Abstract
Post-transcriptional gene silencing (PTGS) agents such as antisense, ribozymes and RNA interference (RNAi) have great potential as therapeutics for a variety of eye diseases including retinal and macular degenerations, glaucoma, corneal degenerations, inflammatory and viral conditions. Despite their great potential and over thirty years of academic and corporate research only a single PTGS agent is currently approved for human therapy for a single disease. Substantial challenges exist to achieving both efficacious and safe PTGS agents. Efficacy, as measured in specific target mRNA and protein knockdown, depends upon a number of complex factors including the identification of rare regions of target mRNA accessibility, cellular colocalization of the PTGS agent in sufficient concentration with the target mRNA, and stability of the PTGS agent in the target cells in which it is delivered or expressed. Safety is commonly measured by lack of cytotoxicity or other deleterious cellular responses in cells in which the PTGS agent is delivered or expressed. To relieve major bottlenecks in RNA drug discovery novel, efficient, inexpensive, and rapid tools are needed to facilitate lead identification of the most efficacious PTGS agent, rational optimization of efficacy of the lead agent, and lead agent safety determinations. We have developed a technological platform using cell culture expression systems that permits lead identification and efficacy optimization of PTGS agents against arbitrary disease target mRNAs under relatively high throughput conditions. Here, we extend the technology platform to include PTGS safety determinations in cultured human cells that are expected to represent the common cellular housekeeping microenvironment. We developed a high throughput screening (HTS) cytotoxicity assay in 96-well plate format based around the SYTOX Green dye which is excluded from healthy viable cells and becomes substantially fluorescent only after entering cells and binding to nuclear DNA. In this format we can test a number of PTGS agents for cellular toxicity relative to control elements. We also developed a HTS 96-well plate assay that allows us to assess the impact of any given PTGS agent on stimulating a variety of common cellular stress signaling pathways (e.g. CRE, SRE, AP-1, NFκB, Myc, and NFAT) that could indicate possible deleterious effects of PTGS agents either dependent or independent of base pairing complementarity with target mRNAs. To this end we exploited the secreted alkaline phosphatase (SEAP) Pathway Profiling System where the expression of the secreted reporter protein is coupled to transcriptional activation of a variety of promoter elements involved in common cell signaling pathways. We found that a variety of lead hammerhead ribozyme (hhRz) and short hairpin (shRNA) expression constructs did not exert cytotoxicity in human cells when driven by highly active RNA Pol-III promoters. We also found that most of the cell signaling pathways tested (CRE, SRE, Myc, and NFAT) did not significantly couple through upregulation to expression of the set of PTGS agents tested. AP-1 and NFκB upregulation both appear to couple to the expression of some PTGS agents which likely reflect the known properties of these pathways to be stimulated by abundant small structured RNAs.
Keywords: post-transcriptional gene silencing, high throughput screening
Introduction
PTGS agents hold vast potential as candidate therapeutics for a multitude of diseases, including those of the eye (Millington-Ward et al., 1997; Lewin et al., 1998; LaVail et al., 2000; Farrar et al., 2002; Gorbatyuk et al., 2007; O'Reilly et al., 2007; Sullivan et al., 2008; Abdelmaksoud et al., 2009). However, despite over three decades of work on development of therapeutic PTGS agents, only one has been FDA-approved for the US pharmaceutical market (Anderson et al., 1996; Jabs and Griffiths, 2002). The design of successful PTGS agents is a challenging task that embraces many complex variables. The most obvious initial hurdles are locating accessible sites in the target mRNA where a small PTGS agent could rapidly anneal as an initial step toward cleavage as well as insuring that the target mRNA and PTGS agent co-localize within the same cellular compartment with similar lifetimes. In addition, the enzyme (PTGS): substrate (target mRNA) concentration ratio must be in sufficient excess to drive a robust intermolecular collision frequency. Even after the accessibility, colocalization, and kinetics of the reaction have been optimized toward maximized cellular efficacy, other factors must also be taken into consideration such as the potential activation of cytotoxic or cellular stress responses by the delivered or expressed PTGS agent.
A PTGS agent is designed to specifically interact with a single target mRNA. Any type of PTGS agent that interacts with a target RNA on the basis of base pair complementarity has the potential to partially or fully interact “off-target” with other mRNAs in the cell, promoting knockdown or suppression of unintended target mRNAs. As a PTGS modality RNAi is particularly prone to off-target effects because the RNA-induced silencing complex is mismatch tolerant, and the seed sequence is short (∼7-8 nt) and commonly has alternative binding sites in the transcriptome. Hammerhead ribozymes, on the other hand, require longer antisense flank spans (≥ 12 nt), are not mismatch tolerant in the region around the phosphodiester bond of the target that is cleaved, and are thought to have higher specificity. Nevertheless, a PTGS agent must interface with the complexity of the cellular biome.] It is difficult, if not impossible, to predict the extent to which a given PTGS agent will impact upon processes in the cellular, animal, or human environment. For example, RNAi technology has been associated with fatalities in mice apparently due not to activation of the RNA-dependent protein kinase/interferon pathway or the presence of a shRNA target, but rather as a result of competition of expressed shRNAs with naturally expressed microRNAs (miRNAs) for intracellular processing and trafficking (Grimm et al. 2006). Competition for cellular machinery and resources is not the only concern with PTGS agents. Though ribozymes do not rely on intrinsic cellular pathways and machinery for target knockdown, there may still be concerns when the agent is present at high doses (Kayikcioglu et al., 2006). Toxicity with antisense oligonucleotide therapeutics has also been reported (Henry et al., 1999).
Recent studies have shown that PTGS agents have potential to stimulate innate immune responses in the cell. In a study for the development of a treatment for choroidal neovascularization (CNV), synthetic short interfering RNAs (siRNA) designed to anneal to and promote cleavage of target mRNAs for vascular endothelial growth factor-A or its receptor, suppressed CNV through a target-independent mechanism that involved binding of the siRNA to surface TLR3 receptors, activation of the TLR3 signaling pathway, and subsequent cellular production of interferon-γ and interleukin-12 which inhibited the pathological angiogenesis (Kleinman et al., 2008). The activation of TLR3 by the specific PTGS agents produced mechanistically unintended, yet therapeutically positive results. NFκB can be activated in general stress pathways and upregulates, for example, in a photoreceptor degeneration that is due to the absence of expression of a photoreceptor gene (Zeng et al., 2008). Induction of a cellular immune response by a PTGS agent may potentially promote adverse effects. Indeed, PTGS agents as nucleic acid drugs have potential to activate innate cellular immune responses that appear to have evolved to signal stresses involved in viral infections (Kawai and Akira, 2006; Yoneyama et al., 2004; Johnsen et al. 2006). One such pathway is mediated by the activation of the dsRNA-activated serine protein kinase (PKR) which can inhibit translation and interface with different signaling pathways, including NFκB (Chu et al., 1999; Gil et al., 2000; Zamanian-Daryoush et al., 2000). We realize that it is necessary to investigate unintended effects that a PTGS agent may have, such as cellular toxicity or activation of stress-related transcriptional pathways, in addition to determining the efficiency with which it can knockdown or suppress its cognate target mRNA and protein.
