Abstract
Lysophosphatidic acid (LPA) is a multi-functional intercellular phospholipid mediator present in blood and other biological fluids. In cancer cells, LPA stimulates expression or activity of inflammatory cytokines, angiogenic factors, matrix metalloproteinases and other oncogenic proteins. In the present study, we showed that LPA upregulated expression of the cyclin-dependent kinase inhibitor p21Waf1 in transforming growth factor beta (TGFβ)-sensitive breast and ovarian cancer cells, but not in TGFβ-resistant ones. We examined the possibility that LPA-induced p21 might contribute to the cytostatic response to TGFβ. In serum-free conditions, TGFβ alone induced p21 expression weakly in TGFβ-sensitive cells. Serum or serum-borne LPA cooperated with TGFβ to elicit the maximal p21 induction. LPA stimulated p21 via LPA1 and LPA2 receptors and Erk-dependent activation of the CCAAT/enhancer-binding protein beta (C/EBPβ) transcription factor independent of p53. Loss or gain of p21 expression led to a shift between TGFβ sensitive and resistant phenotypes in breast and ovarian cancer cells, indicating that p21 is a key determinant of the growth inhibitory activity of TGFβ. Our results reveal a novel crosstalk between LPA and TGFβ that underlies TGFβ sensitive and resistant phenotypes of breast and ovarian cancer cells.
Keywords: LPA, p21Waf1, TGFβ, breast cancer, ovarian cancer
INTRODUCTION
Lysophosphatidic acid (LPA, 1-acyl-sn-glycerol-3-phosphate) is a naturally occurring intercellular mediator of diverse biological processes including neurogenesis, angiogenesis, would healing, immunity, and carcinogenesis (1). LPA is produced by activated platelets during coagulation and thus is a normal constituent of serum (2, 3). LPA is a ligand of at least six G protein-coupled receptors (GPCRs) (4). The LPA1/Edg2, LPA2/Edg4 and LPA3/Edg7 receptors are members of the endothelial differentiation gene (Edg) family, sharing 50–57% homology in their amino acid sequences. GPR23/P2Y9/LPA4 of the purinergic receptor family and the related GPR92/LPA5 and P2Y5/LPA6 have been identified as additional LPA receptors, which are structurally distant from the LPA1–3 receptors (4). The LPA receptors couple to multiple G proteins, Gq, Gi, G12/13 and Gs, which, in turn, activate diverse pathways including Gq-mediated stimulation of phospholipase C (5), Gi-mediated activation of the Ras-mitogen-activated protein kinases and phosphatidylinositol-3-kinase (6, 7), and G12/13-mediated activation of RhoA (8). Activation and integration of these signaling events downstream of LPA receptors leads to cytoskeleton remodeling, cell proliferation, survival, and migration (9, 10). Recent studies demonstrated that LPA exerts its biological actions through transcriptional activation of multiple target genes involved in a wide range of physiological and pathophysiological processes (11).
TGFβ is also a platelet-derived factor that controls a multitude of biological activities including cell proliferation (12), differentiation (13), and apoptosis (14). The complicated role of TGFβ is mediated through the heteromeric complex of transmembrane serine/threonine kinases, the type I and type II receptors (TβRI and TβRII), and the Smad family of transcription factors and non-Smad signaling pathways (15, 16). TGFβ inhibits proliferation of epithelial cells and thus plays a role in early tumor suppression. However, TGFβ frequently fails to induce growth arrest in transformed epithelial cells. On the other hand, TGFβ stimulates migration and invasion of neoplastic cells, thereby promoting the metastatic potentials of advanced cancer (17, 18).
The anti-proliferative effect of TGFβ is mediated by a complex signaling network involving TβRI and TβRII activation of Smad2/3 and ultimately transcriptional modulation of growth control genes such as induction of the cyclin-dependent kinase (CDK) inhibitors p21Waf1 and p15Ink4b, and suppression of the c-Myc, Id1 and Id2 transcription factors (19). Tumor cells tend to escape from the anti-proliferative effect of TGFβ through acquisition of mutations in components of the TGFβ signal transduction pathway or through deregulation of other signaling cascades interconnecting with the TGFβ pathway (20). Mutations in the TβRII receptor gene (19) as well as mis-sense mutation or deletion of Smad2 and 4 (21, 22) have been identified in different types of cancer. There is also evidence for overexpression of oncoproteins in inactivation of the cytostatic effect of TGFβ in cancer, such as Myc-Miz-1 complex (23), Evi-1 (24), FoxG1 (25), CDK (26) and Ski and/or SnoN (27). However, these aberrations seen in only fractions of human tumors do not explain the generally altered responses to TGFβ in a wide spectrum of cancers.
In the present study, we examined the potential crosstalk between LPA signaling and TGFβ in growth regulation of breast and ovarian cancer cells. We report that LPA upregulates expression of the CDK inhibitor p21 in breast and ovarian cancer cells sensitive to TGFβ-induced growth arrest but not in TGFβ-resistant cancer cells. In TGFβ-sensitive cells, LPA cooperates with TGFβ to elicit the maximal induction of p21 to mediate the cytostatic response to TGFβ. Loss or gain of p21 expression led to a shift between TGFβ sensitive and resistant phenotypes in these cells. Our results reveal a novel mechanism underlying the cytostatic program of TGFβ in breast and ovarian cancer cells.
