal cells exerted indirectly by D1 receptors on FS cells can be reversed by D2 agonists apparently acting on those same cells.52,53 There is a possibility that the effect of dysbindin reduction on dopaminergic transmission in the PFC may be to alter the balance of functional interactions between D2 and D1 receptors, which are both expressed in pyramidal and FS GABA cells.54
Despite decades of research, the neural circuit abnormalities underlying schizophrenia remain elusive. Recent neuroimaging55,56 and EEG57 studies suggest that functional connectivity between the PFC and the hippocampus, is abnormal in schizophrenia patients. However, it remains unclear how such findings relate to genetic factors, such as the dysbindin gene. In the present study, the impaired spontaneous excitatory post-synaptic currents in both pyramidal and FS neurons of layer II/III may be viewed as a potential microcircuit pathophysiological mechanism of altered cognitive functions. Future investigation might focus on this intriguing phenomenon.
Dysbindin modulates sensitivity to D2 agents
We have observed that the dysbindin null mutation affected molecular and behavioral responses to D2 pharmacological agents. Compared with pyramidal neurons of dys+/+ mice, dys–/– pyramidal neurons displayed a much larger decrease in neuronal firing after acute treatment of a D2 agonist. It has been reported that D2 activation induces a concentration-dependent decrease in the excitability of pyramidal cells.45 Thus, upon acute activation of D2, dys–/– pyramidal neurons in layer II/III may exhibit a more marked decrease in firing rate due to their increased expression of D2 receptors on the cell surface.
Similarly, we show that the D2 agonist quinpirole decreased startle and PPI primarily in the dysbindin mutants. In contrast, the D2 antagonist eticlopride increased startle and PPI in dys+/+ only, producing minimal effects in dysbindin mutants. In agreement, earlier work has shown that acute D2 inhibition increases, whereas acute D2 stimulation decreases startle amplitude and PPI in rodents.58,59 Similarly, in healthy humans, the D2 DA agonist bromocriptine produces a reduction in PPI that can be blocked by coadministration of the D2 antagonist haloperidol.40 Thus, the higher sensitivity to the D2 agonist effects and the higher resistance to the D2 antagonist effects in dysbindin mutant mice further implicate the role of increased D2 signaling dependent on dysbindin genetic disruption in startle amplitude and PPI. Moreover, these results strongly suggest that these behaviors are dependent on D2 pathways and support previous conclusions implicating a prominent role of D2 receptors in regulating sensorimotor gating.58
In contrast to the effect of acute quinpirole injections, chronic D2 stimulation from quinpirole treatment increased PPI, and chronic D2 stimulation from the dys–/– mutation increased both startle amplitude and PPI. Similarly, acute injection of eticlopride in our wild-type mice increased startle amplitude, whereas chronic genetic disruption of D2 receptors decreased it.60 Thus, these results demonstrate that D2 pathways have opposite effects on startle and PPI with acute or chronic stimulation/inhibition. It is noteworthy that dys–/– mice show features reminiscent of other schizophrenia models in animals, but do not show the typical PPI phenomenon. Acoustic startle and PPI are thought to be distinct behavioral responses with distinct mechanisms.61 Even though the increased startle should not interfere with the PPI levels, a change in sensitization could be relevant to the increased PPI and cannot be conclusively discounted.
In contrast to the other behavioral effects, the dysbindin/D2 link with locomotor activity is less clear. Although dysbindin mutant mice were hyperactive, chronic quinpirole treatment produced a strong suppression of such locomotor activity. It is well established that systemic acute and chronic stimulation or disruption of D2/pathways in mice decreases locomotor activity.62,63 Thus, it is not surprising that chronic injection of a D2 agonist produced hypoactive behavior. Instead, the hyperactive phenotype found following dysbindin disruption might involve more fine and region-specific alteration of the DA D2/pathways or non-dopaminergic effects of the dysbindin defect.
Dysbindin, D2 and CaMK
Dysbindin mutant mice had reduced levels of CaMKII and CaMKKβ in their mPFC but no changes of other CaMK components, such as CaMKIV and CaMKKα. Chronic treatment with the D2 agonist produced the same decrease in CaMKII and CaMKKβ levels, indicating that these molecular changes in dysbindin mutant mice are dependent at least in part on their increased D2 signaling. It has been shown that pharmacologic D2 stimulation increases intracellular Ca2+, activates CaMKII and decreases the CaM-dependent phosphorylation of striatal membranes.28,64 Also, a direct protein–protein interaction between CaMKII and limbic D3 receptors has been demonstrated.26 Thus, these findings point to an important role played by DA D2-like receptors in the regulation of specific CaMK components.