We previously developed a HTS method for evaluating the knockdown efficacy of sets of PTGS agents within the cellular environment to identify lead candidate RNA drugs for subsequent optimization (Yau and Sullivan, submitted). Here we describe a HTS method of investigating for cellular toxicity or potentially adverse cellular signaling effects of candidate therapeutic PTGS agents. These studies are conducted in inexpensive and time-efficient cell culture model systems in 96-well plates in comparison to the evaluation of potentially therapeutic PTGS agents in vivo in animal models. As toxicity and potentially adverse effects of given PTGS agents would commonly occur at the cellular housekeeping level, such a screen in cultured human cells is both rational and valuable and is likely to be useful to anticipate potential adverse effects that might occur in vivo during preclinical testing. This initial proof-of-principle study was conducted in HEK293 cells, but could readily be extended to other cells types in future studies. Changes in cell viability due to the introduction or expression of PTGS agents in the cellular environment can be detected using a SYTOX Green nuclear stain that freely enters cells with compromised membranes undergoing apoptosis. Transcriptional activation of common stress pathways by PTGS agents can be assayed using the SEAP Pathway Profiling System which consists of several plasmids containing a transcriptional enhancer element upstream of a SEAP reporter gene. Measurement of SEAP reporter enzyme secreted into the culture media indicates the level of activation of the particular upstream promoter element. These combined methods provide a rapid means to broadly determine cellular responses to a PTGS agent before testing in an animal model. These approaches can be enhanced in future studies to screen a larger range of transcriptionally modulated signal pathways that are increasingly associated with nonspecific cellular dsRNA toxicity, or toxicity associated with specific pathways pertinent to PTGS (Schlee et al., 2006; Marques and Williams, 2005; Sledz and Williams, 2004; Judge et al., 2005).
Materials & Methods
Vector Development
Using standard plasmid construction approaches hammerhead ribozyme (hhRz) cDNA constructs were directionally ligated into the Sal I/Pst I sites in pUC-VaI, Prislei-VAI and pUC-CELO vectors. pUC-VaI was generated by cloning the gene for VAI as a BssHII-XbaI fragment into pNEB193-T7, which is an engineered version of pNEB-193 (New England Biolabs, Ipswich, MA). pUC-Val is a reengineered version of the pGVAL vector previously described (Lieber and Strauss, 1995; Abdelmaksoud et al., 2009; Yau and Sullivan, submitted). A modified central domain stem-loop structure (designed using RNA secondary structure analysis) was added to as a series of adapters into the adenoviral VAI RNA. The construction of Prislei-VAI followed the prior report of VAI RNA central domain modifications (Prislei et al., 1997). The human adenoviral 5 VAI RNAs are expressed from strong intrinsic promoters (RNA Pol-III). A major role of the VA RNAs is to prevent host cell translational shutdown at the ribosome during late adenoviral infection. CELO VAI (Zakharchuk et al., 1995) was formed by annealing two partially complementary oligonucleotides and filling in with DNA polymerase and dNTPs. CELO VAI was then cloned into the BssHII and XbaI sites of pNEB193-T7. CELO-VAI is expressed from an intrinsic RNA Pol-III promoter. The chicken embryonic lethal orphan (CELO) VAI RNA is smaller (∼90 nt) than the mammalian VAI RNAs (∼160 nt). Such VA RNAs can serve as chimeras to support expression, stability, and function of hhRzs (Lieber and Strauss, 1995; Zakharchuk et al., 1995; Abdelmaksoud et al., 2009; Yau and Sullivan, submitted). Prislei-725HH16 and pCELO725HH16 are hhRzs expressed in Prislei-VAI and pUC-CELO, respectively, and designed to target human rod opsin (RHO) mRNA. The hhRz cDNAs were ligated into respective plasmids such that the hhRz components of the expressed chimeric RNAs would reside in expected loop regions of the Prislei-VAI and CELO-VAI RNAs. shRNA cDNA constructs for RNAi were directionally ligated into the BglII/XhoI sites into pSUPER.puro vector (Oligoengine, Seattle, WA), which expresses shRNAs from a strong extrinsic H1 promoter (RNA Pol-III) (Brummelkamp et al., 2002). Rhoi2 is a shRNA construct previously reported to suppress human RHO mRNA (Cashman et al., 2005).
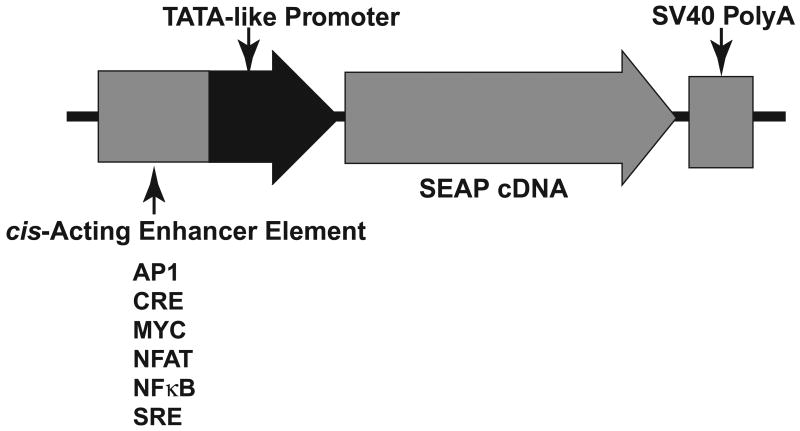
Plasmids containing the SEAP reporter gene downstream of specific transcription factor binding sequences such as cAMP response element (CRE), nuclear factor of activated T-cells (NFAT), activator protein 1 (AP1), Myc, serum response element (SRE), and NFκB were obtained from Clontech (Mountain View, CA) as part of the SEAP Pathway Profiling System which evaluates the induction of a broad spectrum of key cellular signaling pathways. A general schematic of the Pathway Profiling SEAP expression vector element is shown (Fig. 1).
Fig. 1. SEAP Pathway Profiling Vector Expression Element.
The Pathway Profiling vectors contain a cis-acting enhancer element followed by a TATA-like promoter element (TAL) from thymidine kinase which is located upstream of the coding sequence for SEAP. The SV40 late polyadenylation signal ensures efficient processing of SEAP mRNA in eukaryotic cells. The response elements that were investigated in this study are listed.
Cell Culture and Transfection Conditions
Suspension-adapted human embryonic kidney cells (HEK293S) (Stillman and Gluzman, 1985) were engineered to stably express macrophage scavenger receptor 1 (MSR1) for increased cellular adherence to culture substrates (Robbins and Horlick, 1998). HEK293S cells were co-transfected with pCMV6-XL5 (Origene, Rockville, MD) and pPur (Clontech, Mountain View, CA). Transfected cells were selected with 2.5 μg/ml puromycin, and single clonal isolates were obtained and screened for the ability to adhere well to tissue culture plastic. HEK293S-MSR1 cells were cultured at 37°C in Dulbecco's modified essential medium containing F12 salts (DMEM/F12) plus 10% (v/v) heatinactivated bovine serum, L-glutamine (2.5 mM), penicillin and streptomycin, and kept under persistent puromycin selection (2.5 μg/ml) to maintain the stable MSR1 phenotype. For the cell cytotoxicity assay using SYTOX Green (Invitrogen, Carlsbad, CA) cells were seeded into 96 well plates (Black Optilux; Product No: 353220; Becton Dickinson, Franklin Lakes, NJ) and transfected (Lipofectamine 2000, Invitrogen, Carlsbad, CA) in Opti-MEM I reduced serum media (Invitrogen, Carlsbad, CA) according to the manufacturers recommendations with 500 ng of the VAI-Chimera control vector, VAI-Rz-Chimera constructs or shRNA constructs only. For transient transfections with promoter-SEAP plasmids, cells were seeded into 96 well plates (Black Optilux) and co-transfected (Lipofectamine 2000, Invitrogen, Carlsbad, CA) in Opti-MEM I reduced serum media (Invitrogen, Carlsbad, CA) with 500 ng of SEAP reporter plasmid with 500 ng of VAI-Chimera control vector, VAI-Rz-Chimera constructs, shRNA constructs, or empty pCDNA3.1 (Invitrogen, Carlsbad, CA) and 100 ng of pEGFP-NI plasmid (Clontech, Mountain View, CA) per well.