MATERIALS AND METHODS
Materials
Anti-phospho C/EBPβ, phospho-Erk1/2, tubulin α/β antibodies and PD98059 were obtained from Cell Signaling (Danvers, MA). Anti-C/EBPβ, p21, and Erk antibodies were from Santa Cruz Biotech (Santa Cruz, CA). LPA (1-oleoly, 18:1) and S1P were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). Prior to use, LPA and S1P were dissolved in PBS containing 0.5% fatty acid-free bovine serum albumin (BSA) obtained from Roche (Indianapolis, IN). TGFβ was obtained from PeproTech Inc (Rocky Hill, NJ). TPA was from Sigma (St Louis, MI). FBS was obtained from Atlanta Biological (Atlanta, GA). Oligonucleotides were synthesized by Operon Biotechnologies, Inc. (Huntsville, AL). TRIzol and cell culture reagents were obtained from Invitrogen Inc. (Carlsbad, CA). The transfection reagent Dharmafect 1 was obtained from Dharmacon (Lafayette, CO). Plasmid DNA was purified using the endo-free purification kit from Qiagen (Valencia, CA).
Cell Culture
MDA-MB-231 was provided by S Spiegel (Virginia Commonwealth University). SK-BR-3 and BT-549 were obtained from Dr. G. Mills (MD Anderson Cancer Center). MDA-MB-231 cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% FBS and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin). Other breast and ovarian cancer cell lines used in the study were cultured in RPMI 1640 supplemented with 10% FBS and antibiotics as we described previously (28, 29).
Western blotting
Cells were lysed in SDS sample buffer or in ice-cold X-100 lysis buffer [1% Triton X-100, 50 mM HEPES (pH 7.4), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 100 mM NaF, 10 mM Na PPi, and protease inhibitor cocktail]. Total cellular proteins were resolved by SDS-PAGE, transferred to Immun-Blot membrane [poly(vinylidene difluoride)] from BIO-RAD (Hercules, CA), and immunoblotted with antibodies following the protocols of manufacturers. Immunocomplexes were visualized with an enhanced chemiluminescence detection kit from Amersham (Piscataway, NJ).
siRNA knockdown
The siRNA oligos for LPA receptors (LPA1 #4050, LPA2 #44997, LPA3 #136436 and LPA5 #s32725), p21 (#S415) and C/EBPβ (#s2891) were obtained from Applied Biosystems (Carlsbad, CA). siRNA oligos for Erk (Erk1 #L-003592-00 and Erk2 #L-03555-00) were obtained from Dharmacon (Lafayette, CO). They were transfected into cells using Dharmafect 1 following the manufacturers’ protocol. In brief, cells were plated in 6-well plates to reach 50% confluence before transfection for 12–16 hours with specific siRNA (100 pmole) and Dhamafect 1 (4 µL). The transfected cells were cultured in complete medium for approximately 48 hours before experiments.
Quantitative PCR
Total cellular RNA was isolated using Trizol (Invitrogen). Complementary DNA (cDNA) was synthesized from RNA (1 µg, random primers) using the High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems. The relative levels of individual LPA receptors were determined using gene specific probes, the TaqMan Universal PCR Master Mix and the 7900HT Fast Real-Time PCR System (Applied Biosystems).
Statistics
All numerical data were presented as mean ± SD. The statistical significance of differences was analyzed using Student's t test where p<0.05 was considered statistically significant.
RESULTS
Induction of p21 by LPA in TGFβ-sensitive breast and ovarian cancer cells
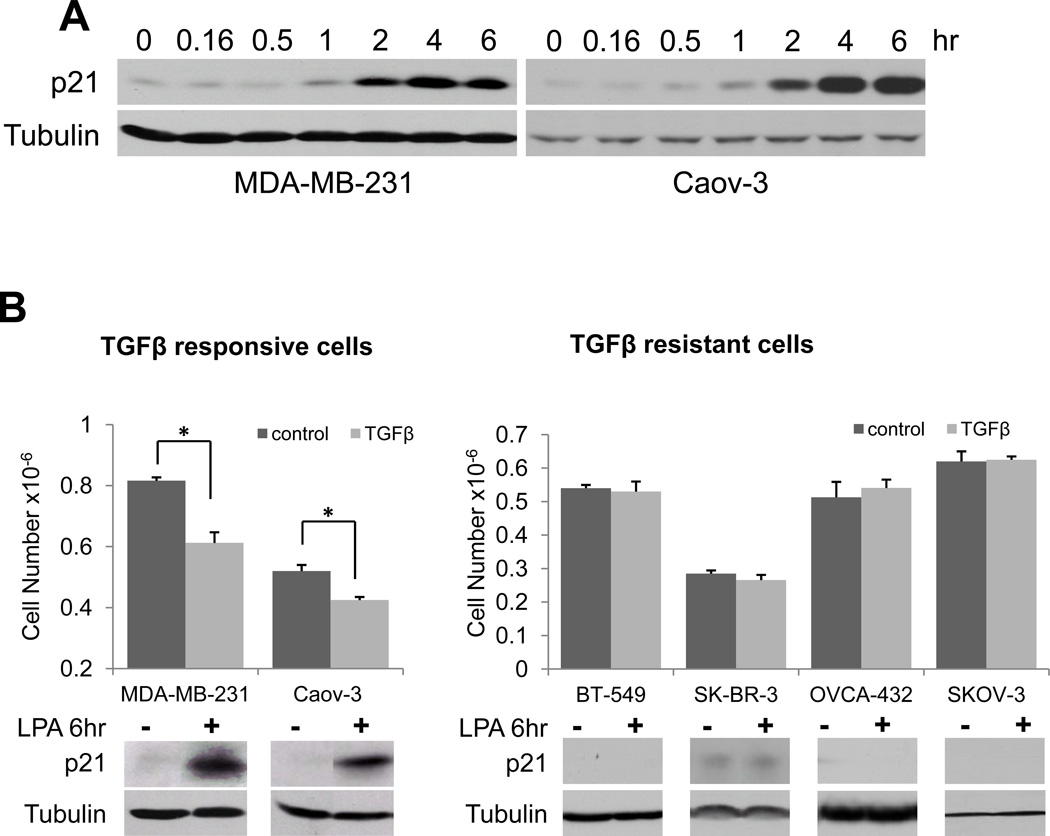
It has been well documented that LPA regulates expression of cytokines, angiogenic factors and many other proteins involved in tumorigenesis and cancer metastasis (28–31). In contrast to these oncogenic mediators, we also found that in a subset of breast and ovarian cancer cell lines, LPA upregulates expression of p21, an inducible inhibitor of CDKs. As shown in Fig. 1A, in the MDA-MB-231 breast carcinoma cells and the Caov-3 ovarian carcinoma cells, LPA stimulated p21 expression in a time-dependent manner. Following addition of 10 µM LPA to serum-starved cells, p21 protein was induced at 1 hour. The p21 protein levels reached the maximum by 4 hours. Although modest levels of p21 may promote assembly of active cyclin-CDK complex (32), excessive expression of p21 generally causes cell cycle arrest. However, the strong and sustained induction of p21 by LPA was not associated with growth inhibition. Instead, LPA treatment led to increased proliferation in MDA-MB-231 and Caov-3 cells (data not shown or see Fig. 5) as well as in other breast and ovarian cancer cell lines in which LPA did not trigger p21 expression (Fig. 1B).