Because of the CaMK involvement in learning and memory processes,24 and the mPFC dependence of the T-maze task used,33 the changes we found in the mPFC of our mice might be one of the mechanisms through which changes in D2 signaling produce the working memory phenotypes. In support of this, and analogous to our findings in dys–/– mice, faster learning of this same mPFC-dependent T-maze task in COMT–/– mice correlated with reduced CaMKII levels and, conversely, slower learning of COMT Val-tg mice correlated with increased CaMKII protein immunoreactivity.27 Regarding task performance with different delays after acquiring the T-maze task, COMT–/– mice with improved working memory performance, manifest increased mPFC CaMKKβ levels,27 whereas both dys–/– and quinpirole-treated mice, which have working memory performance deficits, had decreased mPFC CaMKKβ. Further support for these relationships comes from the observation that CaMKKβ null mutant mice present spatial memory deficits, but normal hippocampal-dependent contextual, fear and passive avoidance memory.65
In conclusion, the present study demonstrates that dysbindin null mutant mice on a B6 background represent an interesting model of dysbindin down-regulation and D2 upregulation, unraveling the impact of DA/D2 pathways on discrete working memory functions, startle and stress reactivity, PPI, locomotor activity and mPFC neural activity and implicating a molecular mechanism for the association of dysbindin with psychosis and cognitive deficits. We have further revealed a previously unexpected role of dysbindin in CaMK regulation in the mPFC. These results thus indicate that genetically induced reductions in dysbindin can impact cognition via an effect on DA/D2 mechanisms in the rodent homolog of the dorsolateral PFC, and thus help clarify how such reductions can promote cognitive deficits and psychosis in schizophrenia.
Acknowledgments
We thank Q Tian, L Erickson, K Jenkins, J Aney and G Carr for technical assistance. This research was supported by the Intramural Program of the NIH, National Institute of Mental Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Talbot K, Ong WY, Blake DJ, Tang J, Louneva N, Carlson GC, et al. Dysbindin-1 and its protein family. In: Javitt D, Kantorowitz J, editors. Handbook of Neurochemistry and Molecular Neurobiology. 3rd edn. Vol. 27. Springer; New York: 2009. pp. 107–241. [Google Scholar]
- 2.Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, et al. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- 4.Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, et al. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31:2002–2010. doi: 10.1038/sj.npp.1301003. [DOI] [PubMed] [Google Scholar]
- 5.Morris DW, Murphy K, Kenny N, Purcell SM, McGhee KA, Schwaiger S, et al. Dysbindin (DTNBP1) and the biogenesis of lysosome-related organelles complex 1 (BLOC-1): main and epistatic gene effects are potential contributors to schizophrenia susceptibility. Biol Psychiatry. 2008;63:24–31. doi: 10.1016/j.biopsych.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M, et al. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72:185–190. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res. 2008;98:105–110. doi: 10.1016/j.schres.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 11.Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet. 2009;18:3851–3863. doi: 10.1093/hmg/ddp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams NM, Preece A, Morris DW, Spurlock G, Bray NJ, Stephens M, et al. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1). Arch Gen Psychiatry. 2004;61:336–344. doi: 10.1001/archpsyc.61.4.336. [DOI] [PubMed] [Google Scholar]
- 13.Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci. 2007;27:12390–12395. doi: 10.1523/JNEUROSCI.1689-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, et al. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci USA. 2009;106:19593–19598. doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann NY Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- 16.Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol (San Diego, Calif) 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 17.Kumamoto N, Matsuzaki S, Inoue K, Hattori T, Shimizu S, Hashimoto R, et al. Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin. Biochem Biophys Res Commun. 2006;345:904–909. doi: 10.1016/j.bbrc.2006.04.163. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murotani T, Ishizuka T, Hattori S, Hashimoto R, Matsuzaki S, Yamatodani A. High dopamine turnover in the brains of Sandy mice. Neurosci Lett. 2007;421:47–51. doi: 10.1016/j.neulet.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 2009;34:2601–2608. doi: 10.1038/npp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang TT, Yang F, Chen BS, Lu Y, Ji Y, Roche KW, et al. Dysbindin regulates hippocampal LTP by controlling NMDA receptor surface expression. Proc Natl Acad Sci USA. 2009;106:21395–21400. doi: 10.1073/pnas.0910499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talbot K. The sandy (sdy) mouse: a dysbindin-1 mutant relevant to schizophrenia research. Prog Brain Res. 2009;179:87–94. doi: 10.1016/S0079-6123(09)17910-4. [DOI] [PubMed] [Google Scholar]