PTGS-Induced Cytotoxicity Assay
After the transfection the media was replaced with complete serum-containing media. At 72 hours post-transfection media was removed and cells were incubated in DMEM/F12 containing 0.5 μM SYTOX Green nuclear stain (Invitrogen, Carlsbad, CA) for 30 minutes at 37°C. SYTOX Green fluorescence (excitation maximum: 504 nm, emission maximum: 523 nm; fluorescence quantum yield: 0.53) was measured on a Nikon TE-300 inverted microscope equipped with a 4× lens and a dichroic cube (Excitation filter peak: 500 nm, Emission filter peak: 530 nm) using IP-Lab software controlling the 12-bit RetinX camera. Following a 1 hour incubation with 1% (w/v) saponin in DMEM/F12 at 37 °C to permeabilize cell surface membranes, SYTOX Green fluorescence was measured again. The ratio of SYTOX Green emission without and with saponin was taken as a measure of percentage of dying cells in each population. The SYTOX Green signal from saponin permeabilized HEK293 cells is linear over several log-orders of cell number in a 96-well plate assay (Butler and Sullivan, in preparation).
PTGS-Induced Signal Pathway Profiling Assay
HEK293S-MSR1 cells were plated onto 96-well dishes at 80% confluence in complete culture medium containing serum. Cells were then transfected in Opti-MEM I reduced serum media (Invitrogen, Calsbad, CA) with 500 ng of plasmids containing CRE, NFAT, AP1, Myc, SRE and NFκB transcriptional response elements modulating expression of a SEAP cDNA (SEAP Pathway Profiling System; Clontech, Mountain View, CA; product number: 631910), 500 ng of various shRNA, VAI-hhRz chimera, and vector cDNAs, and 100 ng of pEGFP-NI (Clontech, Mountain View, CA), and then 150 μl of serum-free DMEM/F12 containing L-glutamine (2.5 mM) replaced an equal volume of the media used in transfection and cells were maintained until harvest. The total amount of DNA was maintained at 1.1 μg with the addition of a control plasmid pCDNA3.1 (Invitrogen, Carlsbad, CA). After 24 hours positive stimulators (controls) of the enhancer elements were added to control wells at the concentrations recommended by the manufacturer. Serum (20% vol/vol) was used to induce Myc- and SRE-dependent SEAP expression, while a combination of serum and phorbol 12-myristate 13-acetate (PMA) (100 nM) was used to stimulate the AP1 response element. CRE-dependent SEAP expression was induced with forskolin (20.5 μg/ml), and NFAT- and NFκB response elements were stimulated with PMA (100 nM) and tumor necrosis factor-alpha (TNF) (20 ng/ml), respectively. Media was collected after 72 hours and assayed for SEAP enzyme activity. Conditioned cell culture media (70 μl) was removed from each culture well, transferred to separate wells in black-walled 96 well plates (Becton Dickinson) and incubated at 65 °C for 30 minutes to inactivate non-specific heat-labile phosphatases. After cooling samples to room temperature, 65 μL of SEAP assay buffer (1 M diethanolamine pH 9.8, 2 mM MgCl2, 1 mM L-homoarginine (an inhibitor of non-SEAP phosphatases) was added per well. 5 μL of 4-methyl umbelliferyl phosphate (4-MUP) substrate was added to each well for a final concentration of 0.04 mM per well and incubated for 85 minutes at room temperature (21°C) in the dark (Yau and Sullivan, submitted). SEAP converts the minimally fluorescent 4-MUP substrate into a strongly fluorescent 4-methyl-umbelliferone product (355 nm peak excitation, 460 nm peak emission). Fluorescence was assayed on a Fluoroskan Ascent FL plate reader (Thermo Scientific, Vantaa, Finland, Model number: 5210462) (excitation: 355 ± 19 nm FWHM (full width of spectral band at half maximum throughput); emission: 460 ± 12 nm FWHM).
Average relative fluorescent units were determined for each transfection and expressed as fold activation relative to control vector-transfected cells. To monitor transfection efficiency, EGFP fluorescence was measured in each well containing cells on a quantitative microscope imaging platform with the excitation, dichroic and emission filters chosen to appropriately quantitate EGFP (Excitation: 500, Emission: 530 nm).
Data Collection and Analysis
All PTGS-induced cytotoxicity and transcriptional signaling experiments were performed in triplicate, and a total of at least 3 independent experiments were conducted to obtain means and standard error of means for each experimental variable. Outcomes of transfection experiments to evaluate cytotoxicity and SEAP Pathway Profiling were initially subjected to one-way ANOVA. For datasets where the ANOVA demonstrated that the sample means were not the same, pairwise t-tests were used to compare sample means of individual test conditions relative to control or to compare between-sample means.
Results
PTGS-induced Cytotoxicity Screen
We first sought to determine the extent to which the transfection and cellular expression of plasmids for VAI-hhRz chimeras, shRNA constructs, and their control vectors were associated with significant human cell death. Plasmids harboring these expression constructs and their controls (plasmid harboring the promoter and RNA chimera but without the target specific PTGS sequence) were transfected into HEK293S-MSR1 cells seeded into 96 well dishes. Cellular apoptosis/viability was assayed 72 hours after transfection with SYTOX Green as a fluorescent reporter that is readily utilized for HTS. SYTOX Green (or other SYTOX dyes: SYTOX Blue, SYTOX Orange) becomes brightly fluorescent only after binding to nuclear DNA (∼1000-fold fluorescence enhancement upon DNA binding). SYTOX dyes are completely excluded from healthy cells. Cells undergoing apoptosis have compromised plasma membranes which allow the SYTOX dye to enter the cell and its nucleus, where it binds to nuclear DNA and elicits a strong fluorescence emission signal. There is a small fraction of cells dying in HEK293S-MSR1 populations in routine cell culture without any adverse stimulus (Fig. 2A). There is no increase in cell death with transfection of a PTGS agent (Fig. 2B). After quantifying cell death due to the transfected agents, the addition of the detergent saponin permeabilized all cells to allow a total cell count with the same dye (Fig. 2C). The ratio of total fluorescence emission in untreated and saponin treated cells gives a percent of total apoptotic cell death in the population.
Fig. 2. High throughput cytotoxicity assay.
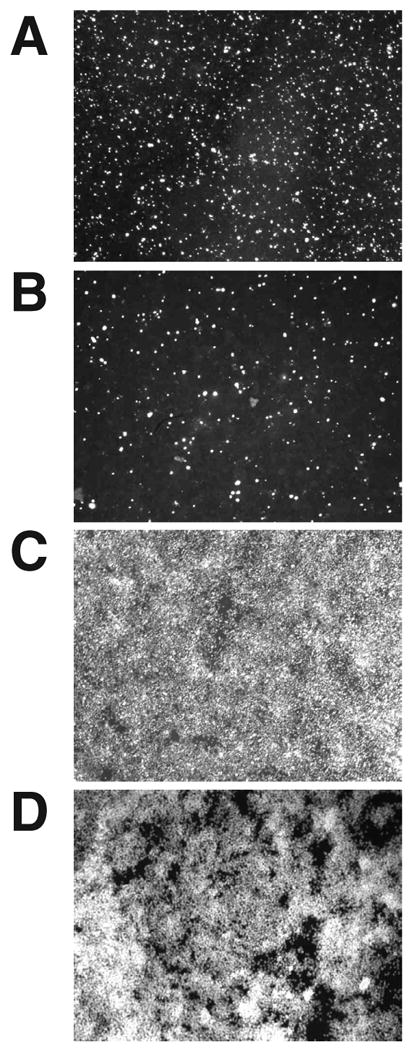
HEK293S-MSR1 cells were transfected with the PTGS agents Rhoi2, Rhoi725, Prislei-725HH16, and pUC-CELO-725HH16, as well as the control vectors pUC-VAI, Prislei-VAI, Rhoiscramble, and pUC-CELO, and later stained with SYTOX Green at 72 hrs post-transfection. SYTOX Green Fluorescence from a representative non-transfected cell sample (A), a cell sample transfected with Prislei-725HH16 (B), and after cell surface permeabilization of the non-transfected (C) and transfected (D) samples with saponin.
