Abstract
Executive functions (EFs)—a set of general-purpose control processes that regulate one’s thoughts and behaviors—have become a popular research topic lately and have been studied in many subdisciplines of psychological science. This article summarizes the EF research that our group has conducted to understand the nature of individual differences in EFs and their cognitive and biological underpinnings. In the context of a new theoretical framework that we have been developing (the unity/diversity framework), we describe four general conclusions that have emerged from our research. Specifically, we argue that individual differences in EFs, as measured with simple laboratory tasks, (1) show both unity and diversity (different EFs are correlated yet separable); (2) reflect substantial genetic contributions; (3) are related to various clinically and societally important phenomena; and (4) show some developmental stability.
Keywords: executive functions, self-regulation, individual differences, behavioral genetics
People differ greatly in their abilities to regulate thoughts and behaviors. For example, some people can resist the temptation to eat chocolate cake, whereas others cannot help it even when they are on a diet and know that they should avoid high-calorie foods. Why do people differ in their ability to control their impulses and urges? What are the cognitive and biological underpinnings of such individual differences?
These are the central questions that we have been addressing through our studies of executive functions (EFs)—a set of general-purpose control mechanisms, often linked to the prefrontal cortex of the brain, that regulate the dynamics of human cognition and action. EFs are important to study because they are a core component of self-control or self-regulation ability (or “willpower”) that has been shown to have broad and significant implications for everyday lives (Mischel et al., 2011; Moffitt et al., 2011).
Since our original study (Miyake et al., 2000), we have learned much about individual differences in EFs. In this article, we present what we have learned so far in the form of four general conclusions. Before doing so, however, we explain our approach.
Assessing Individual Differences in EFs
EF is a challenging topic to study: It is not only elusive to define (Jurado & Rosselli, 2007) but also difficult to measure. Although there are several reasons for this measurement difficulty (Miyake et al., 2000), arguably the most vexing problem is the task-impurity problem. Because any target EF must be embedded within a specific task context, any score derived from an EF task—say, the well-known Stroop task (naming the ink color when the color and word are incongruent, as in GREEN)—necessarily includes systematic variance attributable to non-EF processes (e.g., color processing, articulation speed). Unfortunately, this systematic non-EF variance and measurement error (random noise in the data) are substantial, making it difficult to cleanly measure the EF variance of interest.