Figure 1.
LPA induction of p21 in TGFβ-sensitive breast and ovarian cancer cells. A. MDA-MB-231 cells and Caov-3 cells were starved in serum-free medium and treated with LPA (10 µM) for the indicated periods of time. The cells were lysed with SDS sample buffer and expression of p21 protein was examined by immunoblotting. B. Various breast and ovarian cancer cell lines in 6-well plates were incubated for 48 hours in complete medium with or without TGFβ (2.5 ng/ml). Cell numbers were quantified with a Coulter counter. The induction of p21 protein by LPA in these cell lines was examined by immunoblotting as described in A. In this and the following figures, the statistical significances of the data were indicated with * if p<0.05, or ** if p<0.01.
Figure 5.
p21-dependent inhibition of LPA-induced cell proliferation by TGFβ. A. TGFβ inhibits LPA-afforded cell proliferation. MDA-MB-231 and Caov-3 cells in 6-well plates were incubated with LPA (10 µM) or vehicle (BSA) in the presence or absence of TGFβ (2.5 ng/ml). B. The growth inhibitory effect of TGFβ depends on p21 induction. MDA-MB-231 and Caov-3 cells were transfected with control or p21 siRNA as described in Figure 4. The cells were treated with LPA in the presence or absence of TGFβ. The cell numbers presented in both panels were determined after 48 hours. Efficiency of p21 siRNA knockdown in transfected cells was analyzed by immunoblotting as shown in Fig. 4A.
In an effort to understand the biological significance of LPA-mediated p21 expression, we noticed surprisingly that LPA stimulated p21 expression only in cell lines sensitive to the TGFβ-induced growth arrest but not in cells refractory to TGFβ. As demonstrated in Fig. 1B, treatment of MDA-MB-231 and Caov-3 cells with TGFβ (2.5 ng/ml) for 48 hours resulted in a significant decrease in cell numbers compared to control cells cultured in the absence of TGFβ (Fig. 1B). In contrast, TGFβ did not inhibit the growth of cell lines such as BT-549, SK-BR-3, OVCA-432 and SKOV-3 in which LPA did not induce p21 (Fig. 1B).
Correlation of LPA and TGFβ induction of p21
We next explored the possibility that LPA-driven p21 expression might modulate the sensitivity of breast and ovarian cancer cells to TGFβ. Coincidently, the effect of TGFβ on p21 expression was identical to that of LPA in these breast and ovarian cancer cells. As shown in Fig. 2, TGFβ induced p21 expression at significant levels only in MDA-MB-231 and Caov-3 cells but not in TGFβ-resistant lines in which LPA failed to induce p21 (Fig. 2).
Figure 2.
Correlation of LPA of TGFβ induction of p21. Breast and ovarian cancer cell lines in complete medium were treated for 6 hours with or without TGFβ (2.5 ng/ml) before lysis with SDS sample buffer. Expression of p21 protein and phosphorylated Smad3 was analyzed by immunoblotting.
The loss of p21 inducibility by TGFβ could be due to abnormalities in TGFβ receptors or the TGFβ intracellular signaling through Smads. It is well known that TGFβ superfamily ligands bind to a TβRII, which recruits and phosphorylates a TβRI. TβRI then phosphorylates receptor-regulated Smads (RSmad) such as Smad2 and Smad3, which then bind to the common mediator Smad (coSmad). RSmad forms heterodimeric complexes with coSmads and accumulates in the nucleus where the complexes participate in regulation of TGFβ target genes involved in growth control (19). As shown in Fig. 2, TGFβ induced phosphorylation of Smad3 in all breast and ovarian cancer cell lines examined, irrespective of their status of TGFβ sensitivity. Furthermore, we examined the effect of TGFβ on another TGFβ-target gene plasminogen activator inhibitor-1 (PAI-1). Upon treatment with TGFβ, all cell lines showed variable increases in expression of PAI-1 mRNA (Supplementary Figure S1). This suggests that both TGFβ-sensitive and resistant cells maintain functional TGFβ receptors and the Smad3 signal transducer.