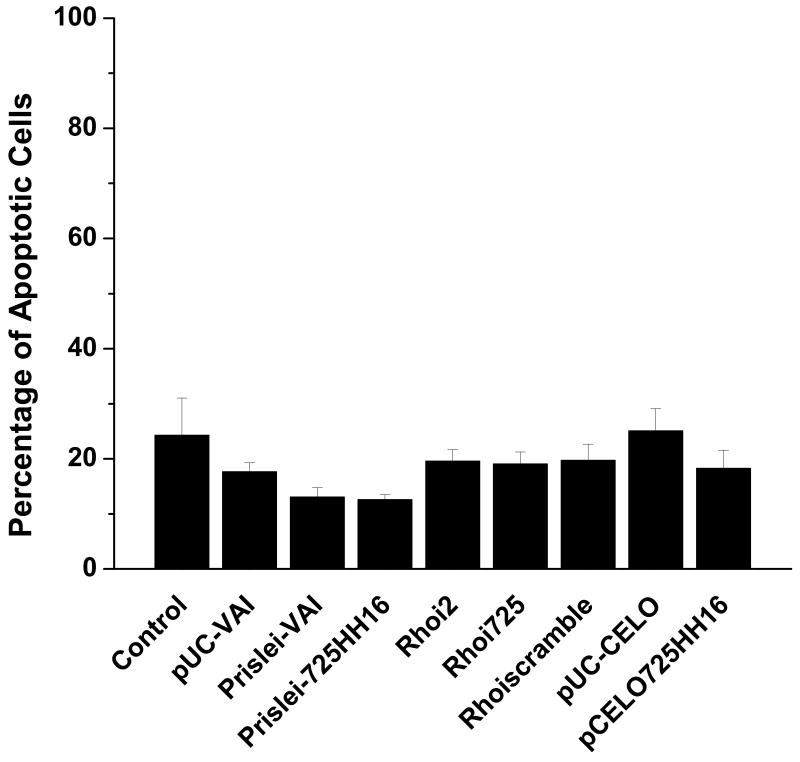
There was no significant cell death associated with the introduction and expression of the PTGS agents or control vectors when compared to the untransfected control cells (Fig. 3). None of the PTGS agents or their control vectors (without PTGS sequences) exerted significant cytotoxicity in human HEK293S-MSR1 cells relative to controls. These results suggest that the VAI RNAs, the VAI-hhRz chimeras, the shRNA constructs, and the plasmid vectors do not cause apoptotic cell death after plasmid transfection and expression into the human cellular environment of this human cell line. All of these agents were expressed from strong RNA Pol-III promoters (intrinsic promoters in the adenoviral VAI and CELO expression constructs and an extrinsic promoter (H1) in the shRNA expression constructs). This suggests that the lead candidate hhRz or shRNA agents against human RHO mRNA do not activate cellular apoptosis on the practical time scale of a transient transfection experiment (72 hrs here) in this model cell line.
Fig. 3. PTGS agents do not exert cytotoxicity in HEK293S-MSR1 cells.
Cell death in response to transfection with VAI-hhRz chimeras and shRNAs was assayed by staining dead cells with SYTOX Green nucleic acid stain and measuring SYTOX Green fluorescence 72 hours post-transfection. Cells undergoing apoptosis have permeable cell surface membranes and take up SYTOX Green which binds to nuclear DNA to result in strong enhanced fluorescence emission. After 1 hr incubation with 1% saponin to permeabilize all cell membranes, an estimate of total cell number was determined by again measuring SYTOX Green fluorescence emission. The VAI-hhRz chimeras and shRNA constructs did not cause any significant cell death when compared to the non-transfected control (ANOVA p= 0.69484).
Transcriptional Activation Assays of PTGS-Induced Cell Stress
In order to determine the extent to which the VAI-hhRz chimeras, shRNA constructs, and control vectors are capable of activating specific cellular stress-related promoter response elements, HEK293S-MSR1 cells were seeded onto 96-well dishes and transiently co-transfected with the PTGS agent plasmids and plasmids containing specific promoter response element sequences upstream of a SEAP reporter cDNA and a polyadenylation signal (see Fig. 1). Measures were made relative to the expression of the signal reporter plasmid in the absence of the PTGS expression plasmid. Activation of the cellular signaling pathway leads to transcriptional activation of the promoter of the specific reporter plasmid, which promotes SEAP expression and its secretion into the cellular culture medium. Culture media was assayed for SEAP enzyme activity at 72 hours after transfection. Results are expressed as foldactivity over control cells transfected with the SEAP reporter constructs alone. SEAP, which is a form of placental alkaline phosphatase engineered for secretion, is an ideal reporter element because 95% of the protein is secreted into the extracellular medium, where it is stable, and the secretion profile reflects the steady-state level of the SEAP mRNA in the cells, which is an index of the strength of promoter activation (transcriptional rate) as the SEAP mRNA degradation rate is expected to be otherwise constant (Berger et al., 1988).
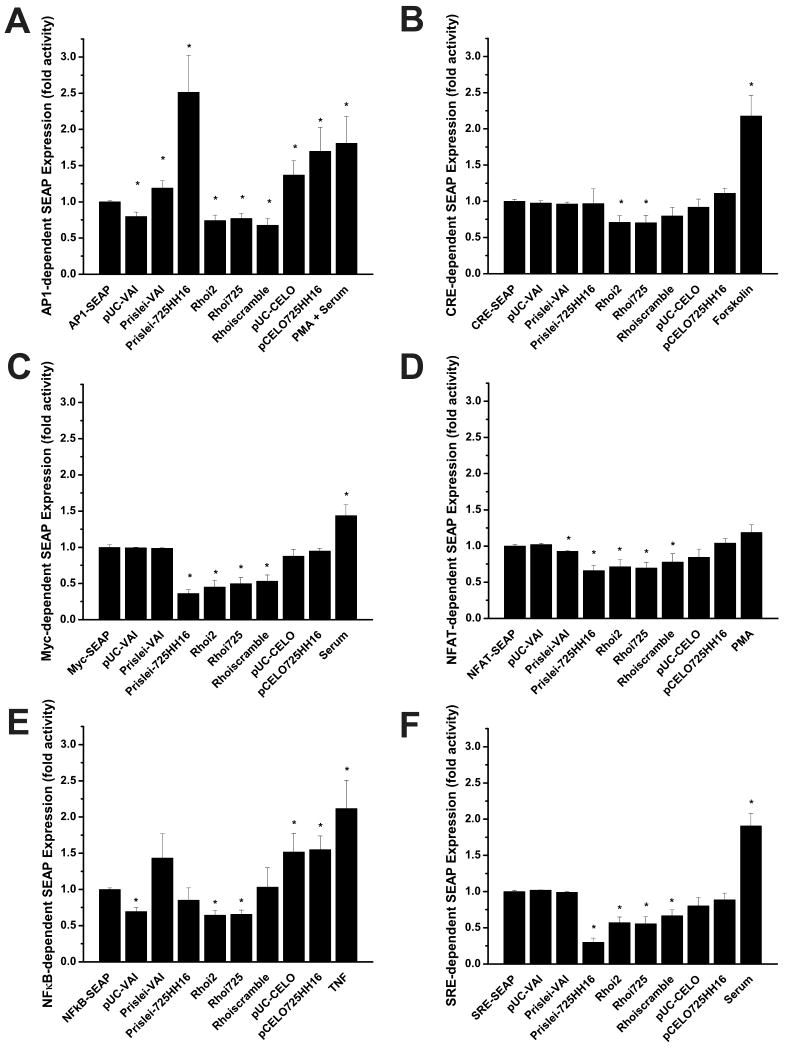
The positive controls for the SEAP pathway profiling were significantly increased in five (AP-1, CRE, Myc, NFκB, SRE) of six of these tests (p≤ 0.05), while the PMA positive control in the NFAT induction experiment lead to a increase in mean SEAP secretion but this was not statistically significant (p = 0.089) (Fig. 4). When co-transfected with the SEAP pathway profiling reporter plasmids, the VAI-hhRz chimeras and shRNA constructs did not induce any significant increases (upregulation) in SRE-, Myc-, CRE-, or NFAT- dependent SEAP expression relative to negative or positive controls on the time scale of 72 hours. In fact, some of the constructs promoted significant decreases in SEAP expression in the CRE, Myc, NFAT, and SRE screens (see legend for Fig. 4). Of the six stress sensing enhancer elements screened in these experiments, only the AP-1 and NFκB elements produced a significant increase in SEAP activity in response to transfection of specific VAI control vectors or VAI-hhRz chimeras. For both the AP-1 and NFκB screens the null hypothesis (no differences) of the ANOVA test was refuted (p< 0.001). With the AP-1 enhancer element the VAI-hhRz chimeras (Prislei-VAI (p < 0.05) and pUC-CELO (p < 0.05)), and their hhRz expression constructs (Prislei-725HH16 (p< 0.01) and pCELO725HH16 (p< 0.01)), produced a significantly greater level of SEAP expression (transcriptional upregulation of AP-1 response elements) relative to control reporter plasmid alone. No other constructs lead to significant