To alleviate this problem, we use a latent-variable approach. In this approach, one selects multiple exemplar tasks that seem different on the surface but still capture the target ability. If exemplar tasks are chosen such that they share little systematic non-EF variance, one can statistically “extract” what is common across those tasks (using such multivariate statistical techniques as confirmatory factor analysis and structural equation modeling) and use the resulting “purer” latent variable as the measure of EF.
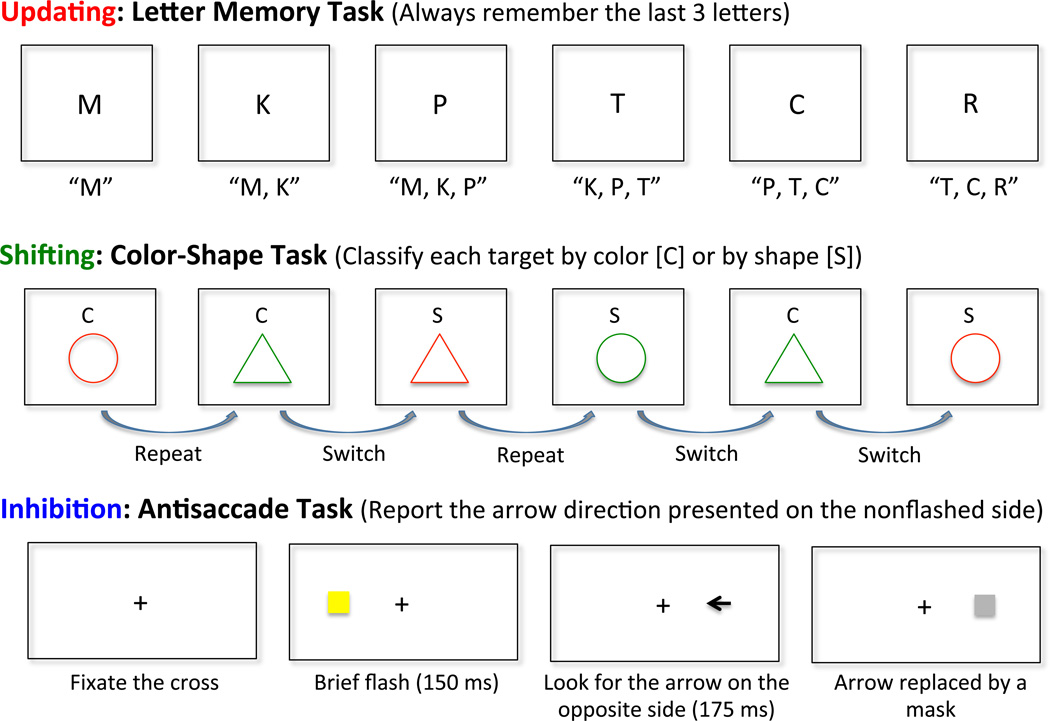
Our research has focused primarily on three EFs: updating (constant monitoring and rapid addition/deletion of working-memory contents); shifting (switching flexibly between tasks or mental sets); and inhibition (deliberate overriding of dominant or prepotent responses). Some tasks we use to capture these EFs are illustrated in Figure 1. Although there are other EFs (e.g., dual-tasking) and other levels of analysis that could be justified and explored,1 these three EFs (updating, shifting, and inhibition) have provided useful insights into the nature and organization of individual differences in EFs.
Figure 1.
Schematic illustrations of three executive function (EF) tasks used in our current EF test battery. (A) In the letter memory task (an example of an updating task), participants are presented with a series of consonant letters one at a time. Their task is to report the last three letters after the presentation of the letter sequence ends. To ensure that participants constantly update their working memory contents, they are required to say aloud what the last three letters are after each letter. The dependent measure is the accuracy of the recalled letters at the end. (B) In the color-shape task (an example of a shifting task), participants see a letter cue first (either C or S) and, depending on the cue, they make a classification decision about the target item presented shortly afterwards in terms of color (green or red) or shape (circle or triangle) by pressing appropriate buttons on a button box. The dependent measure is the switch cost, namely, a reaction time difference between switch and repeat trials. (C) In the antisaccade task (an example of an inhibition task), participants first fixate on the center cross. When a brief flash occurs, they need to avoid looking at that flash and instead move their gaze toward the opposite side of the screen so that they can correctly identify and report the direction of an arrow briefly presented there. The dependent measure is the proportion of correctly reported arrows. More procedural details of these tasks and the details of other EF measures we use are provided inFriedman et al. (2008).