Input of LPA signaling in TGFβ-induced p21 expression
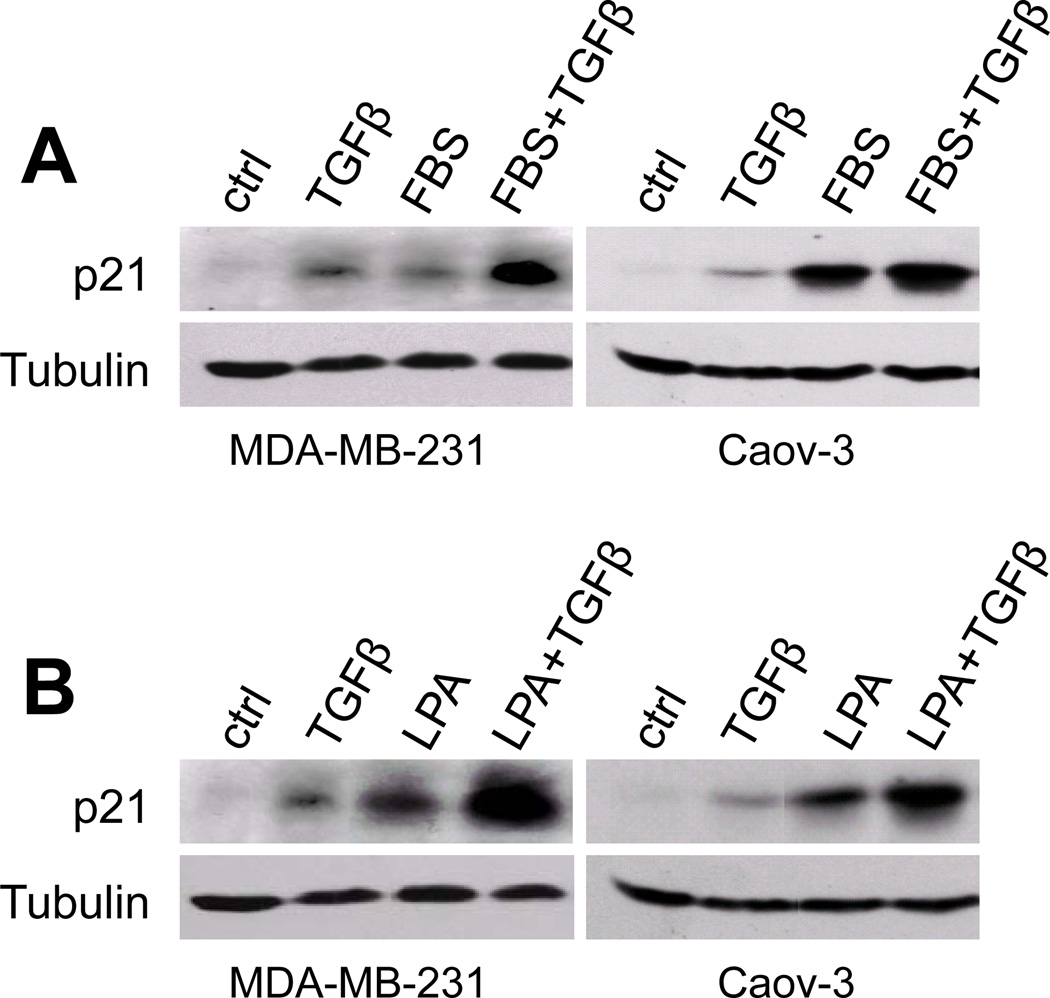
Since phosphorylation of Smad3 by TGFβ was observed in both TGFβ-sensitive and resistant cells, p21 induction by TGFβ seems to involve other signaling routes beyond the canonical Smad pathway in TGFβ sensitive cells. In addition, both MDA-MB-231 and Caov-3 carry mutant p53 (33, 34). TGFβ-induced p21 expression in these cells is apparently mediated by a p53-independent process. We therefore examined the possibility that LPA contributes to TGFβ-induced p21 expression in the TGFβ-sensitive MDA-MB-231 and Caov-3 cells. When these cells were cultured in serum-free medium, TGFβ stimulated only weak to modest levels of p21 (Fig. 3A). The maximal p21 induction by TGFβ was seen when the cells were incubated in complete medium containing fetal bovine serum (FBS) (Fig. 3A), a condition in which the effects of TGFβ on cell proliferation and p21 expression were assessed in earlier experiments (Fig. 1B & Fig. 2). Serum itself induced p21 expression in MDA-MB-231 and Caov-3 cells. This suggests that induction of p21 by TGFβ that we had observed resulted from a combined action of TGFβ and a co-factor present in serum.
Figure 3.
Input of LPA action in TGFβ-mediated p21 induction. A. MDA-MB-231 and Caov-3 cells were serum starved and stimulated for 6 hours with TGFβ (2.5 ng/ml), FBS (5%), or TGFβ+FBS. Expression of p21 in these cells was assessed by immunoblotting. B. MDA-MB-231 and Caov-3 cells were treated and analyzed as described in A except that FBS was replaced with LPA (10 µM).