enhancement of AP-1 mediated SEAP secretion relative to control (all p> 0.05), and some constructs promoted a significant decrease in AP-1 mediated SEAP expression (see legend for Fig. 4). For AP-1 a slightly greater increase in mean SEAP expression was seen with Prislei-725HH16 compared to pCELO725HH16, but this increase was not significant (p> 0.05). With the NFκB enhancer element both the CELO VAI parent construct (p< 0.01) and the pCELO725HH16 PTGS construct (p< 0.001) produced a significant increase in SEAP expression relative to control. No other constructs lead to significant enhancement of NFκB mediated SEAP secretion relative to control (all p> 0.05). Again, some constructs promoted a significant decrease in NFκB signaling (see legend Fig. 4).
Fig. 4. Transcriptional Activation Assays.
VAI-hhRz chimeras and shRNA targeting accessible regions in human rod opsin mRNA (RHO mRNA) were co-transfected into HEK293S-MSR1 cells with plasmids containing a SEAP reporter gene downstream of different transcription factor binding sequences. Transfection media was removed and replaced at 24 hrs post-transfection. Known activators of each response element were also added as positive controls (Forskolin 20.5 μg/μl; Serum 20% (v/v); TNFα 20 ng/ml; PMA 100 nM). SEAP protein was assayed on aliquots of extracellular growth media at 72 hrs post-transfection. Results are expressed as fold activity over the control and are an average of at least three independent experiments. (A) AP1 testing (ANOVA: F= 5.706, p= 1.05E-6; statistically significant upregulated internal t-test: Prislei-VAI t= 2.052 p= 0.049; Prislei-725HH16 t= 3.249, p= 0.003; pUC-CELO t= 2.653 p= 0.014; pCELO725HH16 t= 3.02, p= 0.006; PMA + Serum t = 2.015, p = 0.051; statistically significant downregulated internal t-test: pUC-VAI t= -3.752 p= 8.131E-4; Rhoi2 t= -3.685 p= 8.695E-4; Rhoi725 t= -3.057 p= 0.004; Rhoiscramble t= -3.828 p= 5.873E-4), (B) CRE testing (ANOVA: F= 9.229, p= 6.775E-12; statistically significant upregulated internal t-test: Forskolin t= 4.12, p= 3.038E-4; statistically significant downregulated internal t-test: Rhoi2 t= -3.105 p= 0.004; Rhoi725 t= -2.853 p= 0.008), (C) Myc testing (ANOVA: F= 14.411, p= 2.909E-14; statistically significant upregulated internal t-test: serum t= 2.533, p= 0.018; statistically significant downregulated internal t-test: Prislei725HH16 t= -9.549 p= 8.07E-10; Rhoi2 t= -5.462 p= 1.735E-5; Rhoi725 t= -5.306 p= 2.518E-5; Rhoiscramble t= -5.052 p= 4.647E-5), (D) NFAT testing (ANOVA: F= 6.357 p= 2.67E-8; statistically significant downregulated internal t-test: Prislei-VAI t= -2.368 p= 0.027; Prislei-725HH16 t= -3.953 p= 4.165E-4; Rhoi2 t= -3.804 p= 7.087E-4; Rhoi725 t= -2.897 p= 0.007; Rhoiscramble t= -2.119 p= 0.044), the PMA positive control had increased SEAP expression but this was not statistically significant (t= 1.761, p = 0.089), (E) NFκB testing (ANOVA: F= 4.909, p= 1.08E-5; statistically significant upregulated internal t-test: pUC-CELO t= 2.886, p= 0.008; pCELO725HH16 t= 4.129, p= 3.56E-4; TNF t= 2.854, p= 0.007; statistically significant downregulated internal t-test: pUC-VAI t= -5.971 p= 1.979E-6; Rhoi2 t= -5.384 p= 7.142E-6; Rhoi725 -5.537 p= 3.445E-6), (F) SRE testing (ANOVA: F= 20.912 p= 0; statistically significant upregulated internal t-test: serum t= 4.606, p=1.036E-4; statistically significant downregulated internal t-test: Prislei-725HH16 t= -9.883 p= 4.063E-10; Rhoi2 t= -5.425 p= 1.893E-5; Rhoi725 t= -4.460 p= 1.96E-4; Rhoiscramble t= -3.969 p= 6.510E-4) * denotes a statistically significant increase (upregulation) or decrease (downregulation) in SEAP activity relative to the control (p ≤ 0.05).
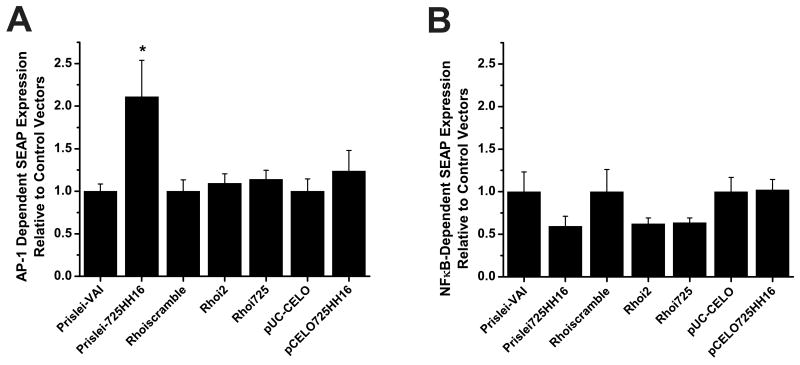
For the AP-1 and NFκB signaling pathways t-tests were conducted to determine the extent to which the PTGS expression vectors were able to activate SEAP reporter expression relative to control vectors that expressed either an empty RNA chimera (e.g. without an embedded hhRz cDNA, e.g. pUC-CELO) or a scrambled irrelevant shRNA sequence (i.e. Rhoiscramble) (Fig. 5). Here, the SEAP expression data were normalized to the mean level of the respective control vector. With the AP-1 enhancer element the increase in SEAP expression was significantly greater for the Prislei-725HH16 compared to the control vector (Prislei-VAI) that lacked the hhRz (p< 0.05) (Fig. 5A). There was no significant increase in AP-1 mediated SEAP expression by pCELO725HH16 when compared to the control vector, pUC-CELO (p>0.05), and there was no significant increase in SEAP expression of either active shRNA expression vector (Rhoi2, Rhoi725) compared to the scrambled shRNA control (Rhoiscramble) (p> 0.05). With the NFκB enhancer element there was no significant increase in SEAP expression of any of the active PTGS expression constructs relative to their respective control vectors (Fig. 5B).