Four General Conclusions about Individual Differences in EFs
Unity and Diversity
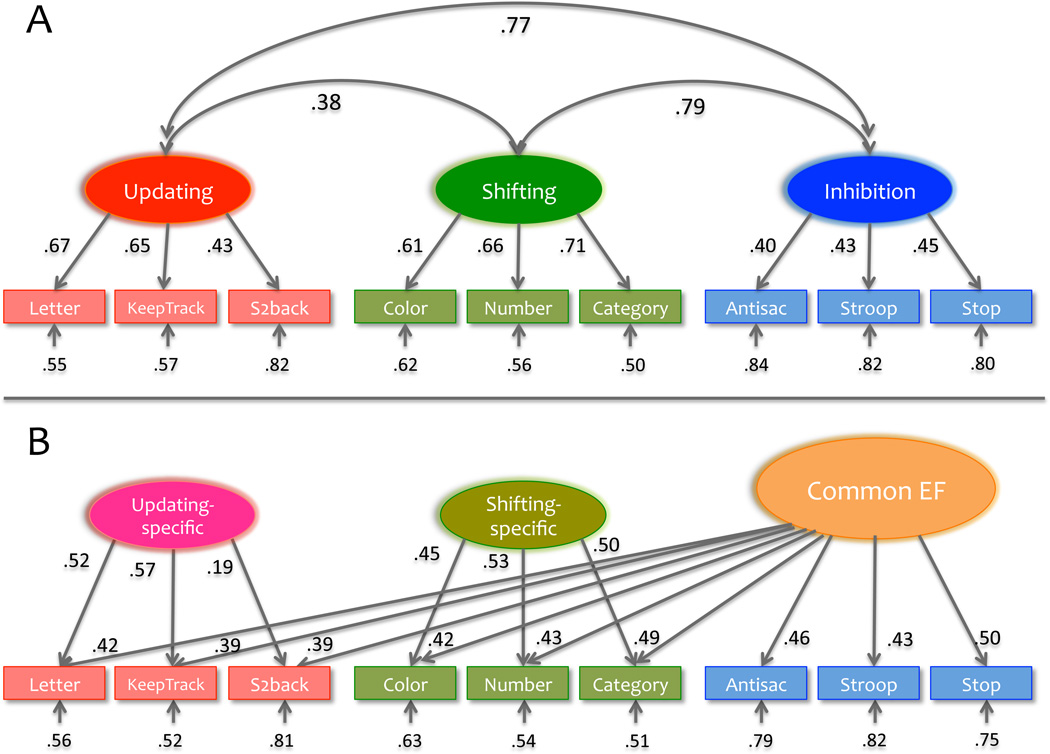
The first conclusion is that individual differences in EFs show both “unity” and “diversity, ” a notion originally proposed by Teuber (1972). That is, different EFs correlate with one another, thus tapping some common underlying ability (unity), but also show some separability (diversity). This pattern is illustrated in Figure 2A with data from a large twin sample (Friedman et al., 2011): The correlations among the three EF latent variables are substantial, but are far from 1.0. This general unity/diversity pattern has been observed in other samples, including preadolescent children (Rose, Feldman, & Jankoswki, 2011) and older adults (Vaughan & Giovanello, 2010), although only a single unitary factor may be evident in preschool children (Wiebe, Espy, & Charak, 2008). The diversity of EFs is further supported by the observation that the three EFs differentially relate to other measures, such as well-known neuropsychological tests of frontal-lobe functioning (Miyake et al., 2000) and IQ (Friedman et al., 2006).
Figure 2.
Two complementary ways of representing the unity and diversity of EFs, adapted from the confirmatory factor analysis results reported inFriedman et al. (2011). Numbers on arrows are standardized factor loadings, those under the smaller arrows are residual variances, and those on curved double-headed arrows are interfactor correlations (task names are abbreviated). (A) This panel illustrates the unity and diversity of EFs by showing that the three types of EFs (updating, shifting, and inhibition) are substantially correlated with each other (unity) but are separable (diversity) in that those correlations are far from 1.0. This was the way our research initially examined individual differences in EFs (e.g., Miyake et al., 2000). (B) This panel illustrates the unity and diversity of EFs in a way that is more consistent with the unity/diversity framework we have been developing. As Panel B shows, there is a Common EF latent variable on which all nine EF tasks load (unity), as well as two “nested” latent variables on which the updating and shifting tasks, respectively, also load (diversity). Because the Common EF variance happened to be perfectly correlated with the inhibition latent variable, no inhibition-specific factor is represented in the figure. Letter = letter memory, Keep = keep track, S2back = Spatial 2-back, Color = color-shape, Number = number-letter, Category = category-switch, Antisac = antisaccade, and Stop = stop-signal (for details about these tasks, see Friedman et al., 2008).