- 24.Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn Mem (Cold Spring Harbor, NY) 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci. 2003;23:867–875. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, et al. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi Y, Fukunaga K, Miyamoto E. Activation of nuclear Ca(2+)/calmodulin-dependent protein kinase II and brain-derived neurotrophic factor gene expression by stimulation of dopamine D2 receptor in transfected NG108-15 cells. J Neurochem. 2002;82:316–328. doi: 10.1046/j.1471-4159.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- 29.D'Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG. Comparative immunohistochemical study of the dopaminergic systems in two inbred mouse strains (C57BL/6J and DBA/2J). J Chem Neuroanat. 2007;33:67–74. doi: 10.1016/j.jchemneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Takao K, Toyama K, Nakanishi K, Hattori S, Takamura H, Takeda M, et al. Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Mol Brain. 2008;1:11. doi: 10.1186/1756-6606-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, et al. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. 2009;8:390–397. doi: 10.1111/j.1601-183X.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 36.Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle JM, et al. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia. 2007;45:454–458. doi: 10.1016/j.neuropsychologia.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- 38.Feenstra MG, Botterblom MH. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Res. 1996;742:17–24. doi: 10.1016/s0006-8993(96)00945-6. [DOI] [PubMed] [Google Scholar]
- 39.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 40.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 41.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Hess G, Jacobs KM, Donoghue JP. N-methyl-D-aspartate receptor mediated component of field potentials evoked in horizontal pathways of rat motor cortex. Neuroscience. 1994;61:225–235. doi: 10.1016/0306-4522(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 43.Miller R. Neural assemblies and laminar interactions in the cerebral cortex. Biol Cybern. 1996;75:253–261. doi: 10.1007/s004220050292. [DOI] [PubMed] [Google Scholar]
- 44.Lewis DA, Gonzalez-Burgos G. Intrinsic excitatory connections in the prefrontal cortex and the pathophysiology of schizophrenia. Brain Res Bull. 2000;52:309–317. doi: 10.1016/s0361-9230(99)00243-9. [DOI] [PubMed] [Google Scholar]
- 45.Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Druzin MY, Kurzina NP, Malinina EP, Kozlov AP. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behav Brain Res. 2000;109:99–111. doi: 10.1016/s0166-4328(99)00166-7. [DOI] [PubMed] [Google Scholar]
- 49.Nagai T, Kitahara Y, Shiraki A, Hikita T, Taya S, Kaibuchi K, et al. Dysfunction of dopamine release in the prefrontal cortex of dysbindin deficient sandy mice: an in vivo microdialysis study. Neurosci Lett. 2010;470:134–138. doi: 10.1016/j.neulet.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 50.Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- 51.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 52.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Le Moine C, Gaspar P. Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Brain Res Mol Brain Res. 1998;58:231–236. doi: 10.1016/s0169-328x(98)00118-1. [DOI] [PubMed] [Google Scholar]
- 55.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 56.Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 57.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 58.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 59.Weber M, Chang WL, Breier M, Ko D, Swerdlow NR. Heritable strain differences in sensitivity to the startle gating-disruptive effects of D2 but not D3 receptor stimulation. Behav Pharmacol. 2008;19:786–795. doi: 10.1097/FBP.0b013e32831c3b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halberstadt AL, Geyer MA. Habituation and sensitization of acoustic startle: opposite influences of dopamine D1 and D2-family receptors. Neurobiol Learn Mem. 2009;92:243–248. doi: 10.1016/j.nlm.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plappert CF, Pilz PK, Schnitzler HU. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behav Brain Res. 2004;152:403–412. doi: 10.1016/j.bbr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 62.Chausmer AL, Katz JL. The role of D2-like dopamine receptors in the locomotor stimulant effects of cocaine in mice. Psychopharmacology. 2001;155:69–77. doi: 10.1007/s002130000668. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc Natl Acad Sci USA. 2001;98:1964–1969. doi: 10.1073/pnas.98.4.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peters M, Mizuno K, Ris L, Angelo M, Godaux E, Giese KP. Loss of Ca2+/calmodulin kinase kinase beta affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J Neurosci. 2003;23:9752–9760. doi: 10.1523/JNEUROSCI.23-30-09752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]