Fig. 5. NFκB and AP1 Activation by PTGS Agents Compared to Activation by Control Vectors.
The increase in AP1-1- and NFκB-dependent SEAP expression by the PTGS agents was examined relative to the SEAP induction produced by their respective control vectors. Here, data for the respective comparisons was normalized to the mean value of the appropriate corresponding control. (A) AP1 testing (t-tests: Prislei vectors t= 2.53582, p = 0.01708; Rhoi2 vs Rhoiscramble t = 0.54065, p = 0.59303; Rhoi725 vs Rhoiscramble t= 0.82301, p = 0.41679; CELO vectors t= 0.8454, p = 0.41035), (B) NFκB testing (t-tests: Prislei vectors t= -1.555 p= 0.131; Rhoi2 vs Rhoiscramble t= -1.397 p= 0.173; Rhoi725 vs Rhoiscramble t= -1.483 p= 0.148; CELO vectors t= 0.101 p= 0.920). * denotes a significant increase in SEAP expression relative to the respective control vector (p ≤ 0.05).
Discussion
Need for High Throughput Screening Tools in RNA Drug Discovery
Initial challenges in the development of PTGS agents revolve around finding accessible sites in the target mRNA and designing an appropriate agent which causes efficient knockdown in the target mRNA and protein levels (Sullivan et al., 2008; Abdelmaksoud et al., 2009). High throughput approaches are useful if not essential in the effective and efficient identification of lead PTGS candidates and their rational optimization. We have directed substantial effort in the development of such approaches (Yau and Sullivan, submitted; Sullivan et al., work in progress). Achieving high potency or efficacy under conditions of minimized cellular PTGS expression is the ideal condition that minimizes potential for cellular toxicity. Toxicity is a critical issue in the development and clinical translation of expressable RNA drugs. Toxicity could arise from many mechanisms which include but are not limited to: 1) off-target effects of the PTGS agent which binds to unintended target mRNAs and promotes their cleavage or translational suppression, 2) nonspecific effects of usurping cellular metabolism or RNA processing streams that creates stress and toxicity for the cell due to expression of the PTGS agent, 3) activation of intrinsic cellular dsRNA immune mechanisms (e.g. PKR), 4) activation of cellular signaling mechanisms that promotes stress responses (e.g. TLR3). Clearly, the capacity to assess the potential for toxicity of PTGS agents in the human cellular milieu under high throughput conditions could provide useful or essential information to begin to determine the safety of a given candidate PTGS agents prior to preclinical or possible future human clinical testing.
We developed a HTS approach to evaluate the potential toxicity of candidate therapeutic PTGS agents as expressable RNA drugs in the human cellular environment. We are testing these agents prior to preclinical testing in appropriate animal models of disease. We developed experimental approaches to assess the impact of PTGS agents on cell viability and also to assess test the activation of key signal transduction pathways that are transcriptionally modulated under conditions of cellular stress. A human cell culture system was chosen as a model system that is expected to embrace much of the critical housekeeping functionality (e.g. cell death activation mechanisms, cell signaling mechanisms) in which toxicity is likely to emerge when such agents are tested in mammalian disease model systems. This particular cell culture system (HEK293S-MSR1) has high transfection efficiency with plasmids, which makes the HTS evaluation of PTGS efficacy or toxicity more reliable and efficient, and is engineered to resist detachment from cell culture surfaces which enables utilization of the 96-well plate environment to screen for cytotoxicity events in attached cells and to measure the extracellular fluid to probe intracellular signaling processes with the SEAP reporter system.
Cytotoxicity Screen
Cell death in response to exposure to PTGS agents is detected through addition of SYTOX Green nuclear stain to the culture medium. SYTOX Green only enters cells with compromised plasma membranes, which occurs during cellular apoptosis, or is induced to occur through the use of gentle detergents (e.g. saponin) that permeabilize the plasma membrane. First, quantitative assessment of the level of dead or dying cells occurs through measured SYTOX Green fluorescence emission enhancement that occurs after dye introduction into cell culture medium followed by binding to nuclear DNA in cells that already have compromised surface membranes. Then, saponin is added to uniformly permeabilize all surface membranes, and SYTOX Green fluorescence is used to determine total cell number. The percentage of dead or dying cells in each sampled population is then determined as the ratio of initial SYTOX fluorescence emission in the transfected cell population to the final SYTOX fluorescence emission after saponin permeabilization. In another study we determined that SYTOX dyes (Green or Blue) could be used to quantify total cell number with saponin in the 96-well environment with linear dynamic range over several log orders (Butler et al., in preparation). Therefore, we expected that the SYTOX Green would be useful when employed as a ratiometric assessment of cytotoxicity of expressed RNA drugs in a simple HTS assay. This assay, with a single fluorescent probe, is performed in 96-well plates, and provides rapid and reproducible detection of cell death. Previously, SYTOX Orange was used and validated in an HTS assay to determine apoptosis-inducing genes (Pierce et al., 2003). Here we have extended the capabilities of SYTOX dyes to evaluate the potential for cellular toxicity of candidate therapeutic PTGS agents (hhRzs, shRNAs) expressed from delivered transgenes. The assay could also be used to test for toxicity of transfected or expressed siRNA or miRNA candidate PTGS agents. Moreover, this single dye assay could readily be extended to robotic HTS platforms.
We have shown here that all of the PTGS agent expression vectors developed to target human RHO mRNA, and their parent control vectors without ribozyme or shRNA cDNA inserts, did not produce any significant cell death when compared to the untransfected control. This outcome illustrates that the process of simply transfecting plasmid DNAs into HEK293S-MSR1 cells with Lipofectamine 2000 does not intrinsically promote enhanced cell death. It also shows that the structured RNAs that are used to support hammerhead ribozyme expression (as chimeras) do not promote significant cell toxicity or death in this model human cell system over the temporal integration period of 72 hours. The insertion of hammerhead ribozymes into these structured RNAs as chimeras is also non-toxic to these cells. Finally, the expression of a scrambled shRNA and two human RHO targeting shRNAs is not toxic to these cells. This novel approach establishes a first order screen for toxicity in a human cell line that can be conducted under a simple HTS protocol. The approach could be readily adapted to other cell lines.
Further commentary is suggested for Figure 3 in that there was greater measured cell death in the control sample set than in samples transfected with diverse PTGS agents. This outcome suggests that the human cell based HTS cytotoxicity assay could potentially be used to quantitatively assess the ability of discrete PTGS agents to rescue cells from specific toxic agents or mutant genes. While “rescue” strategies for gene-based therapeutics are typically considered in the context of an animal model experiment, it may be that cultured cells house sufficient machinery (e.g. housekeeping functions) that may be able to sense stress exerted by expression of particular toxic mutant proteins with gain-of-function properties (e.g. misfolding and ER stress responses). While this was not the initial goal of the experiment and indeed we did no co-express any plasmid to express a target mRNA that would translate a toxic gain-of-function protein, this could be done in the future and further developed and exploited as a potential in cellulo assay of rescue. Clearly, this approach would be constrained by the nature of the target gene and the specific mutation, and the ability of certain cells to harbor machinery that would respond to specific types of cell stress that might be also found in vivo.