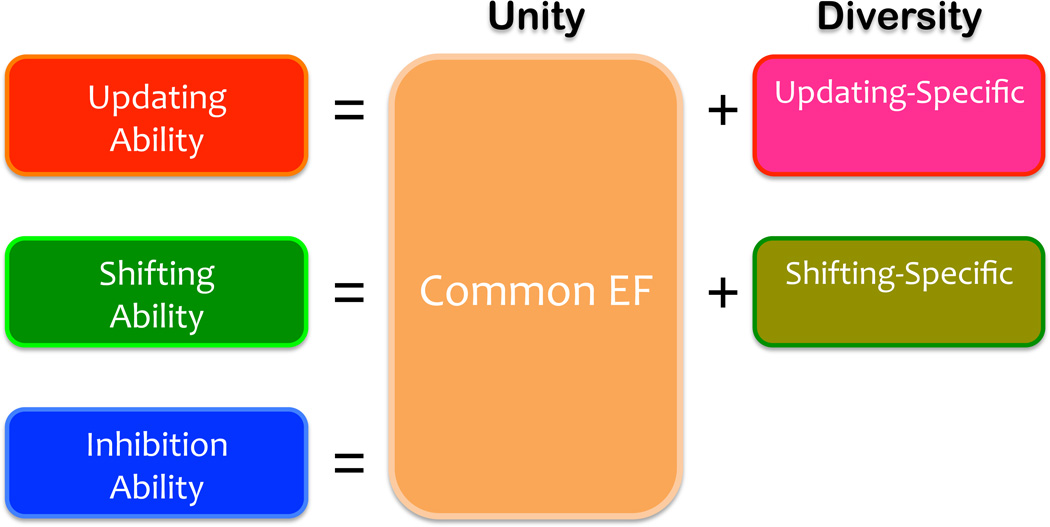
Although our work initially focused on how the three EFs relate to one another and to other individual differences of interest, our goal has shifted recently to specifying the cognitive and biological underpinnings of the unity and diversity more directly. A new framework—the unity/diversity framework—that reflects this recent shift in research goals is illustrated in Figure 3. Each EF ability (e.g., updating) can be decomposed into what is common across all three EFs, or unity (termed Common EF), and what is unique to that particular ability, or diversity (e.g., updating-specific). Instead of the elements on the left side of the equation that reflect mixed influences from both unity and diversity (updating, shifting, inhibition), the unity/diversity framework focuses on the elements on the right side that may more cleanly map onto the underlying cognitive processes (Common EF, updating-specific, shifting-specific) and seeks to specify their underpinnings.
Figure 3.
Schematic representation of the unity and diversity of three executive functions (EFs). Each EF (e.g., updating) is really a combination of what is common to all three EFs (Common EF) and what is specific to that EF (e.g., updating-specific). Although our initial research has focused on three types of EFs (the left side of the equation) and how they relate to other psychological measures of interest, the unity/diversity framework we have been developing focuses on the right side of the equation (Common EF, updating-specific, shifting-specific) so that we can more directly specify the cognitive and biological underpinnings of EF’s unity and diversity. In this figure, the inhibition-specific component is absent, because we have found repeatedly that, once the unity (Common EF) is accounted for, there is no unique variance left for the inhibition-specific factor, a point also illustrated in Figure 2B in the data from a large twin sample (Friedman et al., 2011).
This new way of examining individual differences in EFs is still under development but has already produced some interesting discoveries. First, after accounting for the unity (Common EF), there is no unique variance left for inhibition (Friedman et al., 2008, 2011), hence the absence of the inhibition-specific factor in Figure 3. Stated differently, the inhibition factor happens to correlate virtually perfectly with Common EF, leaving no inhibition-specific variance. This finding, which we have now replicated in two independent college-student samples, is shown in Figure 2B. Another discovery, to be illustrated later, is that the Common EF and shifting-specific components sometimes show opposing patterns of correlations with other measures, hinting at the multifaceted nature of shifting ability (stability vs. flexibility) suggested in the literature (Goschke, 2000).
Using these findings as clues, we have been developing hypotheses regarding what specific ability each EF component may be tapping. According to our current view, Common EF is about one’s ability to actively maintain task goals and goal-related information and use this information to bias lower-level processing. This basic ability is necessary for all three EFs and has also been suggested as a key requirement of response inhibition (Munakata et al., 2011). In contrast, we hypothesize that the shifting-specific component reflects flexibility—ease of transitioning to new task-set representations. At present, we are less certain about what the updating-specific component taps, but two candidate mechanisms are effective gating of information and controlled retrieval from long-term memory.