LPA is a prominent serum-borne factor responsible for many biological activities of serum (35). To determine whether LPA reproduces the action of serum in concert with TGFβ to maximize p21 induction, we examined the effect of LPA and TGFβ on p21 expression in MDA-MB-231 and Caov-3 cells. Indeed, p21 induction was maximized when both LPA and TGFβ were present (Fig. 3B). We also assessed other serum factors such as sphingosine 1 phosphate (S1P) and insulin for their ability to regulate p21 expression (36, 37). In contrast to LPA, S1P and insulin did not increase p21 expression. Nor did S1P and insulin potentiate the effect of TGFβ on p21 (data not shown). Taken together, these results suggest that a significant input of TGFβ-induced p21 is attributable to the action of LPA, which likely underlies the sensitivity of breast and ovarian cancer cells to TGFβ.
Role of p21 in mediating the cytostatic response to TGFβ
To confirm an essential role for p21 in mediating the TGFβ response, we used siRNA to knockdown p21 expression in the TGFβ sensitive MDA-MB-231 and Caov-3 cells. As shown in Fig. 4A, suppression of p21 induction by siRNA converted these cells into a resistant phenotype. The p21 knockdown cells became insensitive to the inhibitory effect of TGFβ, confirming that p21 induction is indeed a key component of TGFβ-induced cytostasis in breast and ovarian cancer cells.
Figure 4.
Essential role of p21 in the cytostatic response to TGFβ. A. The TGFβ-sensitive cells lost sensitivity to TGFβ following siRNA knockdown of p21 expression. MDA-MB-231 and Caov-3 cells in 6-well plates were transfected with control or p21 siRNA. The cells were treated for 48 hours with or without TGFβ (2.5 ng/ml) before quantification of cell numbers with a Coulter counter. Efficiency of p21 siRNA knockdown was confirmed by immunoblotting. B. The TGFβ resistant cell lines gained sensitivity to TGFβ following TPA induction of p21. BT-549 and OVCA-432 in 6-well plates were treated for 48 hours with or without TGFβ in the presence of TPA (0.1 µM) or vehicle before quantification of cell numbers. Expression of p21 in these cells treated with TGFβ, TPA or TGFβ+TPA was analyzed by immunoblotting.
If the p21 inducibility distinguishes TGFβ-sensitive cells from resistant ones, we assume that the resistant cells could be rendered sensitive to TGFβ when p21 is induced somehow by other p21 stimuli. To test this possibility, we took advantage of the fact that phorbol ester (12-O-tetradecanoylphorbol-13-acetate, TPA) induces expression of p21 in cancer cells (38). We treated the TGFβ resistant cell lines with TGFβ alone or TGFβ and TPA (0.1 µM). The presence of TPA led to induction of high and sustained expression of p21 while the cells treated with TGFβ alone did not show p21 expression (Fig. 4B). Treatment with TPA was not associated with inhibition of cell proliferation as shown in Fig. 4B, suggesting that p21 induced by TPA was not sufficient to affect cell growth without TGFβ. However, the presence of TPA-induced p21 expression enables TGFβ to suppress growth of these otherwise TGFβ-resistant cells, consistent with the importance of p21 in mediating TGFβ sensitivity (Fig. 4B). The restoration of the growth-inhibitory effect of TGFβ was not due to induction of apoptotic cell death. TGFβ did not trigger apoptosis in TPA-treated BT-549 or OVCA-432 cells (Supplementary Figure S2).
p21-dependent inhibition of LPA-driven cell proliferation by TGFβ
LPA stimulated p21 expression in MDA-MB-231 and Caov-3 cells (Fig. 3B). However, In spite of the robust and sustained induction of p21, LPA is mitogenic towards these cells. To determine whether TGFβ was able to block the mitogenic effect of LPA, we compared the growth of MDA-MB-231 and Caov-3 cells incubated with LPA in the absence or presence of TGFβ. Fig. 5A showed that TGFβ effectively inhibited cell number increases stimulated by LPA. Moreover, siRNA knockdown of p21 expression resulted in resistance of these cells to TGFβ (Fig. 5B), confirming an essential role for p21 in TGFβ repression of LPA-induced cell proliferation. In TGFβ-resistant breast and ovarian cancer cell lines, LPA also acted as a mitogen. The mitogenic activity of LPA, however, was not affected by TGFβ (data not shown), consistent with the lack of induction of p21 by LPA, TGFβ or LPA and TGFβ in these cells.