Stress Pathway Profiling
The SEAP Pathway Profiling System has been validated and used extensively to assess the effects of agents or perturbations on specific signal transduction pathways in the cellular environment (Ohkubo et al., 2001; Galetic et al., 2003; LaVoie and Selko, 2003; Manna and Ramesh, 2005; Zhu et al., 2006). Here, we assessed the activation of a number of elements and pathways that signal cellular stress. The use of a SEAP reporter, which is stoichiometrically secreted by the cell into the culture medium in proportion to the steady state level of mRNA expressed, allows for rapid and reliable detection of the activation of transcriptional enhancer elements in a convenient assay that can be subjected to an HTS approach. As we had already established a HTS fluorescence enzyme reporter assay for SEAP in 96-well plate format to conduct HTS for PTGS efficacy (Yau and Sullivan, submitted), we were able to rapidly embrace the SEAP Pathway Profiling system as a means to test for the activation of specific signaling pathways that occur through expression of candidate therapeutic chimeric PTGS RNAs or their structured RNA supports (controls). The PTGS agents evaluated in this study were all designed against the therapeutic target of human RHO mRNA and its cognate protein rod opsin, and these agents have demonstrated potency in the human cellular environment (Sullivan et al., work in progress). Here, we tested the capacity of these agents to activate signaling pathways in the absence of expression of the RHO target mRNA and protein. The PTGS agents tested did not produce any significant activation of the, CRE, NFAT, Myc, or SRE transcriptional enhancer elements relative to the individual SEAP reporter plasmids in the absence of PTGS expression plasmid (or their controls).
In initial experiments (outcomes not shown) there was significant activation, relative to reporter control, of both the AP-1 and NFκB enhancer elements after transfection of all PTGS expression plasmids and their structured RNA control plasmids. However, the control vector in these initial experiments was a SEAP reporter expression plasmid that had no enhancer element upstream of the minimal TAL promoter and SEAP cDNA and naturally expressed very low levels of SEAP. In these initial experiments, the most significant SEAP expression was produced by cells transfected with alternative control SEAP reporter plasmids that contained both the specific promoter/enhancer element and the SEAP cDNA, but in the absence of the PTGS control or chimera plasmids (data not shown). There appeared to be a level of background or constitutive activation of both AP-1 and NFκB elements in HEK293 cells in the absence of PTGS vectors or their controls. The AP-1 transcription factor is known to be activated by adenoviral E1A protein, which is constitutively expressed in HEK293 cells (Müller et al., 1989; Stillman and Gluzman, 1985). Because of these initial outcomes, in the subsequent experiments reported here, activation of the enhancer elements by the PTGS agents and their respective controls was evaluated in comparison to the background levels of activation produced by cells transfected with the individual control SEAP reporter plasmids containing the enhancer, the minimal promoter, the SEAP cDNA and polyadenylation signal (i.e. without cotransfection of structured chimeric RNA PTGS expression plasmids, and without shRNA expression plasmids) (Fig. 4). In these later experiments only the Prislei-725HH16 construct and the pCELO725HH16 construct and their respective chimeric control vectors (Prislei-VAI, pUC-CELO) increased or upregulated AP-1 mediated SEAP expression, while both the pUC-CELO and the pCELO725HH16 constructs activated NFκB mediated SEAP expression. For the signaling pathways that were probed in these experiments there was surprisingly little significant stress induced by the series of PTGS expression plasmids and their controls, except for specific instances with AP-1 and NFκB activation. It is prudent to consider possible origins for these discrete effects.
Given the potentially diverse gene regulatory effects possible with introduction or expression of discrete PTGS RNA agents, it is essential to probe for effects of PTGS agents on the cellular environment. Of the several signaling pathways tested here we observed transcriptional activation of only AP-1 and NFκB enhancer elements in response to transfection of specific PTGS expression plasmids or control vectors based on VA RNAs, but not by the shRNA agent or its control. The precise cellular mechanisms that lead to these transcriptional activations, as indicators of cellular stress, are currently unclear and under investigation. Critically, the full set of PTGS DNA expression constructs tested in these experiments do not promote cell death when transfected into human HEK293 cells.
In summary, we report on a method and approach to rapidly investigate the effects of candidate therapeutic PTGS agents on both cell viability and signal transduction pathways. Cytotoxicity of PTGS agents was evaluated using the SYTOX Green nuclear fluor to quantify changes in cell viability that may occur due to expression of PTGS agents. Cellular stress due to PTGS agents was evaluated by transcriptional activation of common pathways with tools available in the SEAP Pathway Profiling system. The tools of this pathway profiling can be enhanced to include additional transcriptional response elements. Together these methods provide an inexpensive and efficient means to begin to evaluate the safety of PTGS agents in vivo. We envision that these approaches will serve as a platform for development of more elaborate and diverse screens of potential toxicity of PTGS agents in the human or mammalian cellular environment as a prelude to preclinical and potentially clinical testing.
Research Highlights.
A HTS assay to assess cellular toxicity of post-transcriptional gene silencing agents
A HTS single well and single dye ratiometric cytotoxicity assay assesses cell death
A HTS single well and single dye reporter assay assesses cellular stress pathways
No cytotoxicity and little stress was observed with PTGS agents targeting human RHO
Use for rapid screens of cellular toxicity and stresses of ribozyme and RNAi agents
Acknowledgments
We acknowledge the construction of pNEB193-T7 plasmid in this lab by Mark C. Butler, M.S. We acknowledge the development in this lab of the HTS SEAP enzyme fluorescence assay, and the pUC-VAL, and pPrislei VAI expression constructs (without and with hhRz 725), and the shRNA constructs (Rhoiscramble, Rhoi2, Rhoi725) by Edwin H. Yau, Ph.D. We acknowledge the strong collegial support of our colleagues at the University at Buffalo and at the Veterans Administration Western New York Healthcare System, where this work was conducted. We also acknowledge the strong support of the Department of Ophthalmology, and the Dean's office at University at Buffalo. The Department of Ophthalmology at the University at Buffalo- SUNY is a participating member of the SUNY Eye Institute. Finally, we thank the reviewers of the manuscript for their helpful and insightful comments.
Funding: National Eye Institute (R01-EY013433, PI: JMS); NEI R24 UB Vision Infrastructure grant (NEI/NIH R24 grant EY016662 (UB Vision Infrastructure Center, PI: M Slaughter, Director- Biophotonics Module: JMS)); Research to Prevent Blindness- Challenge and Unrestricted Grants (to the Department of Ophthalmology at the University at Buffalo); grant from the Oishei Foundation (Buffalo, NY) to the Dept of Ophthalmology at University at Buffalo.