Substantial Genetic Contributions
The second conclusion is that individual differences in EFs reflect substantial genetic contributions at the level of latent variables (Friedman et al., 2008). As is commonly done in behavioral genetic analyses, we used correlations from monozygotic (identical) and dizygotic (fraternal) twins to decompose the sources of individual differences in EFs into three components: genetic variance (heritability) and two types of environmental variances (shared and nonshared environments).
The individual tasks showed moderate heritability (.25–.55), with the remaining variance attributable mostly to nonshared environment (which includes measurement error). However, at the level of latent variables, where measurement error is minimized, the heritability estimates were considerably higher (over .75). More important, substantial genetic contributions were observed at both unity and diversity levels, suggesting that separate sets of genes contribute to the variability in Common EF versus updating-specific and shifting-specific abilities. Such results hold promise for current efforts linking specific genes to EFs (Barnes et al., 2011), but also point to the complexity of the EF genetic structure.
We should emphasize here that high heritability does not mean immutability. Heritability is the portion of variability across individuals within a particular sample attributable to genetic effects at a particular point in time. Thus, it says nothing about the source(s) of a particular individual’s EF ability or the trainability of EFs within each individual or among a group of individuals. In fact, recent studies suggest that EF ability is amenable to some training effects (e.g., Dahlin, Neely, Larsson, Backman, & Nyberg, 2008), although the transferability of training effects is not clearly established.
Clinical and Societal Relevance
The third conclusion is that purely cognitive measures of EFs can predict individual differences in clinically and societally important behaviors (Friedman et al., 2007, 2011; Young et al., 2009). In particular, recent evidence points to the unity component of EFs (Common EF) as the primary source of such predictive power.2
An illuminating example comes from the twin data on behavioral disinhibition (Young et al., 2009). Behavioral disinhibition is a general vulnerability factor hypothesized to underlie different types of so-called externalizing behavior problems, such as attention deficits (often shown by individuals with ADHD), novelty seeking/risk taking, conduct disorder, and substance use. A latent variable for behavioral disinhibition was substantially correlated (−.63) with the Common EF variable, indicating that better general EF ability is associated with fewer such behavioral problems.3 Together with related findings linking individual differences in EFs to diverse self-regulatory behaviors such as the expression and control of implicit racial biases (Klauer et al., 2010) and successful implementation of dieting and exercising intentions (Hall, Fong, Epp, & Elias, 2008), these simple lab-based EF tasks illustrated in Figure 1 are capturing something important and meaningful, especially at the level of latent variables.
Developmental Stability
The final conclusion is that individual differences in EFs show some stability during development. In our research, this conclusion has emerged from two lines of longitudinal analyses. The first concerns the 6-year stability of EFs in the twin sample, measured at ages 17 and 23, during which many twins moved out of their parents’ homes and started to live separately. Preliminary results showed that, despite such major life changes, the age 17/age 23 correlations are substantial at the level of latent variables (.82 for Common EF, 1.00 for updating-specific, and .93 for shifting-specific).
The second line of evidence takes advantage of the fact that we have been tracking our twin sample longitudinally. Specifically, we have been trying to identify early measures that could predict later individual differences in EFs. The earliest precursor we discovered is a simple measure of self-restraint during toddlerhood (Friedman et al., 2011). In this study, self-restraint was assessed with a prohibition task, in which a child was shown an attractive toy and then told not to touch it for 30 seconds, a task that has conceptual similarities to the well-known delay-of-gratification tasks (Mischel et al., 2011) and requires active goal maintenance (e.g., maintaining a goal of not touching a toy).