Mechanisms for LPA induction of p21
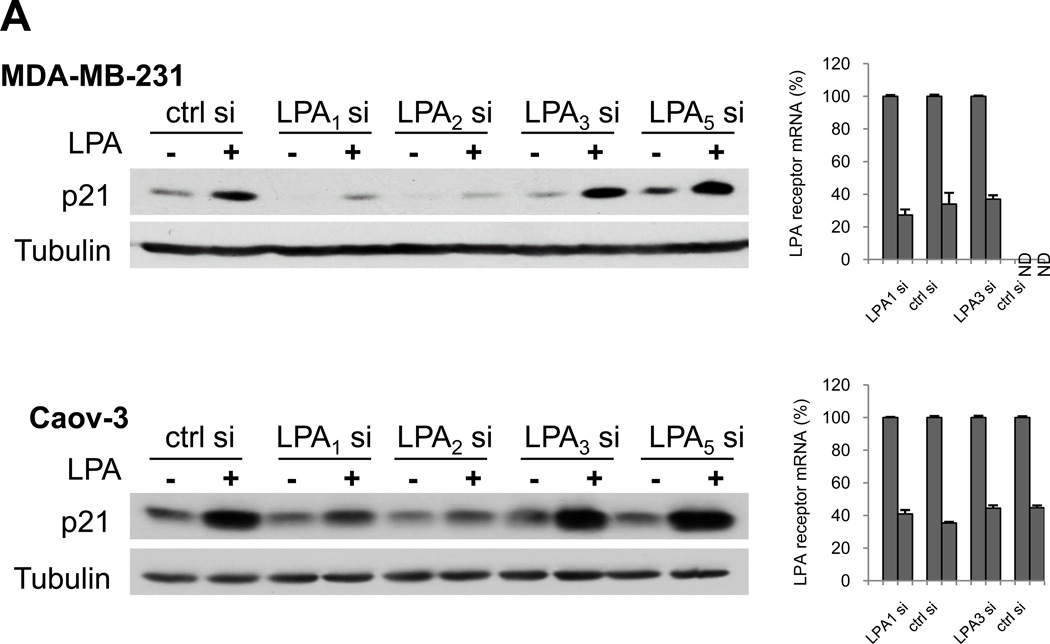
Ovarian and breast cancer cells express multiple LPA receptors including LPA1, LPA2, LPA3 and LPA5 as described previously (39–41). Expression of the LPA4 and LPA6 receptors was undetectable in the breast and ovarian cancer lines (data not shown). We thus used siRNA to knockdown expression of LPA1, LPA2, LPA3 or LPA5. The cells treated with LPA were then examined for p21 protein expression. LPA-induced p21 was drastically reduced by downregulation of LPA1 or LPA2 (Fig. 6A). Knockdown of LPA3 or LPA5 did not attenuate the effect of LPA on p21 expression. Therefore, we conclude that LPA-stimulated p21 expression in MDA-MB-231 and Caov-3 cells occurs through the LPA1 and LPA2 receptors.
Figure 6.
Induction of p21 by LPA through the LPA1/LPA2-Erk-C/EBPβ pathway. A. LPA1 and LPA2 are responsible for LPA stimulation of p21. MDA-MB-231 and Caov-3 cells were transfected with specific siRNA for LPA1–3, LPA5 or non-target control siRNA. The cells were serum starved and stimulated with LPA (10 µM) before immunoblotting analysis of p21 protein expression. The efficiency of knockdown of individual LPA receptors was examined by RT and quantitative PCR as described in Materials and Methods. The mRNA levels of LPA receptors were presented as percentages relative to those in the control siRNA-transfected cells (defined as 100%). ND: not detectable. B. Knockdown of Erk1/2 blocks LPA induction of p21. MDA-MB-231 and Caov-3 cells were transfected with Erk siRNAs or non-target control siRNA. The cells were stimulated for 6 hours with LPA (10 µM) followed by immunoblotting analysis of p21, Erk and tubulin proteins. C. C/EBPβ was critical for LPA induction of p21. MDA-MB-231 and Caov-3 cells were transfected with control or C/EBPβ siRNA. The transfected cells were treated for 6 hours with LPA and analyzed for p21 and C/EBPβ protein expression. D. MDA-MB-231 and Caov-3 cells were serum starved and then treated with LPA (10 µM) in the presence of PD98059 (30 µM) or vehicle (DMSO). PD98059 was added 1 hour before LPA. Cells were then lysed at indicated time points. Levels of phospho-Erk1/2, p21 and phospho-C/EBPβ were examined by immunoblotting.
LPA induced strong and sustained activation of Erk in MDA-MB-231 and Caov-3 cells (29, 42). When Erk1 and Erk2 were silenced by siRNAs, LPA induction of p21 was blocked (Fig. 6B), indicating that the Erk pathway is linked to activation of p21 expression in response to LPA. In contrast to Erk, phosphatidylinositol 3-kinase (PI3K) was dispensable for LPA-induced p21 induction because its inhibitor LY-294002 did not attenuate the effect of LPA on p21 expression (data not shown).
Erk couples directly or indirectly to diverse downstream effectors and transcription factors that could culminate in p21 expression. We used siRNA to screen for transcription factors required for LPA-induced p21 expression including AP-1, SRE, NF-κB and C/EBPβ (30, 43). In this group of transcription factors, C/EBPβ was found to be critical to the p21 induction. Knockdown of C/EBPβ expression prevented LPA-induced p21 expression (Fig. 6C). Finally, inhibition of Erk activity with the MEK inhibitor PD98059 prevented C/EBPβ phosphorylation and the subsequent p21 induction in LPA-treated MDA-MB-231 and Caov-3 cells (Fig. 6D). These findings demonstrate that LPA stimulates p21 expression through the LPA1/2-Erk-C/EBPβ signaling network.
DISCUSSION
TGFβ-mediated cytostasis is induced, at least in part by Smad-dependent activation of TGFβ target genes involved in cell cycle control, primarily CDK inhibitors p15, p21 and p27. In addition, TGFβ activation of Smad represses expression of proteins that promotes cell cycle progression including c-Myc, Id1, Id2, E2F, and Sp-1 (44, 45). These TGFβ-induced cytostatic transcriptional programs, however, are subverted in a majority of cancers, leading to cytostatic resistance to TGFβ (46). In addition to genetic and epigenetic aberrations in TGFβ receptors or Smad proteins, emerging data suggests that in most malignancies, abrogation of TGFβ-induced growth arrest is mediated by abnormal expression or function of intracellular proteins implicated in Smad regulation of its target genes (18). In theory, environmental cues that influence expression or activity of Smad, Smad regulatory circuits or Smad responsive genes could also alter cellular responses to TGFβ. However, there have been few studies to analyze potential crosstalk between extracellular factors such as LPA and TGFβ-Smad to regulate the responsiveness of cancer cells to TGFβ.