Abbreviations
- AP-1
activator protein
- CNV
choroidal neovascularization
- CRE
cyclic AMP response element
- DMEM/F12
Dulbecco's modified essential medium
- dsRNA
double stranded RNA
- HEK293S
human embryonic kidney cells, suspension adapted
- hhRz
hammerhead ribozyme
- HTS
high throughput screening
- miRNA
micro RNA
- MSR1
macrophage scavenger receptor 1
- 4-MUP
4-methyl-umbeliferyl phosphate
- NFAT
nuclear factor of activated T-cells
- NFκB
nuclear factor-kappa beta
- PKR
dsRNA dependent serine protein kinase
- PMA
phorbol myristate acetate
- PTGS
post transcriptional gene silencing
- RHO
human rod opsin
- RIG-1
retinoic acid inducible gene 1
- RNAi
RNA interference
- SEAP
secreted alkaline phosphatase
- shRNA
short hairpin RNA
- siRNA
short interfering RNA
- SRE
serum response element
- TLR3
Toll-like receptor 3
- TNF
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelmaksoud H, Yau EH, Zuker M, Sullivan JM. Development of lead hammerhead ribozyme candidates against human rod opsin for retinal degeneration therapy. Exp Eye Res. 2009;88:859–879. doi: 10.1016/j.exer.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KP, Fox MC, Brown-Driver V, Martin MJ, Azad RF. Inhibition of cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrob Agents Chemother. 1996;40:2004–2011. doi: 10.1128/aac.40.9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Binkley EA, Kumar-Singh R. Towards mutation-independent silencing of genes involved in retinal degeneration by RNA interference. Gene Therapy. 2005;12:1223–1228. doi: 10.1038/sj.gt.3302512. [DOI] [PubMed] [Google Scholar]
- Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- Farrar GJ, Kenna PF, Humphries P. On the genetics of retinitis pigmentosa and on mutation-independent approaches to therapeutic intervention. EMBO J. 2002;21:857–864. doi: 10.1093/emboj/21.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetic I, Maira SM, Andjelkovic M, Hemmings BA. Negative regulation of ERK and Elk by Protein Kinase B modulates c-fos transcription. J Biol Chem. 2003;278:4416–4423. doi: 10.1074/jbc.M210578200. [DOI] [PubMed] [Google Scholar]
- Gil J, Alcami J, Esteban M. Activation of NF-κB by the dsRNA-dependent protein kinase, PKR, involves the IκB kinase complex. Oncogene. 2000;19:1369–1378. doi: 10.1038/sj.onc.1203448. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, Hauswirth WW, Lewin AS. Preservation of photoreceptor morphology and function in P23H rats using an allele independent ribozyme. Exp Eye Res. 2007;84:44–52. doi: 10.1016/j.exer.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Henry SP, Templin MV, Gillett N, Rojko J, Levin AA. Correlation of toxicity and pharmacokinetic properties of a phosphorothioate oligonucleotide designed to inhibit ICAM-1. Toxicol Pathol. 1999;27:95–100. doi: 10.1177/019262339902700117. [DOI] [PubMed] [Google Scholar]
- Jabs DA, Griffiths PD. Fomiversen for the treatment of cytomegalovirus retinitis. Am J Ophthalmol. 2002;133:552–556. doi: 10.1016/s0002-9394(02)01325-9. [DOI] [PubMed] [Google Scholar]
- Johnsen IB, Nguyen TT, Ringdal M, Tryggestad AM, Bakke O, Lien E, Espevik T, Anthonsen MW. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Kayikcioglu OR, Cheng L, Kozak I, Bergeron-Lynn G, Schulteis CT, Rhoades KL, Freeman WR. Toxicity of subretinal ribozyme to the proliferating cell nuclear antigen and 5-fluorouracil in rat eyes. Curr Eye Res. 2006;31:435–440. doi: 10.1080/02713680600672177. [DOI] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Karikó K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Drenser KA, Flannery JG, Lewin AS, Hauswirth WW. Ribozymes rescue of photoreceptor cells in P23H transgenic rats: long-term survival and late-stage therapy. Proc Natl Acad Sci USA. 2000;97:11488–11493. doi: 10.1073/pnas.210319397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Slkoe DJ. The Notch ligands, Jagged and Delta, are sequentially processed by α-Secretase and Presenilin/γ-Secretase and release signaling fragments. J Biol Chem. 2003;278:34427–34437. doi: 10.1074/jbc.M302659200. [DOI] [PubMed] [Google Scholar]
- Lewin AS, Drenser KA, Hauswirth WW, Nishikawa S, Yasamura D, Flannery JG, LaVail MM. Ribozyme rescue of photoreceptor cells in transgenic rat model of autosomal dominant retinitis pigmentosa. Nature Med. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- Lieber A, Strauss M. Selection of efficient cleavage sites in target RNAs by using a ribozyme expression library. Mol Cell Biol. 1995;15:540–551. doi: 10.1128/mcb.15.1.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Ramesh GT. Interleukin-8 induces nuclear transcription factor-.B through a TRAF6-dependent pathway. J Biol Chem. 2005;280:7010–7021. doi: 10.1074/jbc.M410994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JT, Williams BRG. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23(11):1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- Millington-Ward S, O'Neill B, Tuohy G, Al-Jandel N, Kiang AS, Kenna PF, Palfi A, Hayden P, Mansergh F, Kennan A, Humphries P, Farrar GJ. Strategems in vitro for gene therapies directed to dominant mutations. Hum Mol Genet. 1997;6:1415–1426. doi: 10.1093/hmg/6.9.1415. [DOI] [PubMed] [Google Scholar]
- Müller U, Roberts MP, Engel DA, Doerfler W, Shenk T. Induction of transcription factor AP-1 by adenovirus E1A protein and cAMP. Genes Dev. 1989;3:1991–2002. doi: 10.1101/gad.3.12a.1991. [DOI] [PubMed] [Google Scholar]
- Ohkubo N, Mitsuda N, Tamatani M, Yamaguchi A, Lee YD, Ogihara T, Vitek MP, Tohyama M. Apolipoprotein E4 stimulates cAMP response element-binding protein transcriptional activity through the extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:3046–3053. doi: 10.1074/jbc.M005070200. [DOI] [PubMed] [Google Scholar]
- O'Reilly M, Palfi A, Chaderton N, Millington-Ward S, Ader M, Cronin T, Tuohy T, Auricchio A, Hildinger M, Tivnan A, McNally N, Humphries MM, Kiang AS, Humphries P, Kenna PF, Farrar GJ. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Wang C, Rebentisch M, Endo M, Stump M, Kamb A. A high-throughput, homogenous microplate assay for agents that kill mammalian tissue culture cells. J Biomol Screen. 2003;8:283–291. doi: 10.1177/1087057103008003006. [DOI] [PubMed] [Google Scholar]
- Prislei S, Buonomo SM, Michienzi A, Bozzoni I. Use of adenoviral VAI small RNA as a carrier for cytoplasmic delivery of ribozymes. RNA. 1997;3:677–687. [PMC free article] [PubMed] [Google Scholar]
- Robbins AK, Horlick RA. Macrophage scavenger receptor confers an adherent phenotype to cells in culture. Biotechniques. 1998;25(2):240–244. doi: 10.2144/98252st04. [DOI] [PubMed] [Google Scholar]
- Schlee M, Hornung V, Hartmann G. siRNA and isRNA: Two edges of one sword. Mol Ther. 2006;14(4):463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Sledz CA, Williams BRG. RNA interference and double-stranded-RNA-activated pathways. Biochem Soc Trans. 2004;32:952–956. doi: 10.1042/BST0320952. [DOI] [PubMed] [Google Scholar]
- Stillman BW, Gluzman Y. Replication and supercoiling of Simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985;5:2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Yau EH, Taggart RT, Butler MC, Kolniak TA. Bottlenecks in development of retinal therapeutic post-transcriptional gene silencing agents. Vision Res. 2008;48:453–469. doi: 10.1016/j.visres.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau EH, Sullivan JM. Cell-based, high-throughput screening for therapeutic trans-cleaving ribozymes and RNAi. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-1 has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zakharchuk AN, Doronin KK, Karpov VA, Krougliak VA, Naroditsky BS. The fowl adenovirus type 1 (CELO) virus –associated RNA-encoding gene: a new ribozyme expression vector. Gene. 1995;161(2):189–193. doi: 10.1016/0378-1119(95)00251-z. [DOI] [PubMed] [Google Scholar]
- Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol Cell Biol. 2000;20(4):1278–90. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HY, Tso MOM, Lai S, Lai H. Activation of nuclear factor κB during retinal degeneration in rd mice. Mol Vis. 2008;12:1075–1080. [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yao J, Meng Y, Kasai A, Hiramatsu N, Hayakawa K, Miida T, Takeda M, Okada M, Kitamura M. Profiling of functional phosphodiesterase in mesangial cells using a CRE-SEAP-based reporting system. Br J Pharmacol. 2006;148:833–844. doi: 10.1038/sj.bjp.0706785. [DOI] [PMC free article] [PubMed] [Google Scholar]