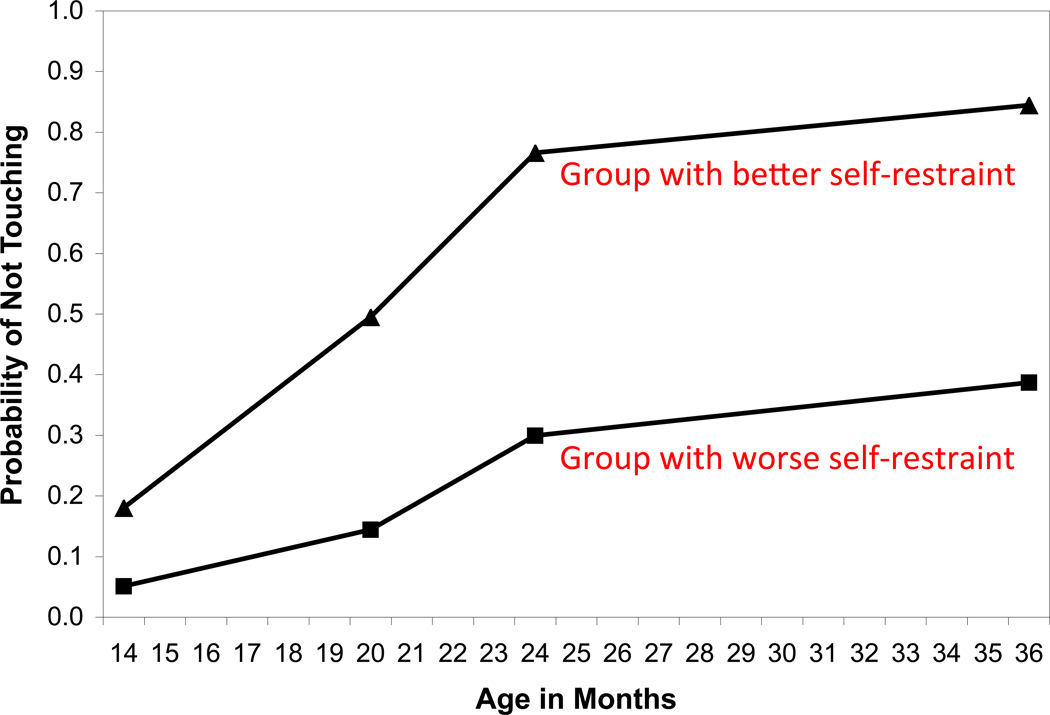
Based on growth modeling of four testing occasions (ages 14, 20, 24, and 36 months), we identified two distinct developmental trajectories, shown in Figure 4. Remarkably, this group difference was still evident at age 17: The better self-restraint group demonstrated significantly better Common EF (by .60 standard-deviation [SD] units), virtually no difference in the updating-specific ability (.05 SDs better), and significantly worse shifting-specific ability (.42 SDs worse) than the worse self-restraint group. Together with other longitudinal data (Mischel et al., 2011; Moffitt et al., 2011), these results suggest that individual differences in self-regulation and EF abilities are relatively stable developmentally. Considering that all participants’ overall self-regulation and EF abilities undoubtedly improved substantially during this 14-year span, this example illustrates that such stability of individual differences does not necessarily mean no change in EF abilities within individuals.
Figure 4.
Growth trajectories of the two groups identified in the latent class growth model illustrating the development of self-restraint ability, as measured by a simple prohibition task (Friedman et al., 2011). In this task, a child is shown an attractive toy and then told not to touch it for 30 seconds. One group (55% of the sample) showed better self-restraint than the other group (45%), and this group difference was significant at all four time points (14, 20, 24, and 36 months of age). As explained in the main text, this group difference was also reflected in their performance of executive function (EF) tasks, assessed 14 years later at age 17. For simplicity, the figure shown here is based on the data based on the dichotomous scoring of the prohibition task performance (pass or fail). The analyses and results reported in theFriedman et al. (2011) article takes into account the durations for which each child was able to not touch the toy.
The evidence of worse shifting-specific ability associated with better childhood self-restraint may be counterintuitive, but it illustrates something we alluded to earlier: The two components of shifting ability—Common EF and shifting-specific—sometimes show opposing patterns of correlations with other constructs. As Goschke (2000) suggested, the ability to actively maintain a single task goal may indeed be a force that makes it difficult for individuals to flexibly switch to a different goal.