Using breast and ovarian cancer cells as model systems, we demonstrated that LPA upregulates expression of the prototype Smad target gene p21, contributing to the TGFβ-mediated growth inhibition. In these cells, the ability of LPA to stimulate p21 expression correlated well with TGFβ induction of p21 and the cytostatic effect of TGFβ. By means of induction and suppression of p21 expression in TGFβ-resistant and sensitive cells, we could reverse the cellular responses to TGFβ confirming an essential role of p21 in mediating the cytostatic response to TGFβ. Previous studies in breast and ovarian cancer cells also supported the involvement of p21 as a key mediator of TGFβ-induced growth inhibition (44). Another observation in ovarian cancer indicates that abrogation of TGFβ induced growth arrest is associated with overexpression of FoxG1, a negative regulator of p21 expression (47). Therefore, p21 seems to be a general mediator of TGFβ-induced growth arrest in multiple types of cancer cells. The findings of the present work highlight the possibility that the sensitivity to TGFβ in breast and ovarian cancer cells could be reconstituted through upregulation of p21 expression. It will be of interest to develop and test agents that can specifically activate p21 expression or stabilize p21 protein in cancer cells.
An interesting finding in the current study is that p21 induction in TGFβ sensitive cells is accomplished through cooperative effects of TGFβ and the serum-borne factor LPA. A significant input of p21 expression is evoked from LPA activation of its receptors, namely LPA1 and LPA2. Using molecular and pharmacological approaches, we further demonstrated that LPA upregulates p21 expression in TGFβ responsive cells through the Erk-C/EBPβ signaling pathway. We have previously shown that C/EBPβ is a transcription factor activated by LPA which accounts for LPA-induced expression of Cox-2 and sphingosine kinase 1 in various cancer cells (30, 48). The results in the present work links C/EBPβ to the induction of p21 by LPA in TGFβ growth arrest program in breast and ovarian cancer cells, suggesting a general role for this transcription factor in regulation of LPA target genes. Consistent with their resistance to TGFβ, the stimulatory effect of LPA on p21 was not seen in most breast and ovarian cancer cell lines. The differential effects of LPA on p21 in different cell lines are not fully understood but could be due to distinct expression patterns of LPA receptors in these cells. The receptor knockdown experiments in the TGFβ-sensitive MDA-MB-231 and Caov-3 cells indicated that both LPA1 and LPA2 receptors are required for induction of p21 by LPA. Among the TGFβ-resistant cell lines, SKOV-3 and BT-549 express low levels of LPA2 (28, 49) and OVCA-432 exhibits elevated LPA3 (28). It is conceivable that co-expression of two or more receptors at appropriate levels is important for optimal induction of p21 by LPA. Alternatively, it is also possible that certain LPA receptors including the conventional LPA3, novel LPA receptor subtypes and other unknown LPA receptors could be present in the resistant cells and serve as negative regulators of certain biological functions of LPA.
Supplementary Material
ACKOWLEDGEMENTS
The work was supported by the NIH/NCI grant 2R01CA102196 (XF), the Department of Defense ovarian cancer research program grant W81XWH-11-1-0541 (XF), the Jeffress Memorial Fund award (XF), and the NIH grant P30 CA16059 to Massey Cancer Center of Virginia Commonwealth University School of Medicine. The authors like to thank Dr. Michael W. Maceyka for reading the manuscript and his constructive suggestions.
REFERENCES
- 1.Panupinthu N, Lee HY, Mills GB. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer. 2010;102:941–946. doi: 10.1038/sj.bjc.6605588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291(Pt 3):677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 4.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 5.van der Bend RL, de Widt J, van Corven EJ, Moolenaar WH, van Blitterswijk WJ. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D, Comparison with the response to endothelin. Biochem J. 1992;285(Pt 1):235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe LR, Marshall CJ. Lysophosphatidic acid stimulates mitogen-activated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J Biol Chem. 1993;268:20717–20720. [PubMed] [Google Scholar]
- 7.Takeda H, Matozaki T, Takada T, Noguchi T, Yamao T, Tsuda M, Ochi F, Fukunaga K, Inagaki K, Kasuga M. PI 3-kinase gamma and protein kinase C-zeta mediate RAS-independent activation of MAP kinase by a Gi protein-coupled receptor. Embo J. 1999;18:386–395. doi: 10.1093/emboj/18.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell. 1999;10:1851–1857. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 10.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Teo ST, Yung YC, Herr DR, Chun J. Lysophosphatidic acid in vascular development and disease. IUBMB Life. 2009;61:791–799. doi: 10.1002/iub.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem. 2005;96:447–462. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- 13.Fei T, Chen YG. Regulation of embryonic stem cell self-renewal and differentiation by TGF-beta family signaling. Sci China Life Sci. 2010;53:497–503. doi: 10.1007/s11427-010-0096-2. [DOI] [PubMed] [Google Scholar]
- 14.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 15.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 16.de Caestecker MP, Piek E, Roberts AB. Role of transforming growth factor-beta signaling in cancer. J Natl Cancer Inst. 2000;92:1388–1402. doi: 10.1093/jnci/92.17.1388. [DOI] [PubMed] [Google Scholar]
- 17.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 18.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 20.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 21.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Yang X. Smad4-mediated TGF-beta signaling in tumorigenesis. Int J Biol Sci. 2010;6:1–8. doi: 10.7150/ijbs.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Letterio J. Transforming growth factor-beta signaling in normal and malignant hematopoiesis. Leukemia. 2003;17:1731–1737. doi: 10.1038/sj.leu.2403069. [DOI] [PubMed] [Google Scholar]
- 25.Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Lundin M, Ristimaki A, Heikkila P, Lundin J, Isola J, Joensuu H, Laiho M. Ski-related novel protein N (SnoN), a negative controller of transforming growth factor-beta signaling, is a prognostic marker in estrogen receptor-positive breast carcinomas. Cancer Res. 2003;63:5005–5010. [PubMed] [Google Scholar]
- 28.Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, LaPushin R, Claret FX, Aggarwal BB, Lu Y, Mills GB. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 29.Lee Z, Swaby RF, Liang Y, Yu S, Liu S, Lu KH, Bast RC, Jr, Mills GB, Fang X. Lysophosphatidic acid is a major regulator of growth-regulated oncogene alpha in ovarian cancer. Cancer Res. 2006;66:2740–2748. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- 30.Oyesanya RA, Lee ZP, Wu J, Chen J, Song Y, Mukherjee A, Dent P, Kordula T, Zhou H, Fang X. Transcriptional and post-transcriptional mechanisms for lysophosphatidic acid-induced cyclooxygenase-2 expression in ovarian cancer cells. Faseb J. 2008;22:2639–2651. doi: 10.1096/fj.07-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Wu J, Oyesanya RA, Lee Z, Mukherjee A, Fang X. Sp-1 and c-Myc mediate lysophosphatidic acid-induced expression of vascular endothelial growth factor in ovarian cancer cells via a hypoxia-inducible factor-1-independent mechanism. Clin Cancer Res. 2009;15:492–501. doi: 10.1158/1078-0432.CCR-08-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. The p21(Cip1) and p27(Kip1) CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. Embo J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui L, Zheng Y, Yan Y, Bargonetti J, Foster DA. Mutant p53 in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D activity and contributes to survival signals generated by phospholipase D. Oncogene. 2006;25:7305–7310. doi: 10.1038/sj.onc.1209735. [DOI] [PubMed] [Google Scholar]
- 34.Yaginuma Y, Westphal H. Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res. 1992;52:4196–4199. [PubMed] [Google Scholar]
- 35.Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 36.Kim DS, Kim SY, Kleuser B, Schafer-Korting M, Kim KH, Park KC. Sphingosine-1-phosphate inhibits human keratinocyte proliferation via Akt/protein kinase B inactivation. Cell Signal. 2004;16:89–95. doi: 10.1016/s0898-6568(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 37.Hiromura K, Monkawa T, Petermann AT, Durvasula RV, Shankland SJ. Insulin is a potent survival factor in mesangial cells: role of the PI3-kinase/Akt pathway. Kidney Int. 2002;61:1312–1321. doi: 10.1046/j.1523-1755.2002.00257.x. [DOI] [PubMed] [Google Scholar]
- 38.Salabat MR, Ding XZ, Flesche JB, Ujiki MB, Robin TP, Talamonti MS, Bell RH, Jr, Adrian TE. On the mechanisms of 12-O-tetradecanoylphorbol-13-acetate-induced growth arrest in pancreatic cancer cells. Pancreas. 2006;33:148–155. doi: 10.1097/01.mpa.0000226896.93945.41. [DOI] [PubMed] [Google Scholar]
- 39.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, Jaffe R, Erickson J, Mills GB. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Murph M, Panupinthu N, Mills GB. ATX-LPA receptor axis in inflammation and cancer. Cell Cycle. 2009;8:3695–3701. doi: 10.4161/cc.8.22.9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 42.Du J, Sun C, Hu Z, Yang Y, Zhu Y, Zheng D, Gu L, Lu X. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PLoS One. 2010;5:e15940. doi: 10.1371/journal.pone.0015940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyesanya RA, Greenbaum S, Dang D, Lee Z, Mukherjee A, Wu J, Dent P, Fang X. Differential requirement of the epidermal growth factor receptor for G protein-mediated activation of transcription factors by lysophosphatidic acid. Mol Cancer. 2010;9:8. doi: 10.1186/1476-4598-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 45.Feng XH, Lin X, Derynck R. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in response to TGF-beta. Embo J. 2000;19:5178–5193. doi: 10.1093/emboj/19.19.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barcellos-Hoff MH, Ewan KB. Transforming growth factor-beta and breast cancer: Mammary gland development. Breast Cancer Res. 2000;2:92–99. doi: 10.1186/bcr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW, Cheung AN, Ngan HY. Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis. J Pathol. 2008;215:245–252. doi: 10.1002/path.2355. [DOI] [PubMed] [Google Scholar]
- 48.Shida D, Fang X, Kordula T, Takabe K, Lepine S, Alvarez SE, Milstien S, Spiegel S. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–6577. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, Towers LN, O'Connor KL. LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells. Am J Physiol Cell Physiol. 2007;292:C1927–C1933. doi: 10.1152/ajpcell.00400.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.