Current and Future Research Directions: Specifying the Biological Basis of EFs
In this review, we presented four general conclusions that summarize what we have learned about individual differences in EFs. As this review indicates, individual differences in EFs are highly relevant to many different subdisciplines of psychological science and hence could have broad implications for both basic and applied research. In this regard, we are currently examining how individual differences in EFs relate to various important phenomena in psychology, such as cognitive control in depression and anxiety (Altamirano, Miyake, & Whitmer, 2010), the expression and control of implicit racial bias, and the regulation of substance use and eating behavior.
We believe that the unity/diversity framework described here provides a useful basis for examining individual differences in EFs in future research, but, to make the framework even more useful, we have been developing the main ideas computationally and trying to link the behavioral, cognitive, and genetic levels of our theoretical explanations more tightly. We end this article by describing such current efforts.
To better understand the neural and genetic mechanisms underlying individual differences in EFs, we are using neural-network modeling in the context of the Prefrontal-Cortex Basal-Ganglia Working-Memory (PBWM) model developed by O'Reilly and colleagues (O'Reilly & Frank, 2006). PBWM provides a biologically plausible model of the brain areas involved in EFs, which exhibit specific properties constrained by known biology. The prefrontal-cortex layer can actively maintain relevant information through persistent activation and recurrent connectivity. Because it can bias lower-level activity through its connections to posterior-cortex layers, which do not have these same active maintenance mechanisms, it is particularly suited to maintaining goals and using these goals to bias ongoing processing. Information is flexibly gated into the prefrontal-cortex layer through the basal-ganglia layers, which learn what information is relevant through dopaminergic reward learning mechanisms.
Using this domain-general PBWM model, we have been simulating the EF tasks used in theFriedman et al. (2008) study and manipulating model parameters to test our hypotheses about the mechanisms influencing individual differences (see Chatham et al., 2011, for a detailed report of our simulation of the N-back task). For example, in this ongoing work, to simulate individual differences in hypothesized Common EF mechanisms of actively maintaining goals and thereby biasing lower-level processing, we manipulated parameters that influence the strength of representations in the prefrontal-cortex layer of the model and how strongly the prefrontal cortex connects with posterior areas. Consistent with our hypothesis that goal maintenance is important for all EFs, these manipulations affected all the modeled tasks. In contrast, manipulations of other parameters in the prefrontal-cortex layer that influenced how long representations persist after they are no longer needed affected only the shifting tasks, consistent with our hypothesis that the shifting-specific factor may reflect the ease of transitioning to new representations in the prefrontal cortex. Hence, we have begun to replicate the unity/diversity pattern observed behaviorally with this biologically constrained model.
An ultimate goal of this computational modeling is to develop hypotheses for testing specific genes that contribute to individual differences in these EFs. Although previous studies have examined the roles of particular genetic variants (especially within the dopamine system), the effects are typically small and often do not replicate (Barnes et al., 2011). One promising approach is to test aggregate effects of multiple genes that, when combined, have larger effects on biological parameters. Because the model can simulate the effects of multiple genetic variants acting independently or even jointly, it can provide a strong theoretical framework with which to develop detailed hypotheses regarding the genetic influences underlying the unity and diversity of EFs.
Acknowledgements
We thank Tiffany Ito, Yuko Munakata, Phil Zelazo, and two anonymous reviewers for providing useful comments on an earlier draft of this article.
Funding
The research described in this article was supported by grants from the National Institutes of Health (MH063207, MH079485, HD010333, DA024002, AA011998) and the National Science Foundation (BCS0847872).
Footnotes
For example, a commonly postulated EF, planning, is a more complex and higher-level construct than those we study and is likely to implicate all three EFs. Each of the three EFs, in turn, could be decomposed into multiple subprocesses (e.g., monitoring, adding, active maintenance, and deletion for updating) and studied at this more fine-grained level if appropriate tasks are available to tap individual differences in those subprocesses.
Interesting to note, individual differences in cognitive abilities, such as IQ, are most strongly related to updating ability (Friedman et al., 2006). In fact, both Common EF and updating-specific ability are equally strongly related to IQ (Friedman et al., 2008), suggesting that Common EF is not the same as so-called general intelligence (g).
The original publication of this study (Young et al., 2009) reported that, among the three EFs, inhibition correlated most strongly with behavioral disinhibition. The result reported here is based on a reanalysis of the data using the unity/diversity framework.
Recommended Readings
Barnes, J. J. M., Dean, A. J., Nandam, S., O’Connell, R. G., & Bellgrove, M. A. (2011). (See References). Provides an in-depth review of recent molecular genetic (polymorphism) research examining the genetic correlates of EFs, including response inhibition, in both clinical (e.g., ADHD) and nonclinical populations.
Diamond, A., & Lee, K. (2011). Interventions shown to aid executive function development in children 4 to 12 years old. Science, 333, 959–964. Provides a concise yet broad-scope review of various intervention efforts to improve children’s EF abilities.
Friedman, N. P., & Miyake, A. (2004). The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General, 18, 893–900. Although the current article focused on one particular type of inhibition (prepotent response inhibition), there are different types of inhibition or interference control processes. This article reports a study demonstrating that different types of inhibition do not necessarily correlate with one another and, hence, should not be treated interchangeably.
Friedman, N. P., Miyake, A., Robinson, J. L., & Hewitt, J. K. (2011). (See References). Provides a nice illustration of behavioral, developmental, and genetic evidence related to all four general conclusions described in this article.
Miyake, A., Emerson, M. J., & Friedman, N. P. (2000). Assessment of executive functions in clinical settings: Problems and recommendations. Seminars in Speech and Language, 21, 169–183. Provides a readable tutorial on various challenges facing the measurement of EFs and offers some concrete recommendations.
References
- Altamirano LJ, Miyake A, Whitmer AJ. When mental inflexibility facilitates executive control: Beneficial side effects of ruminative tendencies on goal maintenance. Psychological Science. 2010;21:1377–1382. doi: 10.1177/0956797610381505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Dean AJ, Nandam S, O’Connell RG, Bellgrove MA. The molecular genetics of executive function: Role of monoamine system genes. Biological Psychiatry. 2011;69:e127–e143. doi: 10.1016/j.biopsych.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Chatham CH, Herd SA, Brant AM, Hazy TE, Miyake A, O’Reilly RC, Friedman NP. From an executive network to executive control: A computational model of the n-back task. Journal of Cognitive Neuroscience. 2011 doi: 10.1162/jocn_a_00047. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, Hewitt JK. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychological Science. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Robinson JL, Hewitt JK. Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioral genetic analysis. Developmental Psychology. 2011;47:1410–1430. doi: 10.1037/a0023750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T. Intentional reconfiguration and involuntary persistence in task set switching. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 331–355. [Google Scholar]
- Hall PA, Fong GT, Epp LJ, Elias LJ. Executive function moderates the intention-behavior link for physical activity and dietary behavior. Psychology and Health. 2008;23:309–326. doi: 10.1080/14768320701212099. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Modeling a cascade of effects: The role of speed and executive functioning in preterm/full-term differences in academic achievement. Developmental Science. 2011;14:1161–1175. doi: 10.1111/j.1467-7687.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: A review. Neuropsychology Review. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Klauer KC, Schmitz F, Teige-Mocigemba S, Voss A. Understanding the role of executive control in the Implicit Association Test: Why flexible people have small IAT effects. Quarterly Journal of Experimental Psychology. 2010;63:595–619. doi: 10.1080/17470210903076826. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, Shoda Y. “Willpower” over the life span: Decomposing self-regulation. Social, Cognitive, and Affective Neuroscience. 2011;6:252–256. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences, U.S.A. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011 doi: 10.1016/j.tics.2011.07.011. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: A computational model of learning in the frontal cortex and basal ganglia. Neural Computation. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Teuber H-L. Unity and diversity of frontal lobe functions. Acta Neurobiologiae Experimentalis. 1972;32:615–656. [PubMed] [Google Scholar]
- Vaughan L, Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychology and Aging. 2010;25:343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, Charak D. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Developmental Psychology. 2008;44:573–587. doi: 10.1037/0012-1649.44.2.575. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]